CT and MRI of adrenal gland pathologies
Introduction
According to their biological behavior, lesions of the adrenal grands can be classified into being benign or being malignant (including primary or metastatic) (Table 1). Different lesions have different treatment options and clinical prognoses, so it is of great clinical value to make a differential diagnosis based on computed tomography (CT) and magnetic resonance imaging (MRI) findings. Thought ultrasound and Nuclear medicine tests are commonly used for adrenal lesion assessment. This article is focused on CT and MRI.

Full table
Adrenal lesions can be classified into two types: symptomatic and asymptomatic. It has been reported that only a small number of adrenal tumors are functional and an even smaller number are malignant (1). Some adrenal lesions can secrete hormones that cause endocrine syndromes, and patients further develop clinical symptoms, such as Conn syndrome and Cushing syndrome (CS) (Table 2).

Full table
CS or hypercortisolism is classically described as the signs and symptoms associated with prolonged exposure to pathologically elevated cortisol levels (2), which represents hypercortisolism stemming from various causes other than a pituitary adenoma (3). CS can result from exogenous administration of glucocorticoids or endogenous overproduction of cortisol (4). The triggering pathways of CS can be divided into pituitary-dependent and pituitary-independent. Females are more likely to have CS than males. CS is most commonly caused by adrenal adenomas; other causes include adrenocortical carcinoma (ACC), pheochromocytomas.
CS is characterized by symptoms such as central obesity, buffalo hump, a rounded face, chromatosis, muscle weakness, hypertension, acne, hirsutism, menstrual irregularities, diabetes mellitus, osteoporosis, immune suppression, gonadal dysfunction, and mood changes (1-3,5). The clinical manifestations of CS are related to the patient’s age and the duration and degree of the hypercortisolism (5).
Conn syndrome, or primary aldosteronism, which is characterized by excessive spontaneous secretion of aldosterone from the adrenal glands, affects 6% of people with high blood pressure and can be either sporadic or familial (6,7). Conn syndrome is related to autonomous aldosterone production causing sodium retention, plasma renin suppression, hypertension, cardiovascular damage, and increased potassium excretion, leading to variable degrees of hypokalemia (8). The prevalence of cardiovascular disease in Conn syndrome patients is higher than that in normal individuals in the same age group. Often, patients are usually asymptomatic, but there may be symptoms of fatigue, muscle weakness, cramping, headaches, and palpitations. Patients may also have symptoms of polydipsia and polyuria due to hypokalemia caused by renal diabetes insipidus.
Addison disease, or primary adrenal insufficiency, is a systemic disease caused by hypoadrenocortical hypofunction. Addison disease has many causes, the most common of which is autoimmune adrenalitis, and other causes include tuberculosis, malignant tumor, infection, hemorrhage, HIV and certain genetic conditions. The incidence of Addison disease is 0.6/100,000 of population per year (9). Patients may experience weight loss, weakness, fatigue, gastrointestinal upset, orthostatic hypotension and pigmentation of skin (10). Dehydration, shock, hyperkalemia, and hyponatremia occurred in patients with adrenal crisis. Different causes of Addison's disease have different CT and MRI findings. CT study of the morphological changes of adrenal glands on patients with Addison's disease might help to define the etiology of the disease and contribute to treatment planning (11). Treatments for Addison disease include etiological treatment, hormone therapy (including glucocorticoids and mineralocorticoids) and treatment of adrenal crisis. When patients develop adrenal crisis, they need a stress dose of hydrocortisone and a large amount of fluid infusion. The treatment corticosteroid replacement and the prognosis following the treatment is the same as the normal population (12).
Normal anatomy of the adrenals
The adrenal glands are situated in the retroperitoneal space, close to the upper pole of the kidney (13). The normal adrenal gland has a linear, inverted V or Y, triangular shape (14). Each adrenal gland consists of two parts: the cortex and medulla, which have different embryological origins, distinct macroscopic and microscopic structures, as well as different functions and properties (15). The cortex derives from the mesoderm and it can be further divided into three areas: the lateral glomerular zone, the middle fascicular zone, and the medial reticulate zone, while the medulla consists of pheochromocytes.
The adrenal glands are highly vascularized. The adrenal artery has three sources: the middle adrenal artery originates from the abdominal aorta; the upper adrenal artery originates from the inferior phrenic artery; and the inferior adrenal artery originates from the renal artery (1,13,16). The branches of these arteries are anastomosed to each other. Venous return is different, the right adrenal vein enters the inferior vena cava, and the left adrenal vein converges with the left renal vein.
