
Prognostic imaging of iatrogenic and traumatic ureteral injury by near-infrared fluorescence
Introduction
Ureters are located near important anatomic structures such as, gonadal and uterine vessels, cervix, iliac arteries, inferior mesenteric and sigmoid vessels, colon, and rectum, which are commonly accessed during gynecologic and general surgery (1,2). Furthermore, ureters have a delicate subadventitial blood supply segmentally provided by the aorta and renal, gonadal, common iliac arteries. Their unique anatomy and proximity to other pelvic and abdominal organs renders them prone to iatrogenic injury (2-6). Common mechanisms of injury include direct trauma caused by transection, suture ligation, crush injury, coagulation, or indirect trauma due to relative ischemia resulting from the use of large caliber instruments, devascularization, or thermal injury (2-6). Earlier studies reported that the incidence of iatrogenic ureteral injury is 0.1% to 7.6% during colorectal and gynecologic surgery, and that more than 80% of cases went unrecognized intraoperatively (7-11). Assimos et al. (11) compared incidences of iatrogenic ureteral injuries during the prelaparoscopic and laparoscopic eras and concluded the incidence of iatrogenic ureteral injuries was significantly greater in the latter (7).
Although traumatic ureteral injury occurs in fewer than 1% of all urologic trauma cases (12-14), if not recognized intraoperatively, it may be life-threatening and lead to permanent urinary tract damage (15) or fistula formation (16). However, traumatic ureteral injury is difficult to diagnose as ureteral injury has no pathognomonic or classic signs nor symptoms, and thus, a high index of suspicion is required in the setting of penetrating abdominal trauma to ensure prompt and appropriate intervention. When symptoms are identified, they often include hematuria, flank pain, ecchymosis, and hypotension, and notably, hematuria may not always be present (documented in 44.4% of cases) (13). Computed tomography (CT) urography is recommended for traumatic ureteral injury (14), while a single shot intravenous pyelogram (IVP) may provide anatomic and functional information intraoperatively regarding ureteral injury (14). However, IVP is unreliable excluding ureteral injury and should only be utilized after hemodynamic stability has been achieved (14). In hemodynamically unstable patients, ureteral injuries should be diagnosed through exploratory laparotomy, either by direct inspection or by visualizing extravasation of excreted intravenous dye such as, methylene blue or indigo carmine (12-14).
Clear identification of ureters during surgery provides the most reliable means of minimizing iatrogenic ureteral trauma because it enables surgeons to monitor ureter status. However, ureter visualization may be limited by anatomical deformations caused by previous surgical procedures, inflammatory pelvic disease, endometriosis, radiotherapy, or tumors (8,14,15). Intravenous pyelography, retrograde pyelography, and urologic CT can be performed preoperatively to help avoid injury, but unfortunately, none of these imaging techniques provides real-time guidance during surgical procedures (7,17). Ureteral catheterization has been proposed as a preoperative prophylaxis to identify ureters in operation fields, but catheter insertion requires technical skill and may cause injury (8,14). In a recent randomized trial, catheterization was found to be inefficient at reducing the incidence of ureter injury and not recommended when compared to direct ureter visualization (7,14).
Intraoperative fluorescence (FL) ureteral identification achieved by administering a suitable dye preoperatively (18,19) is a promising new technique for providing easier and earlier intraoperative visualization of the ureter (7) and enabling ureteral visualization crucial for preventing ureter injury during surgery and for diagnosing iatrogenic or traumatic ureter injury. In particular, near-infrared (NIR) FL imaging has been used for real-time ureteral guidance after the intravenous administration of fluorophores, such as, methylene blue (7), CW800-CA (14,20), ZW700-1 (21), or ZW800-1 (22-25).
ZW700-1 and ZW800-1 are nonsticky zwitterionic fluorescent dyes that are cleared rapidly by the kidneys to the urinary bladder (21-25). Verbeek et al. found that normal ureter visualization to prevent intraoperative iatrogenic injuries, ureteral visualization is feasible after a single injection of cRGD-ZW800-1, which is cleared by kidneys (23). In the present study, we evaluated the feasibility of the preoperative administration of the NIR fluorescent dye ZW800-1C for ureter visualization and as a diagnostic tool for various iatrogenic or traumatic ureteral injuries.
