
Intraoperative cone beam computed tomography of tracheal stenting for stenosis and fistula diseases: a retrospective cohort study
Introduction
The advancement of interventional instruments and the improvement of operative techniques have seen tracheal stent implantation become widely used in the treatment of severe benign and malignant airway stenosis and various types of airway fistulas (1-5). For most patients, stent implantation can significantly alleviate respiratory symptoms; however, some studies have noted its high complications rate (6,7).
Multidetector computed tomography (MDCT) is essential in airway stent placement for stenosis and fistula diseases because it can evaluate the location and extent of a lesion preoperatively and complications after airway stent placement postoperatively (8). However, this procedure must be performed in a CT laboratory, which sometimes delays the detection of complications by the surgeon during the operation. Cone beam CT (CBCT), a functional variant of angiographic C-arm CT, can quickly provide intraoperative 2-dimensional and 3-dimensional (3D) reconstructions similar to CT images (9,10). Using CBCT, a 3D scan of the airway can be obtained, and the complications of airway stenting may also be reduced.
Immediate use of CBCT after placement of an airway stent has been performed in the Department of Interventional Radiology, First Affiliated Hospital of Zhengzhou University, in the last 5 years, and it has sometimes also been used for stent-graft planning in acute cases. Based on the rich experience of interventional radiologists from our hospital, we hypothesized that intraoperative CBCT could be a suitable replacement for MDCT after stent implantation and, in some cases, in stent-graft planning. At the same time, we hypothesized that intraoperative CBCT could reduce the complications of airway stent placement and enhance the surgeon’s confidence of success to perform airway stent placement. To explore these hypotheses, we aimed to evaluate the clinical feasibility of CBCT in airway stent placement by conducting a single-center retrospective cohort study.
We present the following article in accordance with the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-858/rc).
Methods
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Zhengzhou University. Written informed consent was obtained from each patient for the publication of this article and any accompanying images.
The metallic stents received by the patients in this study were made of nickel-titanium alloy memory metal (Nanjing Micro-Tech Medical Company, Nanjing, China). self-expanding, metallic stents (SEMSs) were woven with a temperature-memory nickel-titanium alloy wire and were specially developed according to each individual patient’s airway shape and size as measured by CT.
Case selection
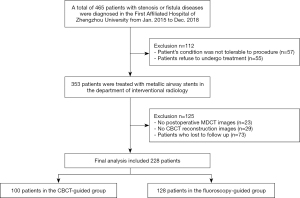
The local database was searched manually by YY and ZQL for records from January 1, 2015, to December 31, 2018. All patients who had been treated in the Western District Intervention Department of the First Affiliated Hospital of Zhengzhou University for tracheal stricture or fistula resulting from disorders that usually occur after intubation or surgery were identified. Patients who had undergone treatment with airway stenting were eligible for inclusion in the study. The case selection and grouping process is presented in Figure 1.
Bronchopleural strictures and fistulas were diagnosed based on clinical manifestations and a chest CT examination. The airway stenting procedures were performed by four international radiologists (ZNL, YL, XZ and DCJ) with 5, 7, 7, and 13 years of intervention experience, respectively.
The exclusion criteria were as follows: (I) postoperative MDCT images could not be obtained in our picture and communication system (PACS); (II) CBCT reconstruction images were not saved; (III) patients with coagulopathy or abnormal cardiopulmonary function; and (IV) patients who could not be followed up as scheduled.
This study was not randomized. Our interventional radiologists rotated daily in the angiography suite (Toshiba, Aquilion 64, Tokyo, Japan) and were equipped with CBCT technology (Artis zee BA Twin; Siemens AG, Munich, Germany; Syngo X-workplace with Syngo CBCT; Siemens AG). They performed airway stent placement using an available imaging system which was assigned to them for the day.
Surgical procedures and image acquisition
All stent placement procedures were performed in a dedicated operating suite—an angiography laboratory either equipped or unequipped with CBCT. All procedures were performed with the patient in a supine position and under conscious sedation. An intramuscular injection of diazepam (10mg) and anisodamine (code name: 654-2; 10mg) was administered 30 minutes before the procedure, to reduce airway secretions and calm the patient. Oxygen was given via a nasal catheter, and a sputum aspirator was prepared.