The lateral glomerular zone cells secrete mineralocorticoids (mainly aldosterone), the middle fascicular zone cells secrete glucocorticoids (mainly cortisol), and the medial reticulate zone cells produce sex hormones, such as dehydroepiandrosterone and estradiol.
Benign conditions
Adrenocortical adenoma
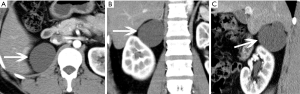
With benign nature, adrenocortical adenomas are the most common adrenal tumors (17). In addition, most incidentally discovered adrenal lesions are also benign adrenal adenomas (18,19).The incidence of adenomas is 3% at autopsy. It has been reported that the prevalence of adrenocortical adenomas is associated with age. The proportion of adenomas in men and women is about 1:2. Adrenocortical adenomas include functional and nonfunctioning adenomas. Functional adenomas can be accompanied by hypercortisolism (or CS) and primary hyperaldosteronism (or Conn’s syndrome). However, most lesions are nonfunctional (20). Nonfunctioning adenomas occur in the cortex, accounting for 25% of adrenal nonfunctioning tumors. Although CT cannot differentiate functional and nonfunctional adenomas, it can suggest a functioning adenoma when the contralateral adrenal gland atrophies.
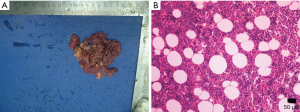
The gross appearance of an adenoma is a solid tumor with hemorrhagic or cystic changes (Figure 1), and occasionally calcification. Under a light microscope, the tumor cells are similar to normal cortical cells, with small nuclei, pale-staining cytoplasm, and arrangement in clusters, and some cells contained large amounts of fat. In addition, the mesenchyme separating the blood vessels is visible.
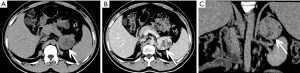
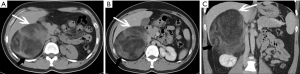
Adenomas are typically smaller in size, well-defined and homogeneous in attenuation. Generally, adenomas are homogeneous on unenhanced and contrast-enhanced CT images, and its density is equal to or slightly lower than that of normal adrenal gland tissue. When the tumor is necrotic and/or cystic, the density is uneven (Figures 2,3). Adenomas can be divided into two types: lipid-rich adenomas (70%, density less than 10 Hounsfield units (HUs) on pre-contrast-enhanced CT scan images) and lipid- poor adenomas (30%, with a density between 10–30 HU). On unenhanced CT scans, the decrease in the density of the lesion due to an increase in the amount of fat (21), and higher density is measured in lipid-poor adenomas than in lipid-rich adenomas.
Adenomas are usually characterized by homogeneity and mild enhancement. Caoili et al. reported that benign adenomas typically demonstrate an absolute percentage washout (APW) ≥60% and a relative percentage washout (RPW) of ≥40%, which can be computed in 10 to 15 minutes on delayed images .If the APW is 60% and/or the RPW 40%, the lesion is characterized as an adenoma (22). The density of the tumor changes with the size of the lesion. The larger and more heterogenous the tumor is, the greater the possibility of cystic and necrotic areas.
On MRI, lipid-rich adrenocortical adenomas have high intensity signal on T1- and T2-weighted images. Chemical shift imaging can detect a large amount of fat in the cytoplasm according to its special principles. Because of the different precession frequencies of protons in water molecules and fat, many adenomas show high signal on in-phase imaging and the signal decreases in the out-of-phase imaging.
Hyperplasia
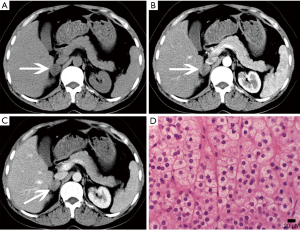
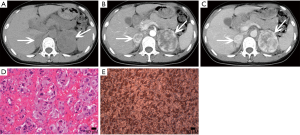
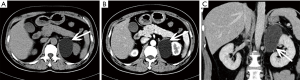
Hyperplasia can be seen as a diffuse process, that may involve the entire adrenal gland, or nodular hyperplasia, and it is typically bilateral (13,23). Diffuse hyperplasia is usually characterized by homogeneous thickening of the entire adrenal gland, maintaining its overall normal inverted-V or inverted-Y appearance (1). As nodular hyperplasia, if the nodules are large enough, they can be identified at cross-sectional imaging.
Most hyperplasia occurs in women, and the proportion in men and women is approximately 1:4, with a prevalence that increases with age and is estimated to be 0.51% (24). Once the hyperplastic adrenal cortex actively produces hormones, hormonal abnormalities in the blood can result, and ultimately CS and Conn syndrome may occur. The treatment of hyperplasia is associated with clinical manifestations. There is no need for treatment when no clinical and biological evidence indicates that hyperplasia is accompanied by adrenal cortical hyperfunction (24). When the patient has symptoms, sampling of the adrenal vein is essential for determining the next step of treatment.