Methods
NIR FL imaging system and agents
In vivo NIR FL imaging was performed using the Fluorescence Imaging Assisted Theranostic Lux (FIAT-L) preclinical imaging system (Nawoo Vision, Gyeongnam, Korea) (26). This system consists of three separate light sources of different wavelengths: a white LED light source generating visible light from 400 to 650 nm light to illuminate the surgical field as well as two NIR laser diodes of wavelength 660 and 760 nm. Visible light and NIR FL images were acquired simultaneously and displayed in real-time, using custom-designed optics and software. Methylene blue was purchased from Sigma-Aldrich (Cat #: M9140, MO, USA), and ZW800-1C was synthesized as reported previously (27). Both contrast agents were filtered through a 0.22 µm sterile PES membrane prior to injection. NIR FL imaging was performed at emission wavelengths 700 nm for methylene blue and 800 nm for ZW800-1C.
Animal care and experiments
Experimental mice (C57BL6, males, 6 weeks old) were housed in the specific pathogen-free animal facility of Lee Gil Ya Cancer and Diabetes Institute, Gachon University, in individually ventilated cages at 21.4 °C and 50% RH. All procedures for maintenance and experimental procedures were approved by the Animal Care and Use Committee of Gachon University (LCDI-2018-0074). Mice were anaesthetized using xylazine (4 mg/kg i.p; Rompun, BAYER KOREA Ltd, Seoul) and ketamine (100 mg/kg; ketamine hydrochloride, Yuhan Co., Korea). Kidneys and bladders were exposed after laparotomy.
Ureter visualization by NIR FL imaging
A total of 140 nmol of methylene blue (n=3) or 25 nmol of ZW800-1C (n=5) were injected intravenously into mice after lateral laparotomy. NIR fluorescent signals were measured at 1, 5, 10, 30, 60, 120, and 180 min post-injection using the FIAT-L preclinical imaging system. Image J Software Ver. 1.52a (NIH, MD, USA) was used to measure FL intensities. Images were analyzed by measuring SBRs, which were calculated by dividing fluorescent intensities of kidneys and ureters by the fluorescent intensities of surrounding tissue.
Ureter injury models and injury site confirmation
ZW800-1C was injected intravenously into mice before ureter injury models were prepared. The ureter obstruction model was made by ligating ureter with 6-0 nylon; the ureter crush injury model was made by keep tie ligation on the ureter for 30 minutes and release it; the ureter transection model was made by transecting ureters in their mid-portions using a microscissors; and the ureter perforation model was made by making a small incision in ureters using a microscissors.
Statistical analysis
At each time point, the FL and background (BG) intensity of a region of interest (ROI) over each organ/tissue was quantified using the custom FIAT-L software. All NIR FL images for each dye were normalized identically for all conditions over the experiments. At least 3–5 animals were analyzed at each time point, and data are expressed as means ± standard deviations (SDs). Comparisons were conducted using the Mann-Whitney U test in SPSS Ver. 22. Statistical significance was accepted for P values <0.05.