Airway stenting was performed based on the method presented by Li et al. (11). Under guidance by fluoroscopy and/or CBCT, a 5F catheter over a wire was introduced transorally into the trachea. First, a transcatheter injection of 5 mL of 2% lidocaine was given as local anesthesia, and then 3 mL of water-soluble contrast was injected to confirm the site and size of the fistula and stenosis. Interventional radiological techniques were employed for stent placement and removal. The Y stent apparatus was inserted by advancing it over the guide wires into the correct position using its SEMS delivery system. The delivery system was slowly rotated left and right until both wires had separated. Once the position had been verified, the SEMS deployment was initiated, first by opening the bronchial part and then the tracheal part, until all stent parts were fully open. The delivery system and guide wires were quickly removed, and the location of the stent was confirmed via bronchograms.
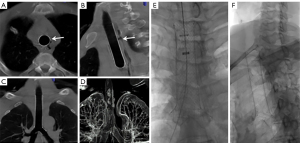
Bronchopleural fistula management was performed as follows: under fluoroscopic or CBCT guidance, an external drainage catheter (8.5, 10.2, or 12 F) with multiple side holes was inserted into the thoracic cavity by percutaneous puncture using the Seldinger technique. The surgical chest tube was then removed. The chest drainage catheter was connected to a syringe by a multidirectional stopcock. The continuous negative pressure of 10–20 mmHg was applied through the syringe for thoracic drainage. According to bacterial culture reports, nebulization and antibiotic treatment were used to reduce phlegm and control infection. Before stent removal, the patient was examined for the presence of granulation tissue, which, if found, was ablated under bronchoscopy. The CBCT and 3DCT images are presented in Figure 2. If the stent was in an unsuitable location or exhibited incomplete deployment, the operator could adjust the position of the stents or introduce a balloon to expand the stents through the guide wire.
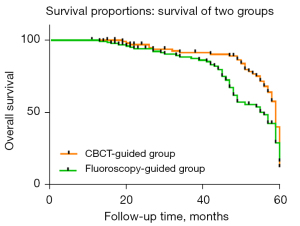
Image analysis
The CBCT image datasets were compared with the post-treatment MDCT images to measure the relevant stent diameters and lengths in suitable planes, and the anatomical details were scored. Each image was independently evaluated by two experienced interventional radiologists (XH, with 14 years of experience; DCJ, with 5 years of experience). The evaluation criteria for the visibility of important anatomical details in the stent graft were derived from “the European Guidelines for Quality Criteria for Computed Tomography” (12), which is a 4-point scale with the following meanings: 1 point = relevant diagnosis impossible; 2 points = acceptable visibility, with relevant diagnosis possible; 3 points = a clear reproduction, with diagnosis possible without restrictions; 4 points = perfect imaging.
Postoperative evaluation and follow-up
The results of imaging follow-up were documented for all patients. Routine MDCT was performed in order to assess possible stent migration or under-expansion within 3 days of the stent placement procedure and again 3 months after. The rate of stent-related complications and the final prognosis for each patient were compared between the fluoroscopy-guided group and the CBCT-guided group.
Technical success was defined as successful placement of the stent in the planned location on the first attempt, as verified on site by fluoroscopy or CBCT. If patients developed adverse clinical conditions, routine MDCT was performed to assess possible stent migration or under-expansion within 3 days of the procedure and again 3 months after. These follow-up studies were performed if adverse clinical conditions developed, irrespective of the planned schedule. Patients were also followed up by telephone to track their progress. Patients who were lost to follow-up were excluded from our study.
Data analysis
The measurement data are presented as the mean ± standard deviation (SD). Categorical data are reported as numbers with percentages in brackets. Comparisons between the two groups were conducted by using the Student’s t-test, the Wilcoxon signed-rank test for continuous variables, and either Pearson’s χ2 test or Fisher’s exact test for categorical variables. Survival time was calculated according to the Kaplan-Meier method, and a P value of <0.05 was considered statistically significant. The variability in relevant measurements and visibility scores between the two interventional radiologists was tested using the kappa or intraclass correlation coefficient (ICC) statistic, with an ICC ≥0.75 considered to be good. The level of agreement for Cohen’s kappa was usually defined as follows: <0.20, poor agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, good agreement; and >0.80, excellent agreement (13). All statistical analyses were performed using the SPSS 23.0 statistical software (SPSS, Chicago, IL, USA).