Nodular hyperplasia is evidenced by multiple nodules in the adrenal glands, and the cells in the nodules are the same as those in the surrounding normal tissue (Figure 4). Diffuse hyperplasia shows enlarged cell volume and increased lipid content in the cytoplasm.
The density and signal of hyperplasia on CT and MRI is the same as that of the normal adrenal gland, but the density of some patients may be lower than that of the normal adrenal gland on unenhanced imaging (24).
Pheochromocytoma
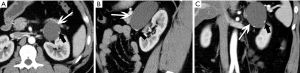
Pheochromocytomas are neural crest cell tumors occurring in the adrenal medulla. Pheochromocytomas are also a rare neuroendocrine tumor that secretes catecholamine, which are potent vasoactive hormones. It is known as the 10% tumor: 10% of these tumors are non-functioning, 10% occur in children, 10% are located outside the adrenal glands, and 10% are bilateral. Goffredo et al. (25) reported that although most cases of pheochromocytoma are benign, approximately 10% to 15% are malignant. Pathologically, there are multi-angle chromaffin cells under the electron microscopy. Under light microscopy, dense chromaffin granules can be seen around the nucleus (Figure 5).
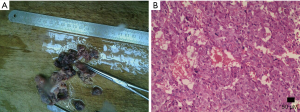
The incidence of pheochromocytoma is 0.1–0.2%, and has no sex difference. The clinical manifestation of these tumors is different from person to person and associated with the excess hormone produced by the tumor. Approximately 10% of pheochromocytomas are asymptomatic (14). Symptomatic patients may experience a feeling of flushing, hypermetabolism, hyperglycemia, hyperhidrosis, headache, palpitations and panic attacks or anxiety. The most common symptom is new onset, malignant, secondary hypertension.
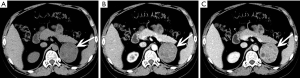
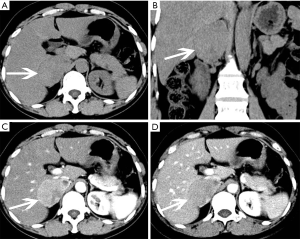
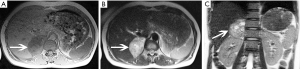
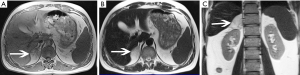
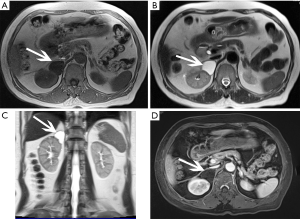
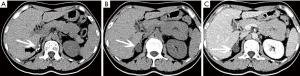
The size of pheochromocytomas is diverse; they are usually larger than adenomas, but smaller than metastatic tumors. A functional lesion is often not as large as a nonfunctional lesion. Pheochromocytoma may be visible as a well-defined mass, which may be solid or cystic to variable degrees (26). CT and MRI can clearly characterize and localize suspected pheochromocytomas. CT is associated with a certain amount of radiation damage; therefore, MRI is the first choice imaging method for children, pregnant women, young people and breast-feeding mothers (22). Attenuation values of pheochromocytomas are often similar to those of muscle tissue, and are significantly higher than those of adrenal adenomas. On CT and MRI, lesions with hemorrhage and necrosis can be heterogeneous (Figures 6,7). Pheochromocytomas are hyperintense on T2-weighted images (light bulb sign) (1,9), however, there is no such characteristic appearance in some pheochromocytomas (Figure 8).
Myelolipoma
Adrenal myelolipomas are rare benign tumors consisting of hematopoietic tissue and fat. These tumors are hormonally silent and clinically asymptomatic and are usually detected incidentally on CT (27). Patients may feel pain only with large myelolipomas (more than 10 cm) or those with intratumoral hemorrhage. Myelolipomas grow slowly, and there is usually no need for treatment. Surgical removal is necessary when the lesion is large and accompanied by clinical symptoms. It has been reported that congenital adrenal hyperplasia may be related to the occurrence of bilateral myelolipomas (1).
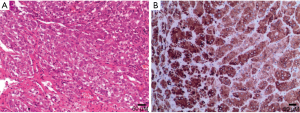
The composition of fat and bone marrow is visible under microscopy (Figure 9). Furthermore, there is internal hemorrhage in some lesions. It has been reported that other lesions can also contain fat, including adenomas, ACCs, pheochromocytomas, adrenal lipomas, or adrenal teratomas. In adrenal nodules containing >50% gross (mature) fat, the diagnosis of adrenal myelolipoma can be reasonably considered (19,28).
