
Semi-quantitative analysis of 99mTc-sestamibi retention level for preoperative differential diagnosis of parathyroid carcinoma
Introduction
Parathyroid carcinoma, which represents approximately 1% of all cases of primary hyperparathyroidism (PHPT) in western countries, is an uncommon malignancy. However, the occurrence of parathyroid carcinoma is as high as 6% in China (1). Unlike local excision for parathyroid adenoma, en bloc resection of carcinoma during the initial operation has been recommended as the best chance for a cure when the tumor is well-localized (2).
Preoperative differential diagnosis between malignant and benign parathyroid lesion is helpful for surgeons to select proper treatment. However, it is not easy to establish a diagnosis of parathyroid carcinomas preoperatively for a number of reasons: (I) The clinical manifestations of parathyroid carcinoma partially overlap with those of benign hyperparathyroidism (1,3,4). (II) Neck ultrasound, 99mTc-sestamibi (99mTc-MIBI) scintigraphy, computed tomography (CT), and magnetic resonance imaging (MRI) are the imaging techniques mainly used for the localization of parathyroid lesions (3). Whole-body positron emission tomography (PET)/CT scan has a complementary role in the staging of parathyroid carcinoma (5,6), and none of these techniques have yet been recommended for the assessment of the malignant potential of parathyroid lesions (7). (III) Fine-needle aspiration cytology in most cases is insufficient to differentiate malignant from benign parathyroid lesions and increases the potential of possible tumor seeding in the biopsy tract (8). Therefore, more effective methods for preoperative differential diagnosis of parathyroid lesions need to be explored.
99mTc-MIBI dual-phase scintigraphy has been widely used in clinic for localizing parathyroid adenoma due to its longer retention level in parathyroid adenoma than healthy thyroid (7) and parathyroid tissue (9). However, to our knowledge, few studies have investigated the retention level of 99mTc-MIBI in parathyroid carcinoma likely due to its low prevalence. Previous studies have suggested that malignant thyroid nodules have a higher retention level of 99mTc-MIBI than benign ones (10,11). Therefore, we hypothesized that there is a difference of 99mTc-MIBI retention level between benign and malignant parathyroid lesions, and this may be useful for the preoperative differential diagnosis of parathyroid lesions. This study was designed to test the above hypothesis.
Methods
Study subjects
From 2011 to 2015, a total of 756 consecutive patients with clinically suspected parathyroid adenoma underwent 99mTc-MIBI dual-phase planar imaging and SPECT/CT in our hospital. Among these patients, 191 patients were excluded as they did not undergo subsequent surgery in our hospital or did not have complete data. A total of 290 patients with negative 99mTc-MIBI scan were treated with follow-up. Forty patients with positive 99mTc-MIBI results who were older or unwilling to undergo surgery were administered with alendronate tablets for palliative treatment. A total of 235 patients underwent surgery in our hospital. Among them, 20 patients were pathologically proven to be parathyroid carcinomas and enrolled in the present study. For comparison, 40 sex- and age-matched patients with benign parathyroid lesions during the same period, including 35 parathyroid adenomas and 5 parathyroid hyperplasias were enrolled as controls for a 1:2 ratio. This retrospective study was approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine.
Image acquisition and analysis
Dual-phase planar scans of the neck and upper mediastinum were performed at 10 min (early images) and 120 min (delayed images) on Symbia T16 SPECT/CT system (Siemens, Erlangen, Germany) after intravenous injection of 740–1,110 MBq 99mTc-MIBI. Planar images were acquired for 10 min in a 128×128 matrix and a low-energy, high-resolution, parallel-hole collimator. Single photon emission CT (SPECT) acquisition was performed immediately after the early or delayed planar scan using the same collimator, a 128×128 matrix, and a total of 120 projections over 360° with 15 s per projection. After the SPECT acquisition, a CT scan was carried out at 140 kV and 2.5 mA. SPECT data were reconstructed based on a Flash 3D algorithm with photon attenuation correction from CT data.