Results
Clinical characteristics and technical success rate of the patient sample
A total of 228 consecutive patients were enrolled during the period from January 2015 to December 2018. Of them, 100 patients received CBCT-guided airway stenting, and 128 patients received fluoroscopy-guided airway stenting. The baseline characteristics of the CBCT-guided group and fluoroscopy-guided group were well balanced (Table 1).
Table 1
| Characteristic | CBCT-guided (n=100) | Fluoroscopy-guided (n=128) | P value |
|---|---|---|---|
| Age (years), mean ± SD [range] | 59.6±13.6 [16–89] | 60.1±12.7 [21–90] | 0.304* |
| Sex | 0.810† | ||
| Male | 71 | 89 | |
| Female | 29 | 39 | |
| Disease | 0.388† | ||
| Stenosis | 63 | 91 | |
| Fistula | 34 | 35 | |
| Both | 3 | 2 | |
| Etiology | 0.606# | ||
| Esophageal cancer | 52 | 66 | |
| Lung cancer | 21 | 27 | |
| Mediastinal tumor | 11 | 9 | |
| Thyroid cancer | 5 | 10 | |
| Tuberculosis | 3 | 7 | |
| Lung infection | 2 | 6 | |
| Tracheal tube injury | 2 | 2 | |
| Neck trauma | 2 | 1 | |
| Other | 2 | 0 | |
| Location of diseases | 0.541† | ||
| Trachea | 59 | 71 | |
| Right bronchus | 12 | 19 | |
| Left bronchus | 17 | 28 | |
| Carina | 12 | 10 | |
| Types of stents | 0.660† | ||
| Y-shaped covered metallic stent | 54 | 72 | |
| Tubular covered metallic stent | 45 | 53 | |
| L-shaped covered metallic stent | 1 | 3 | |
| Comorbid disease | 0.996† | ||
| Hypertension | 21 | 28 | |
| Diabetes | 9 | 12 | |
| Coronary heart disease | 7 | 9 | |
| Renal insufficiency (serum creatinine >140 mmol/L) | 7 | 11 | |
| Hepatic insufficiency | 5 | 8 | |
| Hospitalization (days), mean [range] | 5 [3–7] | 7 [3–11] | 0.073‡ |
P<0.05 was considered statistically significant. *, Student’s t-test; †, Pearson’s χ2 test; #, Fisher’s exact test; ‡, Wilcoxon signed-rank test. CBCT, cone beam computed tomography; SD, standard deviation.
Tracheal stenting procedures for stenosis and fistula diseases were successful on the first attempt for 90 patients in the CBCT-guided group and for 123 patients in the fluoroscopy-guided group; the technical success rates of the two groups were 90% and 96% (P=0.066), respectively. In the CBCT-guided group, interventional radiologists adjusted incompletely deployed stents in 6 patients to ensure the stents completely adhered. For the other 9 patients (4 in the CBCT-guided group and 5 in the fluoroscopy-guided group), stent placement for stenosis and fistula failed on the first attempt, but the second attempt was successful. The duration of hospitalization was shorter in the CBCT-guided group than in the fluoroscopy-guided group [5 (range, 3–7) vs. 7 (range, 3–11) days; P=0.073]; however, the difference between the groups was not significant.
Comparison of relevant measurements and visibility scores between CBCT and MDCT
The results are based on the combined data from two independent investigators and are presented in Tables 2,3. The average diameter of the left and right branches on the CBCT and MDCT images was 13.3 and 13.5 mm [P=0.019; 95% confidence interval (CI): −1.933–1.809], respectively. The average diameter of the right branch on the CBCT and MDCT images was 12.9 and 13.2 mm (P=0.023; 95% CI: 1.897–3.840), respectively. On the CBCT images and MDCT images, the two groups showed significant differences in the diameters of both branches.