Owing to their lipid content, myelolipomas present with specific imaging characteristics with the attenuation less than 0 HU, sometimes less than −50 HU on pre-contrast CT images. Due to the appearance of hematopoietic tissue, the attenuation of myelolipomas is mildly higher than that of ambient fat space (16) (Figure 10). On contrast-enhanced CT images,the hematopoietic tissue shows contrasted enhancement. On MRI, it shows high signal on both T1- and T2-weighted images and there is a loss of signal on fat-saturated MRI (Figure 11).
Cysts
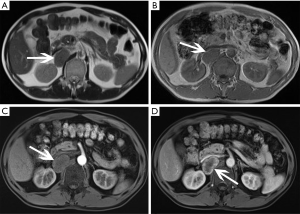
Adrenal cysts are relatively rare, and often occur unilaterally. This is an unusual disease that is usually caused by epithelial or endothelial proliferation, hemorrhage, or parasitic disease. Currently, there are four classifications for cysts: endothelial (more than 80%), epithelial, parasitic, and pseudocysts (24,26). The prevalence of adrenal cyst is 0.064–0.18% and shows a 3:1 female predilection. They are often detected incidentally because adrenal cysts are nonfunctional. For symptomatic patients, the main symptoms include an abdominal mass, hypertension, waist and abdominal pain. When lesions are large or functional, malignancy should be considered.
Adrenal cysts are reasonably simple to characterize radiologically. A cyst appears as a round mass with a clear border and a density close to water (Figure 12), and often have thin walls less than 3 mm as well as internal septa, and both the walls and septa may enhance or contain calcifications (16,29) (Figure 13). Adrenal cysts have features with high signal on T2-weighted images and low signal on T1-weighted images, without contrast enhancement (Figure 14).
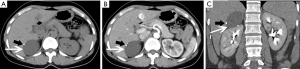
When nodular non-enhancement occurs, a cystic adrenal tumor which is prone to cystic changes, especially pheochromocytoma and cortical adenocarcinoma should be considered (17,20).
Cystic lymphangioma
Adrenal cystic lymphangioma is a type of adrenal endothelial cyst (30). It is a congenital developmental malformation formed by the benign proliferation of primitive lymphatic vessels. Briefly speaking, it is a lesion which composed of dilated lymphatic vessels. The incidence of adrenal cystic lymphangioma is reported to be approximately 0.06% (1), and occur at all ages, with the peak incidence between the third and sixth decades of life (31,32). Some researchers have found that these tumors occur distinctly in females. Like most cysts, adrenal cystic lymphangiomas are commonly asymptomatic and incidentally detected (33). However, if the lesion is large enough to compress surrounding tissues and organs, there are corresponding clinical symptoms, such as a palpable abdominal mass, gastrointestinal symptoms and abdominal pain.
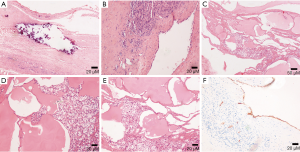
Histologically, almost all of these tumors have polycystic changes of different sizes. The endothelial cells in the inner area can be observed under microscopy (Figure 15). CD31, CD34 (endothelial specific markers) (34) and D2-40 (lymphatic markers) (32) can help diagnose this disease.
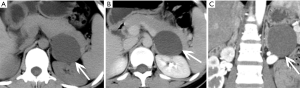
On CT, cystic Lymphangioma shows as sharply demarcated, uniform low-density lumps without enhancement (Figures 16-18). Lymphangiomas usually have smooth thin walls, and calcification may occur (Figures 19,20). On MRI, adrenal lymphangioma are typically T1 hypointense and T2 hyperintense. Therefore, imaging examinations can only prove that adrenal lymphangioma lesion originates from the adrenal gland, but it cannot distinguish cystic lymphangiomas from other cysts.
Teratoma
Teratomas are solid neoplasms originating from germ cells and have the potential to differentiate into somatic cells. These tumors often contain 2 or 3 germ layer tissues, and are most commonly seen in gonads and the sacrococcygeal, mediastinal, retroperitoneal and pineal regions. The biological characteristics of these tumors range from benign to borderline and malignant mature teratomas with malignant transformation, which most often manifest as the development of solid components superimposed on pre-existing cystic components (35). Mature teratomas are usually benign, but there is a possibility of malignancy, the chances of which are greater in adults than in children (36). Primary retroperitoneal teratomas of the adrenal gland are very uncommon (4% of all primary teratomas) (37). The typical gross appearance of teratomas includes hair, teeth, bone, calcification, soft tissue, and fat. Microscopically, muscle cells, glandular epithelium, squamous epithelium and osteocytes can be observed.