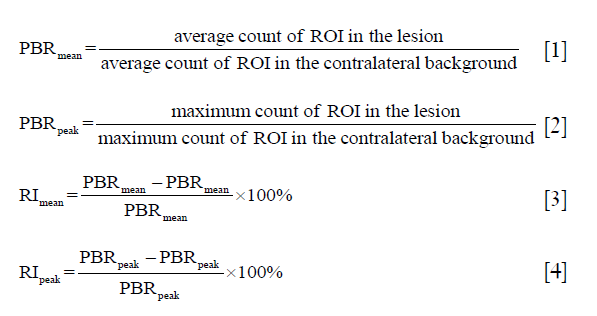
A region of interest (ROI) of the parathyroid lesion was manually delineated on the early and delayed planar images; another ROI of the background was drawn over the deltoid muscle on the contralateral side. The mean and peak of the parathyroid lesion-to-background ratios at 10 min (PBR10mean, PBR10peak) and 120 min (PBR120mean, PBR120peak) after 99mTc-MIBI injection were measured. ThePBR mean and PBRpeak were respectively equal to the average and maximum count in the ROI of the lesion divided by those in the ROI of background as seen in Eq. [1] and Eq. [2]. The mean and peak of retention index (RImean and RIpeak) were subsequently calculated according to the PBRmean and PBRpeak (9) according to Eq. [3] and Eq. [4]. In addition, the maximal axial diameter (12) and the CT value of parathyroid lesions were measured on CT images. For those cases with a negative 99mTc-MIBI planar scan but a visible nodule behind the thyroid lobe on SPECT/CT image, a coronal SPECT/CT fusion image was required as a reference for the ROI delineation of the lesion on the planar image. Two independent nuclear medicine specialists interpreted all the images obtained.
Statistical analysis
Data were analyzed using the SPSS 19.0 software. Continuous data were compared between the benign and malignant groups using independent sample t-test. The χ2 test was used to compare categorical data, such as patients’ sex. The correlation between variables was analyzed using Pearson’s test for linear regression. Receiver-operating-characteristic (ROC) analysis was used to assess the differences in image parameters associated with differential diagnosis, and to determine their thresholds above which malignant parathyroid lesions could be detected. Significance was set at P<0.05.
Results
Table 1 shows a statistical summary of the various measure records of 60 patients’ characteristics. The patients’ weight in the malignant group (53.8±11.2 kg) was significantly lower than that in the benign one (64.7±13.3 kg, P=0.004). No significant difference was found in sex, age, and height between the two groups. The serum levels of parathyroid hormone (PTH) (1,432.9±835.7 pg/mL), calcium (3.0±0.3 mmol/L), and alkaline phosphatase (ALP) (478.1±662.4 IU/L) in the malignant group were respectively higher than those (524.7±540.3 pg/mL, P<0.001; 2.8±0.3 mmol/L, P=0.045; 122.5±82.1 IU/L, P=0.002) in the benign group, but a significantly lower serum phosphorus level was observed in the malignant group (0.6±0.1 mmol/L) than that in the benign group (0.8±0.5 mmol/L, P=0.016). There was no difference in the levels of serum 25(OH)D and creatinine between the two groups.

Full table
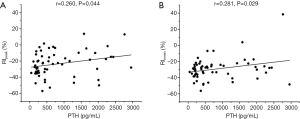
No significant differences in PBR10peak, PBR10mean, PBR120peak, PBR120 mean, and the CT value of parathyroid lesions was observed between the malignant and benign groups, whereas RIpeak (P<0.001, Figure 1A), RImean (P<0.001, Figure 1B), and size (P<0.001, Figure 1C) showed significant differences. In addition, a slight correlation was found between serum PTH level and RIpeak (r=0.260, P=0.044, Figure 2A) or RImean (r=0.281, P=0.029, Figure 2B). There was no significant correlation between other serological indexes and RI of 99mTc-MIBI.

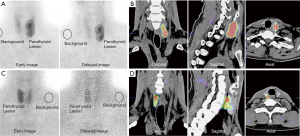
The area under the curve (AUC) from the ROC curves of different variables used to differentiate malignant parathyroid lesions from benign ones is shown in Table 2. Only AUCs for RImean (0.78, P<0.001), RIpeak (0.87, P<0.001), and lesion size (0.87, P<0.001), as well as PTH levels (0.84, P<0.001) showed statistical significance. The cutoff value of RIpeak corresponding to the highest accuracy for discriminating between benign and malignant parathyroid lesions was −19.03%. Sixteen of the 22 parathyroid lesions with the RIpeak above this cutoff had a histologic result with malignancy (Figure 3A,B), whereas 34 of 38 lesions with the RIpeak equal to or below this cutoff were histologically benign (Figure 3C,D). For this threshold, the sensitivity, specificity, PPV, NPV, and accuracy of RIpeak were 80.0%, 85.0%, 72.7%, 89.5%, and 83.3%, respectively. The combination of RIpeak, size and PTH levels as a joint index further increased the diagnostic sensitivity (95.0%) and NPV (97.1%), but its specificity (82.5%), PPV (73.1%), and accuracy (86.7%) were not significantly improved compared with RIpeak, size or PTH levels alone.