Results
NIR fluorescent ZW800-1C for ureter imaging
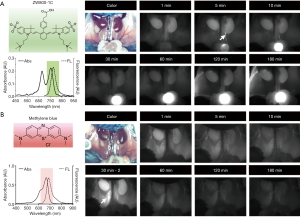
As shown in Figure 1, ZW800-1C is a zwitterionic fluorophore with a chemically stable C-C coupled bond on the mesocarbon of a heptamethine core that emits at 800 nm (27). ZW800-1C has better optical properties than other commercially available NIR fluorophores (22-25), but its optical and physicochemical stabilities under physiological conditions are likely due to the presence of an unstable ether linkage on its mesocarbon. However, ZW800-1C was developed by introducing a rigid carbon bond onto the framework of ether-linked ZW800-1, and has better optical and chemical stabilities in warm serum (100%, 48 h; Figure 1C) and different solvents (Figure 1D). Furthermore, ZW800-1C exhibits a high absorbance peak for H-aggregated dimers and oligomers at ~660 nm (blue arrow) and a monomer peak at 754 nm (red arrow; Figure 1B), which may be advantageous for general single channel tissue imaging; ZW800-1C can be excited by wavelengths ranging from 660 to 760 nm and emit FL photons extensively from 770 to 850 nm. On the other hand, ZW800-1 shows an absorption peak of at ~772 nm and an emission peak of ~788 nm in fetal bovine serum (FBS) (27). In addition, the low logD (−2.8 at pH 7.4) and high TPSA (157.95) values along with zwitterionic net charge (+3/−3) make the final C-C coupled ZW800-1C dissolve well in PBS and FBS (>5 mg/mL), thus freely filtered by the kidneys to the urinary bladder (28).

Ureter visualization
Figure 2 and Tables 1 and 2 compare the intraoperative ureter imaging characteristics of ZW800-1C and methylene blue. Intravenous injection of ZW800-1C at 25 nmol clearly delineated ureters during the passage of urine in the mouse model, and maximal imaging resolution was achieved 30 min post-intravenous injection (Figure 2A). We could confirm the ureter pathway in 1 min post-injection of ZW800-1C. The highest FL intensity was obtained at various time points among different animals, depending on the time when urinary drainage started to the bladder; however, the ureter pathway was clearly visible until 180 min post-injection of ZW800-1C in C57BL6 mice. On the other hand, we could not confirm the ureter structure by injecting 140 nmol of methylene blue until 30 min post-intravenous injection (Figure 2B). A second dose of 140 nmol was injected at 30 min after the first injection. However, ureter was visualized only in one among 3 animals, even after the second dosage (Figure 2B). Methylene blue cleared out through the kidneys very quickly, rendering it difficult to find even the kidney structure 30 min post-intravenous injection. In the mouse colon, feces cause autofluorescence, which is another disadvantage of using methylene blue in pelvic and abdominal surgeries. In contrast, the signal-to-background ratio (SBR) of ZW800-1C of the right kidney was significantly higher than the other over the course of imaging period (Table 1). The statistical comparison of ureter SBR between ZW800-1C and methylene blue was impossible because confirmation of the ureter was only possible in one of the animals when methylene blue was used. The urinary excretion showed the highest signal intensities at 5 post-injection of ZW800-1C in C57BL6 mice (n=5; Table 2).

Full table
Full table
Ureter injury models
Since most ureteral injuries occur during surgery, we created various ureteral injury models in the mice to evaluate the feasibility of ZW800-1C in the clinical setting (Figure 3). These injuries include: (I) an obstruction model by direct ureter ligation; (II) a crush injury model by releasing a suture 30 minutes after ligation; (III) a ureter transection model; and (IV) a perforation model produced by making a small incision. ZW800-1C (25 nmol, i.v.) was injected into each animal before creating the different models. Intraoperative ureteral imaging was followed on the abdominal wall.
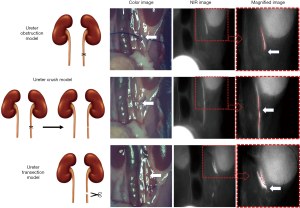
In the ureteral obstruction model (Figure 3A), we could confirm the obstructed site. Urinary flow was abruptly disconnected at the obstruction site, which was difficult to find in the color image. Because of fat tissue which covered the ureter, the exact site of obstruction was hard to confirm in the color image. If surgeons did tie ligation near the ureter, it would be even hard to discriminate whether the ureter jammed in the tissue where the tie ligation was done. In those situations, ZW8001-C imaging would be helpful, because it could help visualize the exact obstruction site.
ZW800-1C also enabled the identification of the crushing injury site of the respective model prepared by tying the ureter and releasing the ligated suture as shown in Figure 3B. The ureter luminal crush injury and partial ureter obstruction resulted in luminal narrowing of the ureter. The luminal narrowing site induced crushing injury site was impossible to be confirmed by the color image.