Unenhanced CT reveals a well-defined mixed density mass with low-density cystic and fatty areas and high density calcifications. Septations are visible in the mass. Egg-shell calcification is a characteristic manifestation of an adrenal teratoma. On MRI, both T1- and T2-weighted images show mixed signals, on which fat is hyperintense. On enhanced CT and MRI, slight enhancement of the substantial part and a hyperdense peripheral rim and internal septations with significant enhancement are visible (Figure 21).
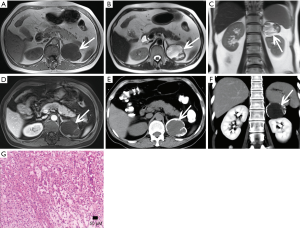
Hemorrhage
Adrenal hemorrhage most commonly appears during the neonatal period and is rarely seen in adults (16), and can be divided into traumatic causes and nontraumatic causes with a prevalence of 1.9–5.5‰. Blunt trauma is the most common causative factor for all adrenal hemorrhage. Traumatic adrenal hemorrhage is usually bilateral, but the right side is more commonly affected (16,24). Non traumatic bleeding is also common on both sides. Coagulation disorders, hemorrhagic diseases or stress can give rise to hemorrhage. Common stress events include surgery, sepsis, as well as severe burns (1). Adrenal hemorrhage can also be a complication of adrenal venous sampling (16,24). On histopathology, bleeding often affects the medulla, accompanied by different degrees of cortical involvement.
Symptoms depend on the degree of hemorrhage, and the patient may have symptoms ranging from mild back pain change to shock. Nevertheless, hemorrhage may appear in both benign and malignant lesions, such as adenomas, myelolipomas, pheochromocytomas, metastases and adrenal cortical carcinomas.
On CT and MRI, adrenal hemorrhage has a round or oval appearance, and the density and signal change according to the different stages of the disease. Acute hemorrhage presents with high density on non-contrast-enhanced CT, and the density decreases along with the size of the lesion (29,38). If the normal abdominal CT manifestations of the abdomen cannot exclude the possibility of hemorrhage, follow-up imaging is necessary.
Adrenal hemorrhage has a different image performance on T1- and T2-weighted imaging during different periods, based on the stage of bleeding and its components. Early hemorrhage is isointense on T1-weighted imaging and shows low signal on T2-weighted imaging. At medium term, lesions show high signal on both T1- and T2-weighted images. Late hemorrhage is hypointense on T1- and T2-weighted images.
Hemangioma
Adrenal hemangiomas are extraordinarily scarce, nonfunctional, benign tumors composed of angioblastic cells. Hemangiomas consist of four types: cavernous hemangioma, venous hemangioma, capillary type hemangioma and mixed hemangioma (33). Capillary and cavernous hemangiomas are the main types, with the latter type being more frequent. The prevalence of adrenal hemangiomas is 1 per 10,000 autopsies (1). Although they are generally asymptomatic and detected by chance, these neoplasms are mainly composed of blood vessels and are inclined to be incredibly vascularized (1), which makes these tumors associated with a high risk of hemorrhage. Therefore, it is important for further management to make the correct pretreatment diagnosis.
Pre-contrast CT scan shows these neoplasms to be well-circumscribed and homogenous with low density. Post-contrast CT scans show nodular enhancement during the arterial phase, and progressive enhancement in venous phase and delayed phases (Figure 22). Some these masses are low density and difficult to distinguish from cysts (Figure 23). Calcifications may also be present. Phleboliths are characteristic. On MRI, these tumors typically have low signal intensity on T1-weighted imaging and high signal intensity on T2-weighted imaging.
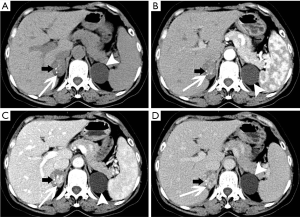
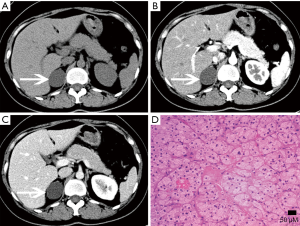
Ganglioneuroma
Ganglioneuromas are a rare benign neurogenic tumor originating from the adrenal medulla, and account for approximately 0.3–2% of all adrenal incidentalomas (39). Only rarely are they hormonally active. These tumors can occur at any age and are more common in young people and adults, and are more common in women than men. Ganglioneuromas are usually detected incidentally and even with large lesions, patients usually do not develop symptoses with clear boundaries and can be a shape of casting mold. Microscopically, they are composed of mature ganglion cells, Schwann cells, mucous matrix and nerve fibers (40).