Full table
Discussion
99mTc-MIBI dual-phase scintigraphy as functional imaging reflects uptake and retention features of 99mTc-MIBI in the parathyroid gland. On the one hand, the uptake of 99mTc-MIBI in parathyroid cells mainly depends on regional blood flow, mitochondrial number, and cell metabolic status (13). However, the uptake level of 99mTc-MIBI on the early or delayed imaging showed no satisfactory discriminatory power for parathyroid lesions in our study. This suggests that high mitochondrial number, metabolic activity, or blood flow might exist in both parathyroid carcinoma and benign lesions, and thus resulted in their comparable uptake level of 99mTc-MIBI. Although the increased tumor-to-background uptake ratio of 99mTc-MIBI has been observed in non-parathyroid tumors (14-16), it was not reliable for the differential diagnosis of parathyroid tumors.
On the other hand, the retention of 99mTc-MIBI is associated with the efflux rate of 99mTc-MIBI from cells. A pioneering study with a small cohort of parathyroid carcinomas showed a lower washout grade of 99mTc-MIBI from malignant parathyroid lesions than that from benign ones (17). Our study, with relatively larger samples, further indicated a significant difference of RI between malignant and benign parathyroid lesions. The membrane multidrug-resistant proteins such as P glycoprotein (P-GP) and multidrug resistance-associated protein 1 (MRP1), which belong to the ATP binding cassette superfamily of membrane transporters that rapidly eliminate lipophilic cations such as chemotherapeutic agents from cells, are thought to likely play an essential role in the efflux of 99mTc-MIBI. Previous studies have reported negative P-GP expressions in the parathyroid adenomas with positive 99mTc-MIBI scan (18-20). However, the association between them is still controversial (9). A recent study showed that negative MRP1 expression was more likely to have an RI greater than 0, but the correlation between MRP1 expression intensity and 99mTc-MIBI retention level was not established (21). In line with the above studies, most parathyroid samples with positive 99mTc-MIBI scans in our study demonstrated a negative expression of P-GP (data not shown), which made the correlation analysis between RI and P-GP expression level difficult to perform.
Meanwhile, various levels of positive MRP1 expression were observed in our parathyroid samples, but no significant correlation between RI and MRP1 expression intensity was found either (data not shown). Therefore, the mechanism of a higher retention level in parathyroid carcinoma than benign lesions needs to be further investigated. In addition, RI showed a weak correlation with serum PTH level and had no significant association with serum calcium level in our study, suggesting that the RI could not reflect the disease severity of PHPT.
Lesion size and PTH levels were also significant variables for differentiating parathyroid carcinoma from benign lesions. A previous report from our hospital has also shown that the size of parathyroid carcinomas determined on ultrasound was significantly larger than that of benign ones (2). Meanwhile, the patients with parathyroid carcinomas usually experience more severe clinical manifestations with PTH levels 3 to 10 times the upper limit of the norm (1). However, these two features still partially overlap with those of benign hyperparathyroidism. A combination of RIpeak, size, and PTH levels could further improve the diagnostic performance, especially of NPV.
Although 99mTc-MIBI dual-phase planar imaging with SPECT/CT is a classic radionuclide imaging method for localization of parathyroid nodules, 18F-fluorocholine PET/CT is a becoming potential alternative for parathyroid imaging. Recent studies have indicated that 18F-Fluorocholine PET/CT had a better diagnostic accuracy than 99mTc-MIBI SPECT/CT for localization of parathyroid adenoma (21-23), especially in the detection of small parathyroid adenomas. However, few cases of 18F-fluorocholine PET/CT in parathyroid carcinoma have been reported until now (24,25). Therefore, the value of 18F-fluorocholine PET/CT for differentiating parathyroid carcinoma from adenoma is still uncertain, and needs to be further explored in a larger cohort of patients with PHPT.
In conclusion, parathyroid carcinomas have a significantly higher retention level of 99mTc-MIBI than benign parathyroid lesions. When the RIpeak of the parathyroid lesion is >−19%, it strongly raises the suspicion of parathyroid carcinoma, and management should be altered; RIpeak may contribute to the preoperative differential diagnosis of parathyroid carcinoma.