Table 2
| Stent graft measurement | CBCT (n=200) | MDCT (n=200) | P value |
|---|---|---|---|
| Stent length | 58.7 (±16.82) | 58.5 (±17.06) | 0.883 |
| Upper diameter | 18.2 (±2.81) | 19.0 (±2.33) | 0.161 |
| Bottom diameter | 18.2 (±2.77) | 18.8 (±2.65) | 0.161 |
| Left branch length | 24.7 (±8.86) | 24.4 (±8.42) | 0.223 |
| Left branch diameter | 13.3 (±3.79) | 13.5 (±3.32) | 0.019 |
| Right branch length | 16.9 (±7.81) | 17.2 (±7.42) | 0.563 |
| Right branch diameter | 12.9 (±2.05) | 13.2 (±1.91) | 0.023 |
Measured lengths and diameters in mm (± SD). n, total number of observations made for each measurement. For example, 200 for CBCT means 100 patients all observed by two radiologists. P<0.05 was considered statistically significant. CBCT, cone beam computed tomography; MDCT, multidetector computed tomography; SD, standard deviation.
Table 3
| Stent graft measurement | ICC (95% CI) |
|---|---|
| Stent length | 0.998 (0.996–0.999) |
| Upper diameter | 0.849 (0.776–0.899) |
| Bottom diameter | 0.861 (0.726–0.922) |
| Left branch length | 0.988 (0.979–0.993) |
| Left branch diameter | 0.943 (0.883–0.970) |
| Right branch length | 0.992 (0.975–0.997) |
| Right branch diameter | 0.812 (0.672–892) |
ICC, intraclass correlation coefficient; CI, confidence interval.
We also evaluated the visibility score of CBCT images to determine whether CBCT was diagnostically relevant, the threshold for which was a score of 2 or more on the visibility scale. When the visibility scores of the anatomical areas in the two types of images were compared, significant differences were observed in the visibility of the distal bronchus (P=0.001; 95% CI: −0.1689–0.1389), pulmonary parenchyma (P<0.001; 95% CI: 0.451–1.25), and airway above the upper stent graft (P<0.001; 95% CI: −0.1545–0.1945). However, 2 of the 3 anatomical areas were reproduced in a diagnostically relevant way, both achieving a score of 3.3 or more on the visibility scale.
Interobserver variation
A summary of the interobserver variability between the two investigators is shown in Tables 4,5. In the assessment of relevant measurements, the overall agreement between the investigators was almost perfect (ICC: 0.812–0.998). A higher degree of interobserver agreement was observed for all anatomical areas than relevant measurements; the overall agreement was moderate (k: 0.652–0.772), except for assessing the numbers and positions of markers in the stent graft, for which almost perfect agreement (k =0.824) was seen.
Table 4
| Anatomical area | Kappa (95% CI) |
|---|---|
| Is the whole stent-graft recorded in the volume? 4—yes with good margin; 3—just barely; 2—<10% is missing; and 1—>10% is missing |
0.776 (0.628–0.9) |
| Markers in the stent graft: number and positions are assessed | 0.824 (0.711–0.934) |
| Airway above upper stent graft | 0.692 (0.567–0.814) |
| Airway under bottom stent graft | 0.652 (0.494–0.786) |
| Bifurcation | 0.687 (0.531–0.822) |
| Distal bronchus | 0.646 (0.497–0.772) |
| Pulmonary parenchyma | 0.673 (0.533–0.796) |
CI, confidence interval.
Table 5
| Anatomical area | CBCT | MDCT | P value |
|---|---|---|---|
| Is the whole stent-graft recorded in the volume? | 3.7 (±0.48) | 4.0 (±0.12) | 0.178‡ |
| Markers in the stent graft: number and positions are assessed | 3.6 (±0.59) | 3.9 (±0.20) | 0.180‡ |
| Airway above upper stent graft | 1.8 (±0.41) | 4.0 (±0.20) | 0.000‡ |
| Airway under bottom stent graft | 3.6 (±0.56) | 3.9 (±0.24) | 0.167‡ |
| Bifurcation | 3.6 (±0.53) | 4.0 (±0.17) | 0.253‡ |
| Distal bronchus | 3.7 (±0.49) | 3.9 (±0.34) | 0.001‡ |
| Pulmonary parenchyma | 3.3 (±0.71) | 3.9 (±0.31) | 0.000‡ |
Score-points on a scale from 1–4 for visibility of anatomical areas (± SD). P<0.05 was considered statistically significant. ‡, Wilcoxon signed-rank test. CBCT, cone beam computed tomography; MDCT, multidetector computed tomography; SD, standard deviation.