CT can reveal a well-circumscribed, homogeneous solid tumor with clear boundaries (Figures 24,25), which may be companied by punctate or discrete calcifications (in 20% of ganglioneuromas). Enhancement varies and lesions often encircle blood vessels, rather than directly encroaching or occluding them. On MRI, these lesions show hypointense on T1-weighted images and have varied signal on T2-weighted images.
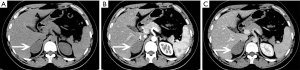
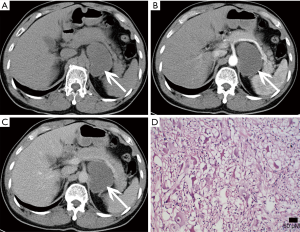
Malignant conditions
Neuroblastoma
Pheochromocytoma and other adrenal benign or malignant tumors can also occur in children, but neuroblastoma is the second most common abdominal mass in children besides Wilms' tumor. Neuroblastomas originate from the neural crest of the embryo with malignant nature, and usually occur in the adrenal medulla (40). Generally, neuroblastomas have no clinical symptoms unless they invade surrounding organs or metastasize. Sometimes, patients may have symptoms of flushing, tachycardia or high blood pressure due to excessive catecholamine production. The prognosis of neuroblastoma varies depending on whether the tumor has spread or metastasized (such as to liver or bone).
Under microscopy, tumor cells are blue stained small round cells, arrayed around the neuropil and have an appearance similar to that of a chrysanthemum.
Lesions always present with calcifications, necrotic, hemorrhagic and cystic changes, which lead to density/signal inhomogeneity (Figure 26). CT shows a large mass extending across the midline, engulfing abdominal vessels and dislocating surrounding structures (Figure 27). On MRI, these tumors are nonhomogeneous and hyperintense on T2-weighted images and hypointense on T1-weighted images (Figure 28). Heterogeneous enhancement on post-contrast imaging may occur. Hemorrhagic areas are hyperintense on T1-weighted images, and cystic and necrotic areas are hyperintense on T2-weighted images.
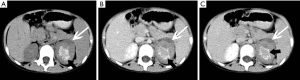
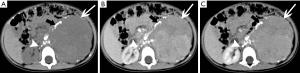
Metastases
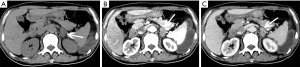
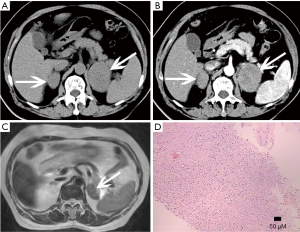
The adrenal blood supply is plentiful, and accordingly, it is a common organ for hematogenous metastasis of malignant tumors (41,42). While all malignant tumors have the possibility of metastasizing to the adrenal gland, the most common primary malignancies that affect the adrenal gland include lung cancer, breast cancer, gastric cancer, liver cancer and pancreatic cancer. The bilateral adrenal glands are usually involved, but unilateral involvement may occur. Metastases occur more commonly in the left gland than in the right gland. On autopsy, about 27% of malignant tumors cause adrenal metastasis.
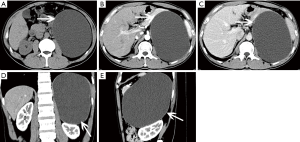
Adrenal metastasis has no specific imaging findings in routine CT or MRI. On pre-contrast CT scan, the attenuation of metastatic tumors is usually greater than 10 HU. There may be calcification and areas of hemorrhage. There is irregular peripheral enhancement following contrast administration (Figure 29), but most lesions usually display obviously enhanced on enhanced CT scan (Figures 30,31). On MRI, adrenal metastases show low signal on T1-weighed imaging and high signal on T2-weighed imaging; sometimes, it may be isointense on T1- and T2-weighed imaging with ring or uneven enhancement after contrast administration (Figure 32).
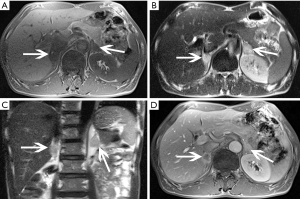
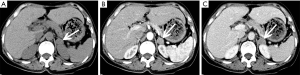
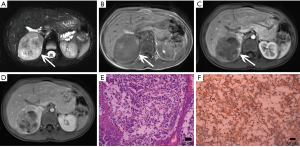
ACC
ACC is a malignant tumor occurring in the adrenal cortex. The incidence of the disease is low, approximately 1–2 out of every one million people (43) and occurs slightly more in females than males and can occur at any age but has distinct bimodal characteristics. The majority of ACCs have endocrine function, so it is typically detected early. The mass may lead to CS or primary aldosteronism or abnormal sexuality. Other symptoms include weight loss, upper abdominal pain, or gastrointestinal complaints. ACCs are typically large masses, usually >6 cm and people often present with palpable mass.