Acknowledgments
We would like to thank Feng Wang from Siemens Healthineers and DaYong Huang from TianSi, Ltd. for their technological support of the semi-quantitative measurement.
Funding: This work was supported by grants from the National Rare Diseases Registry System of China (2016YFC0901500 and 2016YFC0901503), the National Natural Science Foundation of China (81501499), Shanghai Municipal Health Bureau Project (20124235), Shanghai Jiao Tong University Med-X Interdisciplinary Research Funding (YG2017MS61), and Shanghai Pujiang Program (18PJD030).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of Shanghai Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. The Ethics Committee confirmed that formal written consent from patients was not required, because it was a retrospective study.
References
- Zhao L, Liu JM, He XY, Zhao HY, Sun LH, Tao B, Zhang MJ, Chen X, Wang WQ, Ning G. The changing clinical patterns of primary hyperparathyroidism in Chinese patients: data from 2000 to 2010 in a single clinical center. J Clin Endocrinol Metab 2013;98:721-8. [Crossref] [PubMed]
- Xue S, Chen H, Lv C, Shen X, Ding J, Liu J, Chen X. Preoperative diagnosis and prognosis in 40 Parathyroid Carcinoma Patients. Clin Endocrinol (Oxf) 2016;85:29-36. [Crossref] [PubMed]
- Shane E. Clinical review 122: Parathyroid carcinoma. J Clin Endocrinol Metab 2001;86:485-93. [Crossref] [PubMed]
- Liu JM, Cusano NE, Silva BC, Zhao L, He XY, Tao B, Sun LH, Zhao HY, Fan WW, Romano ME, Ning G, Bilezikian JP. Primary Hyperparathyroidism: A Tale of Two Cities Revisited - New York and Shanghai. Bone Res 2013;1:162-9. [Crossref] [PubMed]
- Morand GB, Helmchen BM, Steinert HC, Schmid C, Broglie MA. 18F-Choline-PET in parathyroid carcinoma. Oral Oncol 2018;86:314-5. [Crossref] [PubMed]
- Hatzl M, Roper-Kelmayr JC, Fellner FA, Gabriel M. 18F-Fluorocholine, 18F-FDG, and 18F-Fluoroethyl Tyrosine PET/CT in Parathyroid Cancer. Clin Nucl Med 2017;42:448-50. [Crossref] [PubMed]
- Hindié E, Ugur O, Fuster D, O'Doherty M, Grassetto G, Urena P, Kettle A, Gulec SA, Pons F, Rubello D. Parathyroid Task Group of the E. 2009 EANM parathyroid guidelines. Eur J Nucl Med Mol Imaging 2009;36:1201-16. [Crossref] [PubMed]
- Schulte KM, Talat N. Diagnosis and management of parathyroid cancer. Nat Rev Endocrinol 2012;8:612-22. [Crossref] [PubMed]
- Jorna FH, Hollema H, Hendrikse HN, Bart J, Brouwers AH, Plukker JT. P-gp and MRP1 expression in parathyroid tumors related to histology, weight and (99m)Tc-sestamibi imaging results. Exp Clin Endocrinol Diabetes 2009;117:406-12. [Crossref] [PubMed]
- Saggiorato E, Angusti T, Rosas R, Martinese M, Finessi M, Arecco F, Trevisiol E, Bergero N, Puligheddu B, Volante M, Podio V, Papotti M, Orlandi F. 99mTc-MIBI Imaging in the presurgical characterization of thyroid follicular neoplasms: relationship to multidrug resistance protein expression. J Nucl Med 2009;50:1785-93. [Crossref] [PubMed]
- Erdil TY, Ozker K, Kabasakal L, Kanmaz B, Sonmezoglu K, Atasoy KC, Turoglu HT, Uslu I, Isitman AT, Onsel C. Correlation of technetium-99m MIBI and thallium-201 retention in solitary cold thyroid nodules with postoperative histopathology. Eur J Nucl Med 2000;27:713-20. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Petrovic NS, Grujicic D, Artiko VM, Sobic-Saranovic DP, Gajic MM, Jaksic E, Grajic MM, Antonovic OJ, Petrovic MN, Obradovic VB. Investigation of blood perfusion and metabolic activity of brain tumours in adults by using 99mTc-methoxyisobutylisonitrile. Nucl Med Commun 2010;31:962-73. [Crossref] [PubMed]