In the ureter transection model, transection sites and urinary leakage were well visualized by ZW800-1C (Figure 3C), although urine leakage from the ureter was not visible by eye.
In the ureteral perforation injury model (Figure 4), ZW800-1C (25 nmol, i.v.) enabled sites of perforation and urinary leakage to be visualized, whereas urine leakage was observed by eye.
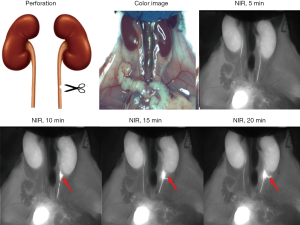
Overall, we evaluated that iatrogenic ureter injuries during abdominal surgeries could be avoided by clear ureter visualization by a single intravenous injection of ZW800-1C. Even if the ureter injured such as ureter ligation, crushing injury, perforation or even transaction iatrogenically, it might be easily diagnosed by NIR FL with ZW800-1C during surgery and early treatment or intervention could be possible.
Discussion
The present study was undertaken to determine whether the near-infrared fluorophore ZW800-1C better enables the visualization of different types of ureteral injuries. It was found ZW800-1C provides better ureter visualization than methylene blue and that it has considerable potential for the early diagnosis of various injuries, including ureter perforation, obstruction, crush injury, and transection. Furthermore, our observations indicate iatrogenic ureter injuries during abdominal surgeries might be avoided by enabling clearer ureter visualization if a single intravenous injection of ZW800-1C was administered.
Indocyanine green (ICG) is a Food and Drug Administration-approved fluorescent dye that is injected intravenously during retinal angiography and cardiac and hepatic function testing and to determine tissue viability (14). However, intravenously administered ICG is not useful for ureteral FL imaging because it is rapidly cleared by the liver and has no detectable urinary excretion. Siddighi et al. injected ICG intraurethrally through a ureteral catheter for ureter visualization in 10 patients prior to robot-assisted laparoscopic surgery (28). However, this technique introduces risk of ureteral damage during catheter insertion.
Methylene blue is another clinically available dye (Figure 2). Matsui et al. presented the possibility of using NIR-guided surgery using methylene blue to identify ureters in pig models (29), which was possible because, unlike ICG, which is cleared via the liver and bile, methylene blue is excreted through the renal route (30-32). Verbeek et al. reported similar results in 12 patients that underwent lower abdominal surgery (33), both ureters were clearly visualized within 10 min of infusing methylene blue in all patients and signals lasted for up to 60 min. However, in another study it was concluded ureteral FL imaging using methylene blue did not provide any practical advantage over conventional laparoscopic imaging in terms of identifying ureters during laparoscopic colorectal surgery (7). Presumably, this discrepancy arose because pigs have a thinner ureteral wall and subretroperitoneal layer and less intra-abdominal fat than humans and because the human trial involved open abdominal surgery not in the laparoscopic surgery (7). Since the penetration depth of NIR FL imaging using 700 nm light and methylene blue is limited to 3–5 mm, ureter identification can be challenging (33). Furthermore, methylene blue sometimes causes severe adverse reactions, such as, methemoglobinemia or anaphylaxis (30-33). These shortcomings suggest renally clearable contrast agents that fluoresce at 800 nm with high extinction coefficients and quantum yields would be advantageous (33).