Macroscopically, necrosis and cystic degeneration often occur in ACC. Under microscopy, the cells are heteromorphic, and there are a large number of multinucleated giant cells and nuclear division.
On pre-contrast-enhanced CT, ACC is usually characterized by a well-demarcated heterogeneous mass with the attenuation greater than 10 HU (1,18). On post-contrast-enhanced CT, ACCs show heterogeneous enhancement. Necrosis and cyst formation are common, particularly in the central part of the lesion (Figure 33).
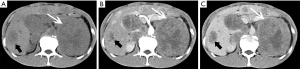
The MRI manifestations of ACC are characterized heterogenous hyperintensity on T2-weighted images and hypointensity on T1-weighted images. As a result, necrosis and hemorrhage, both T1- and T2-weighted images may appear heterogeneous. Areas of hemorrhage may appear hyperintense on T1-weighted images and necrosis may demonstrate high signal intensity on T2-weighted images. One study suggested that invasion of the inferior vena cava is a common complication of ACC.
Lymphoma
Lymphoma is a malignant tumor originating from lymphohematopoietic system that infrequently involves of the adrenal gland. The incidence of non-Hodgkin’s lymphoma (NHL) is higher than that of Hodgkin's lymphoma (HL) (18). There are two types of this condition: primary lymphoma and secondary lymphoma. Primary adrenal lymphoma is extremely rare, and is defined as malignant neoplastic proliferation of the lymphoid cells exclusively in the adrenal glands. Furthermore, primary lymphoma is confined to a single organ but may involve the neighboring lymph nodes. If not, it is thought to be secondary. If invasion by lymphoma is suspected, other nodal stations should be scanned and commonly affected organs (spleen, liver) should be closely scrutinized (25). Patients may present with fever, night sweats, emaciation, itching, adrenal insufficiency and other systemic symptoms.
The pathological characteristics of NHL and HL are different. NHL shows mainly lymphocytes, tissue cells or reticular cells with different degrees of differentiation. However, the tumor tissues of HL contain lymphocytes, eosinophils, plasma cells and specific Reed-Steinberg cells.
Lymphoma lesions are typically large and homogeneous. Like neuroblastomas and cortical cancer, the density of adrenal lymphoma is heterogeneous because of hemorrhagic, necrotic and cystic changes. The enlargement of the retroperitoneal lymph nodes is also common. On MRI, the typical manifestation of adrenal lymphoma is low intensity on T1-weighted images and high-intensity on T2. A mild to moderate enhancement can be seen on enhanced scan (Figure 34).
Conclusions
Lesions in the adrenal glands can secrete hormones and cause corresponding endocrine syndrome. CS and Conn syndrome are two of them. We summarize the adrenal lesions associated with Cushing’s syndrome and conn syndrome in this article. In symptom patients, imaging examination is performed to search for lesions. However, most adrenal lesions are hormonally silent and clinically asymptomatic and are usually detected incidentally in routine health check care or tests for other purposes. Radiologists should be familiar with imaging characteristics of adrenal incidentaloma.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China (grant No. 81760309), Science and Technology Foundation of Health and Family Planning Commission of Guizhou Province (grant No. gzwjkj2017-1-013), and innovation team of medical imaging center of education department of Guizhou province [Talent team of Guizhou Education (2014) 37].
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lattin GE Jr, Sturgill ED, Tujo CA, Marko J, Sanchez-Maldonado KW, Craig WD, Lack EE. From the radiologic pathology archives: Adrenal tumors and tumor-like conditions in the adult: radiologic-pathologic correlation. Radiographics 2014;34:805-29. [Crossref] [PubMed]
- Lau D, Rutledge C, Aghi MK. Cushing's disease: current medical therapies and molecular insights guiding future therapies. Neurosurg Focus 2015;38. [Crossref] [PubMed]
- Wagner-Bartak NA, Baiomy A, Habra MA, Mukhi SV, Morani AC, Korivi BR, Waguespack SG, Elsayes KM. Cushing Syndrome: Diagnostic Workup and Imaging Features, With Clinical and Pathologic Correlation. AJR Am J Roentgenol 2017;209:19-32. [Crossref] [PubMed]
- Bansal V, El Asmar N, Selman WR, Arafah BM. Pitfalls in the diagnosis and management of Cushing's syndrome. Neurosurg Focus 2015;38. [Crossref] [PubMed]
- Eckstein N, Haas B, Hass MD, Pfeifer V. Systemic therapy of Cushing's syndrome. Orphanet J Rare Dis 2014;9:122. [Crossref] [PubMed]
- Monticone S, Buffolo F, Tetti M, Veglio F, Pasini B, Mulatero P. Genetics in endocrinology: The expanding genetic horizon of primary aldosteronism. Eur J Endocrinol 2018;178:R101-11. [Crossref] [PubMed]
- Aristizabal Prada ET, Castellano I, Susnik E, Yang Y, Meyer LS, Tetti M, Beuschlein F, Reincke M, Williams TA. Comparative Genomics and Transcriptome Profiling in Primary Aldosteronism. Int J Mol Sci 2018;19. [Crossref] [PubMed]
- Vilela LAP, Almeida MQ. Diagnosis and management of primary aldosteronism. Arch Endocrinol Metab 2017;61:305-12. [Crossref] [PubMed]
- Munir S, Waseem M. Addison Disease. StatPearls. Treasure Island (FL): StatPearls Publishing StatPearls Publishing LLC., 2018.