- Yüksel M, Cermik TF, Karlikaya C, Salan A, Cakir E, Gultekin A, Berkarda S. Monitoring the chemotherapeutic response in primary lung cancer using 99mTc-MIBI SPET. Eur J Nucl Med 2001;28:799-806. [Crossref] [PubMed]
- Ak I, Gulbas Z, Altinel F, Vardareli E. Tc-99m MIBI uptake and its relation to the proliferative potential of brain tumors. Clin Nucl Med 2003;28:29-33. [Crossref] [PubMed]
- Rowe SP, Gorin MA, Gordetsky J, Ball MW, Pierorazio PM, Higuchi T, Epstein JI, Allaf ME, Javadi MS. Initial experience using 99mTc-MIBI SPECT/CT for the differentiation of oncocytoma from renal cell carcinoma. Clin Nucl Med 2015;40:309-13. [Crossref] [PubMed]
- Cheon M, Choi JY, Chung JH, Lee JY, Cho SK, Yoo J, Park SB, Lee KH, Kim BT. Differential findings of tc-99m sestamibi dual-phase parathyroid scintigraphy between benign and malignant parathyroid lesions in patients with primary hyperparathyroidism. Nucl Med Mol Imaging 2011;45:276-84. [Crossref] [PubMed]
- Sun SS, Shiau YC, Lin CC, Kao A, Lee CC. Correlation between P-glycoprotein (P-gp) expression in parathyroid and Tc-99m MIBI parathyroid image findings. Nucl Med Biol 2001;28:929-33. [Crossref] [PubMed]
- Gupta Y, Ahmed R, Happerfield L, Pinder SE, Balan KK, Wishart GC. P-glycoprotein expression is associated with sestamibi washout in primary hyperparathyroidism. Br J Surg 2007;94:1491-5. [Crossref] [PubMed]
- Yamaguchi S, Yachiku S, Hashimoto H, Kaneko S, Nishihara M, Niibori D, Shuke N, Aburano T. Relation between technetium 99m-methoxyisobutylisonitrile accumulation and multidrug resistance protein in the parathyroid glands. World J Surg 2002;26:29-34. [Crossref] [PubMed]
- Xue J, Liu Y, Yang D, Yu Y, Geng Q, Ji T, Yang L, Wang Q, Wang Y, Lu X, Yang A. Dual-phase 99mTc-MIBI imaging and the expressions of P-gp, GST-pi, and MRP1 in hyperparathyroidism. Nucl Med Commun 2017;38:868-74. [Crossref] [PubMed]
- Beheshti M, Hehenwarter L, Paymani Z, Rendl G, Imamovic L, Rettenbacher R, Tsybrovskyy O, Langsteger W, Pirich C. (18)F-Fluorocholine PET/CT in the assessment of primary hyperparathyroidism compared with (99m)Tc-MIBI or (99m)Tc-tetrofosmin SPECT/CT: a prospective dual-centre study in 100 patients. Eur J Nucl Med Mol Imaging 2018;45:1762-71. [Crossref] [PubMed]
- Araz M, Soydal C, Ozkan E, Kir MK, Ibis E, Gullu S, Erdogan MF, Emral R, Kucuk ON. The efficacy of fluorine-18-choline PET/CT in comparison with 99mTc-MIBI SPECT/CT in the localization of a hyperfunctioning parathyroid gland in primary hyperparathyroidism. Nucl Med Commun 2018;39:989-94. [Crossref] [PubMed]
- Thanseer N, Bhadada SK, Sood A, Mittal BR, Behera A, Gorla AKR, Kalathoorakathu RR, Singh P, Dahiya D, Saikia UN, Rao SD. Comparative Effectiveness of Ultrasonography, 99mTc-Sestamibi, and 18F-Fluorocholine PET/CT in Detecting Parathyroid Adenomas in Patients With Primary Hyperparathyroidism. Clin Nucl Med 2017;42:e491-7. [Crossref] [PubMed]
- Deandreis D, Terroir M, Al Ghuzlan A, Berdelou A, Lacroix L, Bidault F, Troalen F, Hartl D, Lumbroso J, Baudin E, Schlumberger M, Leboulleux S. (1)(8)Fluorocholine PET/CT in parathyroid carcinoma: a new tool for disease staging? Eur J Nucl Med Mol Imaging 2015;42:1941-2. [Crossref] [PubMed]