Several 800 nm emitting preclinical dyes have been tested for ureter visualization. Intravenous injection of CW800-CA provided clear delineation of the course of both ureters by laparoscopy in a swine model (20). CW800-CA (3 µmol, 100 µg/kg) was injected into a 35 kg Yorkshire pig and urinary excretion was observed at 10 min post-injection. The clearance half-life of CW800-CA was found to be ~36 min (20,34), but despite this, CW800-CA is useful for pelvic surgery because ureters are normally identified early during procedures. However, CW800-CA is eliminated by hepatobiliary clearance, which sometimes contaminates intraoperative images of the abdominal cavity (24,35). On the other hand, zwitterionic fluorophores are near ideal for ureteral imaging because they are rapidly cleared via the renal route and do not bind to serum proteins or cell surfaces. Verbeek et al. reported ureters were clearly visualized 30 min after intravenously injecting cRGD-ZW800-1 and that the ideal window for ureter visualization appeared to lie between 10 min and 8 h (23). Ureter imaging for extended times after a single i.v. injection of an NIR fluorophore offers a powerful imaging technique for pelvic and abdominal surgeries because they usually take several hours. A single dose injection could also avoid potential toxicity from additional dosage. In addition, the dosage of cRGD-ZW800-1 required to obtain clear ureter visualization is smaller than that of other fluorophores (<30 nmol per mouse) (23). When dosages used in mice and rats are converted to human doses by body surface area, 0.25, 1, and 30 nmol are equivalent to 0.096, 0.39, and 1.33 mg, respectively (23,35,36), which are much lower than commonly used ICG and methylene blue doses (10–25 and 15–60 mg, respectively). In addition, a dose of 0.25 nmol in mice corresponds to a human equivalent dose of <100 µg, which is below the FDA threshold for micro-dosing (23,35,36).
In the present study, we compared abilities of ZW800-1C and methylene blue to visualize ureters and confirmed the feasibility of using ZW800-1C to visualize various ureteral injuries. In most cases, the ureter pathway was observed after a single injection of 25 nmol ZW800-1C i.v. from 5 min to 3–4 h post-injection. Previous studies that have tried to visualize the ureter have focused on how clearly the ureter can be shown by various dyes to avoid iatrogenic ureteral injury during surgeries. In our study, we evaluated the ZW800-1C could be beneficial as diagnostic tool after iatrogenic or traumatic injury occurred.
The traumatic ureteral injury is rare but hard to do diagnosis. If the trauma patients’ vital signs are not stable, it is difficult to do IVP for diagnosis of traumatic ureteral injury. In this situation, NIR imaging with ZW800-1C would be helpful to enhance the surgeon’s visualization and diagnose the traumatic injury of ureter during surgeries quickly.
One limitation of using ZW800-1C for ureter visualization is that the technique is limited when urine flow is absent or small as in cases of oliguria. Moreover, as urine flow in the ureter is pulsatile and as fluorescent signals are related to flow, signals in ureters vary over time (33). However, this disadvantage is a fundamental limitation of fluorophores when used for ureter imaging.
Acknowledgments
We thank Ivey Choi for manuscript editing.
Funding: This work was partly supported by Institute for Information & communications Technology Promotion (IITP) grant funded by the Korea government (MSIP No. 2016-0-00452, development of creative technology based on complex 3D printing technology for labor, the elderly and the disabled), the Gachon University Gil Medical Center (grant No. FRD2016-05-02), and the US NIH grants NIBIB #R01-EB022230 and NCI #R21CA223270.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All procedures for maintenance and experimental procedures were approved by the Animal Care and Use Committee of Gachon University (LCDI-2018-0074).