Complications
No procedure-related death, choking, stent rupture, severe bleeding, prolonged hypoxia, or airway perforation occurred in any patient during stent insertion or removal. Minor complications in the CBCT-guided group included slight airway hemoptysis, chest or throat pain, laryngeal foreign body sensation, and eating disorder; the incidence rates were 7/100 (7%), 4/100 (4%), 2/100 (2%), and 1/100 (1%), respectively. The incidence rates in the fluoroscopy-guided group were 9/128 (7%), 5/128 (4%), 2/128 (2%), and 3/128 (2%), respectively (P>0.05). There was a significant difference in the incidence of major complications (P=0.019) between the two groups, especially for stent migration (P=0.019), which was much less common in the CBCT-guided group than in the fluoroscopy-guided group (Table 6).
Table 6
| Complications | CBCT-guided (n=100) | Fluoroscopy-guided (n=128) | P value |
|---|---|---|---|
| Major complications | 7 (0.07) | 24 (0.19) | 0.010† |
| Migration | 3 (0.03) | 13 (0.10) | 0.036† |
| Sputum retention | 2 (0.02) | 5 (0.04) | 0.471# |
| Segmental atelectasis | 1 (0.01) | 3 (0.02) | 0.633# |
| Pneumothorax | 1 (0.01) | 3 (0.02) | 0.633# |
| Minor complications | 14 (0.14) | 23 (0.18) | 0.420† |
| Slight hemoptysis | 7 (0.07) | 9 (0.07) | 0.993† |
| Chest or throat pain | 4 (0.04) | 5 (0.04) | 0.971† |
| Laryngeal foreign body sensation | 2 (0.02) | 2 (0.02) | >0.99# |
| Difficulty eating | 1 (0.01) | 3 (0.02) | 0.633# |
Data are numbers of events. Data in parentheses are percentages. P value of <0.05 was considered statistically significant. †, Pearson’s χ2 test; #, Fisher’s exact test. CBCT, cone beam computed tomography.
Outcome and follow-up
The median follow-up durations were 51 (range, 11–60) and 45 (range, 13–60) months for the CBCT-guided group and the fluoroscopy-guided group, respectively. By the end of data collection, 38 patients (38.0%) and 51 patients (39.8%) had died in the CBCT-guided group and the fluoroscopy-guided group, respectively. In the CBCT-guided group and the fluoroscopy-guided group, 47 patients (47%) and 51 patients (39.8%), respectively, showed improvements in clinical symptoms, and 15 (15%) and 26 (20.3%) patients, respectively, were cured, as confirmed by MDCT. None of these variables showed a significant difference between the two groups (Table 7). The 1-, 3- and 5-year overall survival (OS) rates of the CBCT-guided group were 100.0%, 72.4%, and 61.0%, respectively, and those of the fluoroscopy-guided group were 100.0%, 69.8%, and 57.0%, respectively (P>0.05; Figure 3).
Table 7
| Patient outcome | CBCT-guided | Fluoroscopy-guided | P value |
|---|---|---|---|
| Death | 38 (0.38) | 51 (0.398) | 0.777† |
| With improvement | 47 (0.47) | 51 (0.398) | 0.279† |
| Cured | 15 (0.15) | 26 (0.203) | 0.534† |
P<0.05 was considered statistically significant. †, Pearson’s χ2 test. CBCT, cone beam computed tomography.
