
Accuracy of quantitative vessel analysis in endovascular treatment for femoropopliteal lesions
Introduction
In the area of coronary angiography, quantitative coronary analysis (QCA) is used to assess the severity and progression of coronary artery disease, optimize device selection, and evaluate angiographic outcomes (1,2). In contrast, quantitative vessel analysis (QVA) has not been established sufficiently for use in the femoropopliteal area. Several devices used for treating femoropopliteal lesions including stents and drug-coated balloons (DCB) come in sizes with gradations of 1 mm; therefore, an accuracy of ±1 mm is required for device selection when measuring vessel size in the femoropopliteal area. Previous reports have demonstrated various ways to calibrate an angiogram. Some of them include fixing a radiopaque ruler on a patient’s upper thigh (3,4), fixing a radiopaque ruler below a patient’s upper thigh (5), and using a guide catheter or sheath (6-8). However, the accuracy of such methods remains unknown. Additionally, the vessel diameter appears to be influenced by the distance between the vessel and calibration point because they are not the same distance from the angiography table. Therefore, the aim of this study was to investigate the accuracy of QVAs in measuring the reference vessel diameter (RVD) and evaluate the relationship between the distance of a vessel from the angiography table and the accuracy of QVAs.
Methods
Between October 2014 and September 2015, 30 lesions in 25 consecutive patients who underwent endovascular therapy (EVT) for superficial femoral artery (SFA) lesions with intravascular ultrasound (IVUS) guidance were included in this study. Stent implantation was performed for de novo lesions and balloon dilatation was performed for in-stent restenosis lesions. Non-contrast lower extremity computed tomography (CT) was also performed after the procedure to measure the distance between the angiography table and the treated blood vessel. Patients were excluded if they had acute or subacute lower limb ischemia and contraindications to angiography. All patients had symptoms after they received exercise and drug therapy. If angiography revealed stenosis >50% of the diameter of the femoropopliteal artery, vascular specialists, including vascular surgeons and interventional cardiologists, decided on the applicability of EVT. This study was performed in accordance with the Declaration of Helsinki (as revised in 2013); it was approved by the ethics committee of our hospital and registered in the University Hospital Medical Information Network Clinical Trial Registry (UMIN ID: 000016578). All patients provided written informed consent.
Intervention
EVTs were performed using the crossover approach. A 55-cm long, 6-Fr sheathless catheter (SheathLess PV; Asahi Intecc, Aichi, Japan) was inserted and unfractionated heparin (5,000 units) was injected intra-arterially. Subsequently, a 0.014-inch guidewire was passed through the target lesion and balloon dilatation or stent implantation was performed. When two or more stents were used for a long lesion, the overlap was ≤10 mm. Post-dilatation was performed routinely in all lesions. At the end of the procedure, angiography was performed using a digital angiographic system (Allura Xper FD10 systems, Phillips Healthcare, Amsterdam, The Netherlands) and the original angiographic images were stored electronically. Angiography was performed with a radiopaque ruler under the thigh of the patient on the angiography table. Multiple planar images were not routinely obtained. IVUS was also performed, and the measuring points of IVUS were marked on angiographic images to compare the same point between IVUS and angiography. In all cases, IVUS images were recorded using a commercially available IVUS console (VISIWAVE™; Terumo Corporation, Tokyo, Japan) and a phased-array 35-MHz IVUS catheter (View IT; Terumo Corporation, Tokyo, Japan) as automatic pullback through the stented segment was performed at 2 mm/s. The working length of this IVUS is up to 15 cm. When the stent length was more than 15 cm, we performed automatic pullback of IVUS again from the beginning point of the second pullback according to the division of the ruler on the catheter table. All patients underwent non-contrast CT of the lower extremity within 2 days of the procedure.
QVA
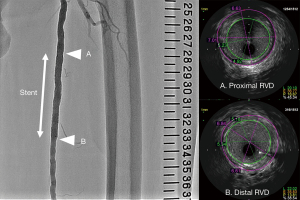
Analysis of the angiographic data was performed using CAAS 5.7 (Pie Medical Imaging, Maastricht, The Netherlands). QVA was evaluated by two experienced observers and performed using two methods of calibration—a ruler along the femur on the catheter table and a 6-Fr sheathless catheter tip in the common femoral artery. The values of proximal and distal reference RVDs were measured. The proximal and distal RVDs selected for analysis were the most normal cross-sections within 10 mm of the proximal and distal margins of the stent.
IVUS analysis
For analysis of the IVUS data, VISIWAVE™ (Terumo Corporation) was used. Two experienced observers who were blinded to the angiographic findings performed IVUS analysis. The IVUS parameters measured or calculated were the proximal and distal reference lumen diameters (Figure 1).