References
- Delacroix SE Jr, Winters JC. Urinary tract injures: recognition and management. Clin Colon Rectal Surg 2010;23:104-12. [Crossref] [PubMed]
- Gild P, Kluth LA, Vetterlein MW, Engel O, Chun FKH, Fisch M. Adult iatrogenic ureteral injury and stricture-incidence and treatment strategies. Asian Journal of Urology 2018;5:101-6. [Crossref] [PubMed]
- Tyritzis SI, Wiklund NP. Ureteral strictures revisited trying to see the light at the end of the tunnel: a comprehensive review. J Endourol 2015;29:124-36. [Crossref] [PubMed]
- Manoucheri E, Cohen SL, Sandberg EM, Kibel AS, Einarsson J. Ureteral injury in laparoscopic gynecologic surgery. Rev Obstet Gynecol 2012;5:106-11. [PubMed]
- Selli C, Turri FM, Gabellieri C, Manassero F, De Maria M, Mogorovich A. Delayed-onset ureteral lesions due to thermal energy: an emerging condition. Arch Ital Urol Androl 2014;86:152-3. [Crossref] [PubMed]
- Parpala-Spårman T, Paananen I, Santala M, Ohtonen P, Hellstrom P. Increasing numbers of ureteric injuries after the introduction of laparoscopic surgery. Scand J Urol Nephrol 2008;42:422-7. [Crossref] [PubMed]
- Al-Taher M, van den Bos J, Schols RM, Bouvy ND, Stassen LP. Fluorescence Ureteral Visualization in Human Laparoscopic Colorectal Surgery Using Methylene Blue. J Laparoendosc Adv Surg Tech A 2016;26:870-5. [Crossref] [PubMed]
- Park JH, Park JW, Song K, Jo MK. Ureteral injury in gynecologic surgery: A 5-year review in a community hospital. Korean J Urol 2012;53:120-5. [Crossref] [PubMed]
- Palaniappa NC, Telem DA, Ranasinghe NE, Divino CM. Incidence of iatrogenic ureteral injury after laparoscopic colectomy. Arch Surg 2012;147:267-71. [Crossref] [PubMed]
- da Silva G, Boutros M, Wexner SD. Role of prophylactic ureteric stents in colorectal surgery. Asian J Endosc Surg 2012;5:105-10. [Crossref] [PubMed]
- Assimos DG, Patterson LC, Taylor CL. Changing incidence and etiology of iatrogenic ureteral injuries. J Urol 1994;152:2240-6. [Crossref] [PubMed]
- Bryk DJ, Zhao LC. Guideline of guidelines: a review of urological trauma guidelines. BJU Int 2016;117:226-34. [Crossref] [PubMed]
- Pereira BM, Ogilvie MP, Gomez-Rodriguez JC, Ryan ML, Peña D, Marttos AC, Pizano LR, McKenney MG. A review of ureteral injuries after external trauma. Scand J Trauma Resusc Emerg Med 2010;18:6. [Crossref] [PubMed]
- Phillips B, Holzmer S, Turco L, Mirzaie M, Mause E, Mause A, Person A, Leslie SW, Cornell DL, Wagner M, Bertellotti R, Asensio JA. Trauma to the bladder and ureter: a review of diagnosis, management, and prognosis. Eur J Trauma Emerg Surg 2017;43:763-73. [Crossref] [PubMed]
- Selzman AA, Spirnak JP. Iatrogenic ureteral injuries: a 20-year experience in treating 165 injuries. J Urol 1996;155:878-81. [Crossref] [PubMed]
- Kim JH, Moore C, Jones JS, Rackley R, Daneshgari F, Goldman H, Vasavada S. Management of ureteral injuries associated with vaginal surgery for pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct 2006;17:531-5. [Crossref] [PubMed]
- Brandes S, Coburn M, Armenakas N, McAninch J. Diagnosis and management of ureteric injury: An evidence-based analysis. BJU Int 2004;94:277-89. [Crossref] [PubMed]
- Troyan SL, Kianzad V, Gibbs-Strauss SL, Gioux S, Matsui A, Oketokoun R, Ngo L, Khamene A, Azar F, Frangioni JV. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol 2009;16:2943-52. [Crossref] [PubMed]
- Dzurinko VL, Gurwood AS, Price JR. Intravenous and indocyanine green angiography. Optometry 2004;75:743-55. [Crossref] [PubMed]
- Korb ML, Huh WK, Boone JD, Warram JM, Chung TK, de Boer E, Bland KI, Rosenthal EL. Laparoscopic fluorescent visualization of the ureter with intravenous IRDye800CW. J Minim Invasive Gynecol 2015;22:799-806. [Crossref] [PubMed]