Discussion
Our study found that measurements of the diameters and lengths of the central airway based on CBCT images were not significantly different from those obtained with MDCT. Although there were significant differences between CBCT and MDCT in the visibility of the distal bronchus, pulmonary parenchyma, and airway above the upper stent graft, 2 of these 3 anatomical areas were reproduced in a diagnostically relevant way, which resulted in low scores on CBCT images for the visibility of the distal bronchus, pulmonary parenchyma, and airway above the upper stent graft which, in some cases, were outside the imaging field (14,15). Therefore, our results indicate that CBCT provides sufficient image quality to replace MDCT for safety control after stent implantation. However, because of the significant differences in the left and right branch diameter measurements between CBCT and MDCT, CBCT guidance is not recommended for conventional stent-graft planning. Nevertheless, we believe that CBCT has a potential advantage as an imaging modality for patients in extremely urgent conditions. In our study, 5 patients with a choking risk or cachexia underwent stent-graft planning with CBCT guidance at the decision of the oncologist, respiratory specialist, and interventional radiologist. These patients could be taken directly into the operating suite, where cross-sectional imaging could be performed without needing to first transfer the patient to the department of radiology, and a decision about airway stents could be made while the patient was being prepared for surgery.
In our study, airway stenting was successful on the first attempt in 90/100 (90%) patients in the CBCT-guided group and in 123/128 (96%) patients in the fluoroscopy-guided group. The ratios differed between the groups, although there was no significant difference. In the CBCT-guided group, the interventional radiologist tended to adjust the stent position or introduce the balloon to expand the stent when its placement was not perfect. Patient outcomes and survival rates in the CBCT-guided group did not appear to be significantly different from those in the fluoroscopy-guided group (P>0.05). However, compared to the fluoroscopy-guided group, the rate of major complications in the CBCT-guided group was significantly reduced (P=0.010). This approach, especially 3D volume reconstruction technology, has the advantage of minimizing migration following tracheal airway stent placement (16). For a few patients with sputum retention, a bronchoscope was used to clear the airway. To avoid life threatening situations, such as severe hemorrhage or dyspnea, our interventional radiologists had experience and training with tracheal intubation and interventional embolization.
Although our study showed that fluoroscopy is an effective alternative method for airway stenting, rigid bronchoscopy remains the main therapeutic approach for both benign and malignant etiological airway diseases. In addition, the rigid bronchoscopy also plays a crucial role in stent complications. Mucus plugs are the most common complication observed in many studies (17,18). Rigid bronchoscopy is recommended in life-threatening situations. A 3-mm inner aspiration catheter or even debulking with the tip of the instrument can solve mucus plugs rapidly. Pseudo-membranes are formed by the ischemic and necrotic mucosa particularly after the removal of longstanding stents. They may be sticky and hard to expectorate, and can lead to airway obstruction and suffocation. Rigid bronchoscopy is the safest and most efficient way to remove them. Also, rigid bronchoscopy is often recommended as the preferred method for the evaluation and management of massive hemoptysis (19). However, urgent rigid bronchoscopy is not recommended unless the bleeding is caused by an apparent endoluminal lesion, and the success of the procedure depends directly on the expertise of the operator and the assisting team. For clot removal, rigid bronchoscopy can be extremely quick and safe. Granulation tissue proliferation can be removed directly with forceps or ablated during bronchoscopy (20). These techniques are the quickest and safest way to remove granulation tissue and control bleeding. However, treating these complications requires a highly trained and experienced team. Specific training should be offered to interventional pulmonologists to manage these rare but life-threatening situations before CBCT-guided airway stenting.
To the best of our knowledge, little research has been presented regarding the application of CBCT during airway stent placement. The technical image quality of CBCT is inferior to that of MDCT (21). However, clinical experience from other centers has demonstrated the usefulness of CBCT imaging to detect complications in various medical contexts, such as during neuro-endovascular procedures (14,22-26), showing that it can improve clinical outcomes (27). A new study from Zhou et al. (28) showed that CBCT can provide 3D images that enable accurate positioning of the cryoprobe for transbronchial cryobiopsy and interstitial lung disease as well as reduce the risks of pneumothorax and massive bleeding. In our study, we came to the same conclusion, finding that intraoperative CBCT decreased the rates of major complications, especially stent migration.
The current study has some limitations. First, it is a retrospective study with inevitable selection bias. Second, although this work has a relatively large sample size to previous researches (3-5), it is limited by the lack of a randomized controlled design, which may weaken the statistical power of our results. However, in this study cohort, the locations of airway diseases were wide ranging. Therefore, our findings with statistical significance are credible. Furthermore, the process of operation may increase the radiation exposure of interventional radiologists. Nevertheless, we strictly monitored the radiation exposure time and always strived to improve the clinical prognosis of patients.