CT analysis
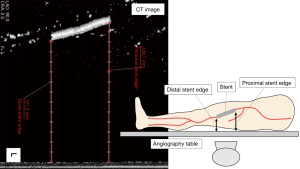
Non-contrast CT of the lower extremity was performed using Aquilion 64-slice CT scanner (Canon Medical Systems, Japan). Data were acquired with collimation of 0.5×64 mm and gantry rotation time of 500 ms. The tube current was determined with auto exposure control at 100 kV, pitch value was 95.0, and scan direction was craniocaudal. Ziostation 2 (Ziosoft Inc., Tokyo, Japan) was used to analyze the distance between the CT table and each of the stent edges. The distance from the CT table to each of the stent edges was substituted as the distance from the angiography table to each of the stent edges (Figure 2). All analyses were performed by two experienced observers who were blinded to the angiographic and IVUS findings.
Vessel diameter analysis
Vessel diameters obtained based on IVUS analysis were used as the standards for comparisons. The discrepancies in proximal and distal RVDs in QVAsheath, QVAruler, and IVUS were evaluated.
Inter-observer reproducibility
In QVAsheath measurements, the inter-observer agreement for RVD was 0.910 (95% confidence interval: 0.849–0.946). In QVAruler measurements, the inter-observer agreement for RVD was 0.876 (0.792–0.926). In IVUS measurements, the inter-observer agreement for RVD was 0.955 (0.915–0.976). In CT measurements, the inter-observer agreement for distance from the CT table to each of the stent edges was 0.998 (0.997–0.999).
Statistical analysis
Data are expressed as mean ± standard deviation for continuous variables and frequency (percentage) for discrete variables. QVAsheath- and QVAruler-measured RVDs were compared with IVUS-measured RVD using Bland-Altman analysis (9). In brief, the differences between individual measurements of the two different measuring systems were calculated, and the means and standard deviations were derived. The 95% limits of agreement (i.e., 95% prediction intervals of the differences or errors) were obtained from the means and standard deviations. We also evaluated the proportions of the agreement with a tolerance of ±1.00 mm. We subsequently explored the association between the measurement difference and the distance from the angiography table using Pearson’s correlation analysis. All statistical analyses were performed and graphs were plotted using Microsoft Excel v2019 (Microsoft Corporation, Washington, USA).
Results
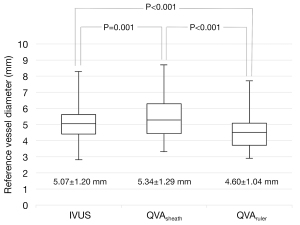
The characteristics of the study population are summarized in Table 1. The overall mean age was 76.2±8.4 years and 36% of the study participants were female. The prevalence of diabetes mellitus, dialysis, and critical limb ischemia was 32%, 20%, and 24%, respectively. The rates of de novo lesions, Trans-Atlantic Inter-Society Consensus (TASC) II class C or D lesions, and popliteal lesions were 40%, 50%, and 23%, respectively. The mean IVUS-measured RVD was 5.07±1.20 mm. The mean distance from the table was 117±26 mm.
Table 1
| Parameters | Value |
|---|---|
| Age, years | 76.2±8.4 |
| Women, n [%] | 9 [36] |
| Body mass index, kg/m2 | 22.7±4.5 |
| Hypertension, n [%] | 21 [84] |
| Dyslipidemia, n [%] | 10 [40] |
| Smoking, n [%] | 6 [24] |
| Diabetes mellitus, n [%] | 8 [32] |
| Chronic kidney disease, n [%] | 13 [52] |
| Dialysis, n [%] | 5 [20] |
| Critical limb ischemia, n [%] | 6 [24] |
| Lesion characteristics (n=30) | |
| Ankle brachial index | 0.63±0.29 |
| De novo lesion, n [%] | 12 [40] |
| TASC II class C/D, n [%] | 15 [50] |
| Calcified lesion, n [%] | 3 [10] |
| Involving popliteal lesion, n [%] | 7 [23] |
| Poor runoff, n [%] | 13 [43] |
| IVUS-measured RVD, mm | 5.07±1.20 |
| Distance from table, mm | 117±26 |
TASC, Trans-Atlantic Inter-Society Consensus; IVUS, intravascular ultrasound; RVD, reference vessel diameter.
The mean QVAsheath-measured RVD was 5.34±1.29 mm, which was significantly larger than the IVUS-measured RVD (P=0.001, paired t-test). In contrast, the mean QVAruler-measured RVD was 4.60±1.04 mm, which was significantly smaller than IVUS- and QVAsheath-measured RVDs (both P<0.001, paired t-test) (Figure 3). Figure 4 illustrates the Bland-Altman analysis between QVA- and IVUS-measured RVDs. The lower and upper 95% limits of agreement versus IVUS were −0.94 and 1.49 mm for QVAsheath, and −1.69 and 0.76 mm for QVAruler, respectively. The agreement with tolerance of ±1.00 mm accounted for 88% (95% confidence interval using Clopper-Pearson’s exact method, 77–95%) of QVAsheath and 83% (71–92%) for QVAruler (P=0.60, Fisher’s exact test).