- Hyun H, Henary M, Gao T, Narayana L, Owens EA, Lee JH, Park G, Wada H, Ashitate Y, Frangioni JV, Choi HS. 700-nm zwitterionic near-infrared fluorophores for dual-channel image-guided surgery. Mol Imaging Biol 2016;18:52-61. [Crossref] [PubMed]
- Ikeda M, Wakasaki R, Schenning KJ, Swide T, Lee JH, Miller MB, Choi HS, Anderson S, Hutchens MP. Determination of renal function and injury using near-infrared fluorimetry in experimental cardiorenal syndrome. Am J Physiol Renal Physiol 2017;312:F629-39. [Crossref] [PubMed]
- Verbeek FP, van der Vorst JR, Tummers QR, Boonstra MC, de Rooij KE, Löwik CW, Valentijn AR, van de Velde CJ, Choi HS, Frangioni JV, Vahrmeijer AL. Near-infrared Fluorescent Imaging of Both Colorectal Cancer and Ureters Using a Low-Dose Integrin Targeted Probe. Ann Surg Oncol 2014;21:S528-37. [Crossref] [PubMed]
- Choi HS, Nasr K, Alyabyev S, Feith D, Lee JH, Kim SH, Ashitate Y, Hyun H, Patonay G, Strekowski L, Henary M, Frangioni JV. Synthesis and in vivo fate of zwitterionic near-infrared fluorophores. Angew Chem Int Ed Engl 2011;50:6258-63. [Crossref] [PubMed]
- Hyun H, Bordo MW, Nasr K, Feith D, Lee JH, Kim SH, Ashitate Y, Moffitt LA, Rosenberg M, Henary M, Choi HS, Frangioni JV. cGMP-Compatible preparative scale synthesis of near-infrared fluorophores. Contrast Media Mol Imaging 2012;7:516-24. [Crossref] [PubMed]
- Kim TH, O’Brien C, Choi HS, Jeong MY. Fluorescence molecular imaging systems for intraoperative image-guided surgery. Appl Spectrosc Rev 2018;53:349-59. [Crossref]
- Hyun H, Owens EA, Narayana L, Wada H, Gravier J, Bao K, Frangioni JV, Choi HS, Henary M. Central C-C bonding increases optical and chemical stability of NIR fluorophores. RSC Adv 2014;4:58762-8. [Crossref] [PubMed]
- Siddighi S, Yune JJ, Hardesty J. Indocyanine green for intraoperative localization of ureter. Am J Obstet Gynecol 2014;211:436.e1-2. [Crossref] [PubMed]
- Matsui A, Tanaka E, Choi HS, Kianzad V, Gioux S, Lomnes SJ, Frangioni JV. Real-time, near-infrared, fluorescence-guided identification of ureters using methylene blue. Surgery 2010;148:78-86. [Crossref] [PubMed]
- DiSanto AR, Wagner JG. Pharmacokinetics of highly ionized drugs. I. Methylene blue whole blood, urine, and tissue assays. J Pharm Sci 1972;61:598-602. [Crossref] [PubMed]
- DiSanto AR, Wagner JG. Pharmacokinetics of highly ionized drugs. II. Methylene blue absorption, metabolism, and excretion in man and dog after oral administration. J Pharm Sci 1972;61:1086-90. [Crossref] [PubMed]
- Mark S. Soloway. Pathology examination cannot be done without a urologist's help. Cent European J Urol 2014;67:310-3.
- Verbeek FP, van der Vorst JR, Schaafsma BE, Swijnenburg RJ, Gaarenstroom KN, Elzevier HW, van de Velde CJ, Frangioni JV, Vahrmeijer AL. Intraoperative near-infrared fluorescence-guided identification of the ureters using low-dose methylene blue: A First-in-Human Experience. J Urol 2013;190:574-9. [Crossref] [PubMed]
- Marshall MV, Draney D, Sevick-Muraca EM, Olive DM. Single-dose intravenous toxicity study of IRDye 800CW in Sprague-Dawley rats. Mol Imaging Biol 2010;12:583-94. [Crossref] [PubMed]
- Choi HS, Gibbs SL, Lee JH, Kim SH, Ashitate Y, Liu F, Hyun H, Park G, Xie Y, Bae S, Henary M, Frangioni JV. Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nat Biotechnol 2013;31:148-53. [Crossref] [PubMed]
- Scheuer W, van Dam GM, Dobosz M, Schwaiger M, Ntziachristos V. Drug-based optical agents:infiltrating clinics at lower risk. Sci Transl Med 2012;4:134ps11. [Crossref] [PubMed]