Conclusions
CBCT imaging is of sufficient quality to replace MDCT for timely safety control after stent implantation and for stent-graft planning in some cases. It also reduces the complications of airway stent placement. In our opinion, a CBCT apparatus might also represent a valuable adjunct to the standard equipment that is normally available in an operating room for airway stent procedures.
Acknowledgments
Funding: This work was supported by the Young and Middle-aged Health Science and Technology Innovation Talent Project of Henan Province in 2020 (No. yxkc2020037); This work was supported by the youth project jointly supported by Henan Provincial Health Commission and Ministry (No. sb201902014).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-21-858/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-858/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Zhengzhou University. Written informed consent was obtained from each patient for the publication of this article and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Han X, Yin M, Li L, Zhu M, Ren K, Qi Y, Li X, Wu G. Customized airway stenting for bronchopleural fistula after pulmonary resection by interventional technique: single-center study of 148 consecutive patients. Surg Endosc 2018;32:4116-24. [Crossref] [PubMed]
- Li Y, Zhou X, Ren K, Ren J, Han X. Bronchopleural Fistula Cured by Customized Airway Metallic Stent. Chest 2019;156:1031. [Crossref] [PubMed]
- Kim J, Shin JH, Kim JH, Song HY, Song SY, Park CK. Metallic stent placement for the management of tracheal carina strictures and fistulas: technical and clinical outcomes. AJR Am J Roentgenol 2014;202:880-5. [Crossref] [PubMed]
- Marchese R, Poidomani G, Paglino G, Crimi C, Lo Nigro C, Argano V. Fully covered self-expandable metal stent in tracheobronchial disorders: clinical experience. Respiration 2015;89:49-56. [Crossref] [PubMed]
- Saad CP, Murthy S, Krizmanich G, Mehta AC. Self-expandable metallic airway stents and flexible bronchoscopy: long-term outcomes analysis. Chest 2003;124:1993-9. [Crossref] [PubMed]
- Kim S, Gotway MB, Webb WR, Gordon RL, Golden JA. Tracheal compression by the stomach following gastric pull-up: diagnosis with CT and treatment with expandable metallic stent placement. Chest 2002;121:998-1001. [Crossref] [PubMed]
- Thornton RH, Gordon RL, Kerlan RK, LaBerge JM, Wilson MW, Wolanske KA, Gotway MB, Hastings GS, Golden JA. Outcomes of tracheobronchial stent placement for benign disease. Radiology 2006;240:273-82. [Crossref] [PubMed]
- Godoy MC, Saldana DA, Rao PP, Vlahos I, Naidich DP, Benveniste MF, Erasmus JJ, Marom EM, Ost D. Multidetector CT evaluation of airway stents: what the radiologist should know. Radiographics 2014;34:1793-806. [Crossref] [PubMed]
- Meyer BC, Frericks BB, Albrecht T, Wolf KJ, Wacker FK. Contrast-enhanced abdominal angiographic CT for intra-abdominal tumor embolization: a new tool for vessel and soft tissue visualization. Cardiovasc Intervent Radiol 2007;30:743-9. [Crossref] [PubMed]
- Anayama T, Yamamoto M, Hirohashi K, Miyazaki R, Okada H, Doi A, Orihashi K. The accuracy of cone-beam computed tomography and augmented fluoroscopy-guided bronchoscopic marking of multiple small-sized pulmonary nodules in a hybrid operating room: a retrospective cohort study. Quant Imaging Med Surg 2021;11:725-36. [Crossref] [PubMed]
- Li YD, Han XW, Li MH, Wu G. Bronchial stump fistula: treatment with covered, retrievable, expandable, hinged stents--preliminary clinical experience. Acta Radiol 2006;47:922-6. [Crossref] [PubMed]