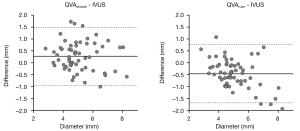
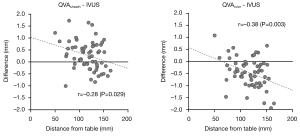
As illustrated in Figure 5, the difference between QVA- and IVUS-measured RVD was inversely correlated with the distance from table.
Discussion
In QVA, the measured vessel diameter varies according to the calibration point and the source of X-rays. It has been reported that a projected image of an object is affected by its distance between the calibration point and the source of X-rays. Takagi et al. reported that QCA is likely to overestimate the minimum stent diameter in the left circumflex artery (LCx) because LCx is anatomically closest to the X-ray source (10). Similar principles apply to the femoropopliteal diameters; the arterial diameter varies according to the calibration point. Therefore, it can be inferred that vessel diameter may be both overestimated and underestimated by QVA.
Some previous studies have compared the assessment of vessel diameter of the femoropopliteal artery between QVA and IVUS. Pliagas et al. reported that angiographic imaging consistently underestimated the vessel size (11), and Arthurs et al. reported that angiography and IVUS provided similar luminal diameters (12). In the present study, the mean QVAsheath-measured RVD was significantly larger than the IVUS-measured RVD (5.34±1.29 vs. 5.07±1.20 mm, respectively, P=0.001) and the mean QVAruler-measured RVD was significantly smaller than the IVUS-measured RVD (4.60±1.04 vs. 5.07±1.20 mm, respectively, P<0.001). These differences appear to have occurred because of differences in the QVA method used.
Furthermore, the findings of the present study, in which femoropopliteal segments were evaluated, provide additional and new information regarding the level of expected error with QVAsheath and QVAruler compared with IVUS. Bland-Altman analysis between QVA- and IVUS-measured RVDs revealed that the lower and upper 95% limits of agreement versus IVUS were −0.94 and 1.49 mm for QVAsheath and −1.69 and 0.76 mm for QVAruler, respectively. The agreement with a tolerance of ±1.00 mm accounted for 88% (77–95%) of QVAsheath and 83% (71–92%) of QVAruler; however, there was no significant difference in the accuracy between QVAruler and QVAsheath (P=0.60). Therefore, more than 1 mm of discrepancy can occur in as high as 12% (5–23%) of cases with QVAsheath and 17% (8–29%) of cases with QVAruler; over- and under-estimation of RVD is not rare with QVA, irrespective of the method used. This inherent issue might result in selecting oversized or undersized devices compared with the actual vessel size.
Undersized balloon dilatation may result in suboptimal vessel expansion and insufficient gains in the lumen. Undersized DCB may result in the lack of apposition between the balloon and the vessel wall and insufficient drug delivery to the tissue, which may contribute to poor clinical outcomes. Undersized stents may result in stent malapposition and risks of restenosis and late thrombosis. In contrast, oversized balloon dilatation may result in severe vessel dissection, and the opportunity of drug balloon angioplasty may be lost. It has been reported that oversized stents were related to in-stent restenosis following self-expandable stenting for femoropopliteal lesions (6). Oversized interwoven nitinol biomimetic Supera stents (Abbott Vascular, Santa Clara, CA, USA) cause elongation and increases the rate of restenosis (13). Therefore, it is crucial to choose correctly sized devices to improve the clinical outcomes of EVT. The findings of this study demonstrate that QVA does not always guarantee accurate estimation of RVD in the selection of device size. IVUS enables choosing accurately sized devices. On the other hand, whether routine IVUS usage during EVT would lead to improvements in treatment of femoropopliteal lesions remains unclear. Further research is needed in this area.
The discrepancy between measuring methods correlates to the distance from the angiography table. For QVAruler, the further the distance from the table, the greater the underestimation of the vessel diameter. In contrast, for QVAsheath, the vessel diameter was overestimated. A significantly large error can appear in people with a larger body size as well. As such, these factors should be recognized when estimating vessel diameter for EVT of femoropopliteal lesions.
There were some limitations to this study. First, this study included a small number of patients. Second, angiography was performed in only one direction, which can possibly increase the estimation of the error; however, angiography in two directions is not usually performed in routine practice. Third, diameter measurement value on angiographic images can be influenced by many factors such as the injection speed and volume thus to some extent. Forth, the distance from the CT table to each of the stent edges was substituted as the distance from the angiography table to each of the stent edges in this study. There may be a slight error between them because the site measured during EVT cannot be accurately located on non-contrast CT images. Finally, QVA with calibration using a ruler on the thigh was not evaluated in this study because the ruler in such cases is not along the horizontal plane. Further studies are required to explore this approach and corroborate our findings.
Conclusions
The accuracies of QVAsheath and QVAruler in measuring RVD were similarly suboptimal. Over- and under-estimation of RVD is not rare in QVA.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/qims-20-1097). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was performed in accordance with the Declaration of Helsinki (as revised in 2013); it was approved by the ethics committee of our hospital and registered in the University Hospital Medical Information Network Clinical Trial Registry (UMIN ID: 000016578). All patients provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE. SIRIUS Investigators. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003;349:1315-23. [Crossref] [PubMed]
- Regar E, Serruys PW, Bode C, Holubarsch C, Guermonprez JL, Wijns W, Bartorelli A, Constantini C, Degertekin M, Tanabe K, Disco C, Wuelfert E, Morice MCRAVEL Study Group. Angiographic findings of the multicenter Randomized Study With the Sirolimus-Eluting Bx Velocity Balloon-Expandable Stent (RAVEL): sirolimus-eluting stents inhibit restenosis irrespective of the vessel size. Circulation 2002;106:1949-56. [Crossref] [PubMed]
- Amighi J, Schillinger M, Dick P, Schlager O, Sabeti S, Mlekusch W, Haumer M, Mathies R, Heinzle G, Schuster A, Loewe C, Koppensteiner R, Lammer J, Minar E, Cejna M. De novo superficial femoropopliteal artery lesions: peripheral cutting balloon angioplasty and restenosis rates--randomized controlled trial. Radiology 2008;247:267-72. [Crossref] [PubMed]
- Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, Schlager O, Cejna M, Lammer J, Minar E. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med 2006;354:1879-88. [Crossref] [PubMed]
- Krankenberg H, Schlüter M, Steinkamp HJ, Bürgelin K, Scheinert D, Schulte KL, Minar E, Peeters P, Bosiers M, Tepe G, Reimers B, Mahler F, Tübler T, Zeller T. Nitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the femoral artery stenting trial (FAST). Circulation 2007;116:285-92. [Crossref] [PubMed]
- Soga Y, Yokoi H, Urakawa T, Iwabuchi M, Nobuyoshi M. Clinical impact of self-expandable stent diameter after femoropopliteal stenting. Cardiovasc Interv Ther 2011;26:38-44. [Crossref] [PubMed]
- Pavlovic C, Futamatsu H, Angiolillo DJ, Guzman LA, Wilke N, Siragusa D, Wludyka P, Percy R, Northrup M, Bass TA, Costa MA. Quantitative contrast enhanced magnetic resonance imaging for the evaluation of peripheral arterial disease: a comparative study versus standard digital angiography. Int J Cardiovasc Imaging 2007;23:225-32. [Crossref] [PubMed]
- Duda SH, Pusich B, Richter G, Landwehr P, Oliva VL, Tielbeek A, Wiesinger B, Hak JB, Tielemans H, Ziemer G, Cristea E, Lansky A, Bérégi JP. Sirolimus-eluting stents for the treatment of obstructive superficial femoral artery disease: six-month results. Circulation 2002;106:1505-9. [Crossref] [PubMed]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307-10. [Crossref] [PubMed]
- Takagi K, Shannon J, Basavarajaiah S, Latib A, Al-Lamee R, Hasegawa T, Godino C, Ferraro M, Figini F, Carlino M, Montorfano M, Chieffo A, Colombo A. Discrepancies in vessel sizing between angiography and intravascular ultrasound varies according to the vessel evaluated. Int J Cardiol 2013;168:3791-6. [Crossref] [PubMed]
- Pliagas G, Saab F, Stavroulakis K, Bisdas T, Finton S, Heaney C, McGoff T, Hardy K, Adams G, Mustapha JA. Intravascular ultrasound imaging versus digital subtraction angiography in patients with peripheral vascular disease. J Invasive Cardiol 2020;32:99-103. [PubMed]
- Arthurs ZM, Bishop PD, Feiten LE, Eagleton MJ, Clair DG, Kashyap VS. Evaluation of peripheral atherosclerosis: a comparative analysis of angiography and intravascular ultrasound imaging. J Vasc Surg 2010;51:933-8; discussion 939. [Crossref] [PubMed]
- Garcia LA, Rosenfield KR, Metzger CD, Zidar F, Pershad A, Popma JJ, Zaugg M, Jaff MRSUPERB investigators. SUPERB final 3-year outcomes using interwoven nitinol biomimetic supera stent. Catheter Cardiovasc Interv 2017;89:1259-67. [Crossref] [PubMed]