- European Commission. European guidelines on quality criteria for computed tomography. Luxembourg: Office for Official Publications of the European Communities, 2000.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. [Crossref] [PubMed]
- Shan Q, Huang W, Shang M, Wang Z, Xia N, Xue Q, Wu Z, Ding X, Mao A, Wang Z. Customization of stent design for treating malignant airway stenosis with the aid of three-dimensional printing. Quant Imaging Med Surg 2021;11:1437-46. [Crossref] [PubMed]
- Eide KR, Ødegård A, Myhre HO, Lydersen S, Hatlinghus S, Haraldseth O. DynaCT during EVAR--a comparison with multidetector CT. Eur J Vasc Endovasc Surg 2009;37:23-30. [Crossref] [PubMed]
- Chen Y, Zhou ZQ, Feng JX, Su ZQ, Zhong CH, Lu LY, Chen XB, Tang CL, Digumarthy SR, Fiorelli A, Natour E, Lococo F, Petrella F, Harris K, Nakada T, Zhong NS, Li SY. Hybrid stenting with silicone Y stents and metallic stents in the management of severe malignant airway stenosis and fistulas. Transl Lung Cancer Res 2021;10:2218-28. [Crossref] [PubMed]
- Guibert N, Saka H, Dutau H. Airway stenting: Technological advancements and its role in interventional pulmonology. Respirology 2020;25:953-62. [Crossref] [PubMed]
- Diaz-Mendoza J, Peralta AR, Debiane L, Simoff MJ. Rigid Bronchoscopy. Semin Respir Crit Care Med 2018;39:674-84. [Crossref] [PubMed]
- Bi Y, Zhu X, Yu Z, Yi M, Han X, Ren J. Clinical outcomes of self-expandable metallic stents for malignant obstructive atelectasis. Sci Rep 2020;10:3600. [Crossref] [PubMed]
- Smyth J, Sutton D, Houston J. Evaluation of the quality of CT-like images obtained using a commercial flat panel detector system. Biomed Imaging Interv J 2006;2:e48. [Crossref] [PubMed]
- Biasi L, Ali T, Hinchliffe R, Morgan R, Loftus I, Thompson M. Intraoperative DynaCT detection and immediate correction of a type Ia endoleak following endovascular repair of abdominal aortic aneurysm. Cardiovasc Intervent Radiol 2009;32:535-8. [Crossref] [PubMed]
- Gupta V, Chugh M, Walia BS, Vaishya S, Jha AN. Digital subtraction angiography laboratory with inbuilt CT (DynaCT): application during intracranial anurysm embolization. Neurol India 2008;56:90-1. [Crossref] [PubMed]
- Wang CY, Xia JG, Chen WH, Lu YF, Han ZH, Wang Q. Value of Dyna CT in guiding embolization during transarterial uterine artery embolization of fibroids. Exp Ther Med 2020;20:1353-8. [Crossref] [PubMed]
- Ritter M, Rassweiler MC, Michel MS. The Uro Dyna-CT Enables Three-dimensional Planned Laser-guided Complex Punctures. Eur Urol 2015;68:880-4. [Crossref] [PubMed]
- Rotolo N, Floridi C, Imperatori A, Fontana F, Ierardi AM, Mangini M, Arlant V, De Marchi G, Novario R, Dominioni L, Fugazzola C, Carrafiello G. Comparison of cone-beam CT-guided and CT fluoroscopy-guided transthoracic needle biopsy of lung nodules. Eur Radiol 2016;26:381-9. [Crossref] [PubMed]
- Ashrafi-Asgarabad A, Safiri S. Prognostic Value of Primary Tumor Volume Changes on Kilovoltage Onboard Cone Beam Computed Tomography during Definitive Chemoradiotherapy for stage III Non-Small Cell Lung Cancer: Methodological Issues. J Thorac Oncol 2018;13:e26-7. [Crossref] [PubMed]
- Li TF, Ma J, Han XW, Fu PJ, Niu RN, Luo WZ, Ren JZ. Application of High-Resolution C-Arm CT Combined with Streak Metal Artifact Removal Technology for the Stent-Assisted Embolization of Intracranial Aneurysms. AJNR Am J Neuroradiol 2019;40:1752-8. [Crossref] [PubMed]
- Zhou G, Ren Y, Li J, Yang T, Su N, Zhao L, Wang D, Li Y, Tian Z, Liu R, Dai H, Wang C. Safety and diagnostic efficacy of cone beam computed tomography-guided transbronchial cryobiopsy for interstitial lung disease: a cohort study. Eur Respir J 2020;56:2000724. [Crossref] [PubMed]