- Michels A, Michels N. Addison disease: early detection and treatment principles. Am Fam Physician 2014;89:563-8. [PubMed]
- Wang YX, Chen CR, He GX, Tang AR. CT findings of adrenal glands in patients with tuberculous Addison's disease. J Belge Radiol 1998;81:226-8. [PubMed]
- Brandão Neto RA, de Carvalho JF. Diagnosis and classification of Addison's disease (autoimmune adrenalitis). Autoimmun Rev 2014;13:408-11. [Crossref] [PubMed]
- Elsayes KM, Mukundan G, Narra VR, Lewis JS Jr, Shirkhoda A, Farooki A, Brown JJ. Adrenal masses: mr imaging features with pathologic correlation. Radiographics 2004;24 Suppl 1:S73-86. [Crossref] [PubMed]
- Korivi BR, Elsayes KM, de Castro SF, Garg N, Qayyum A. An Update of Practical CT Adrenal Imaging: What Physicians Need to Know. Current Radiology Reports 2015;3:12. [Crossref]
- Michalopoulos N, Pazaitou-Panayiotou K, Boudina M, Papavramidis T, Karayannopoulou G, Papavramidis S. Mixed corticomedullary adrenal carcinoma. Surg Today 2013;43:1232-9. [Crossref] [PubMed]
- Song JH, Mayo-Smith WW. Current status of imaging for adrenal gland tumors. Surg Oncol Clin N Am 2014;23:847-61. [Crossref] [PubMed]
- Ctvrtlik F, Koranda P, Tichy T. Adrenal disease: a clinical update and overview of imaging. A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2014;158:23-34. [Crossref] [PubMed]
- Song JH, Chaudhry FS, Mayo-Smith WW. The incidental adrenal mass on CT: prevalence of adrenal disease in 1,049 consecutive adrenal masses in patients with no known malignancy. AJR Am J Roentgenol 2008;190:1163-8. [Crossref] [PubMed]
- Schieda N, Siegelman ES. Update on CT and MRI of Adrenal Nodules. AJR Am J Roentgenol 2017;208:1206-17. [Crossref] [PubMed]
- Johnson PT, Horton KM, Fishman EK. Adrenal mass imaging with multidetector CT: pathologic conditions, pearls, and pitfalls. Radiographics 2009;29:1333-51. [Crossref] [PubMed]
- Park JJ, Park BK, Kim CK. Adrenal imaging for adenoma characterization: imaging features, diagnostic accuracies and differential diagnoses. Br J Radiol 2016;89. [Crossref] [PubMed]
- Caoili EM, Korobkin M, Francis IR, Cohan RH, Dunnick NR. Delayed enhanced CT of lipid-poor adrenal adenomas. AJR Am J Roentgenol 2000;175:1411-5. [Crossref] [PubMed]
- Wang YX, He GX, Du LJ, Jiang H, Tang AR. CT findings in congenital adrenal hyperplasia due to 11 beta hydroxylase deficiency at puberty age. JBR-BTR 1999;82:11-2. [PubMed]
- Liu H, Wang AP, Bo YH, Li YR, Tang ZM, Zhang TJ. CT. J Clin Radiol 2015;34:591-5.
- Goffredo P, Sosa JA, Roman SA. Malignant pheochromocytoma and paraganglioma: a population level analysis of long-term survival over two decades. J Surg Oncol 2013;107:659-64. [Crossref] [PubMed]
- Farrugia FA, Martikos G, Surgeon C, Tzanetis P, Misiakos E, Zavras N, Charalampopoulos A. Radiology of the adrenal incidentalomas. Review of the literature. Endocr Regul 2017;51:35-51. [Crossref] [PubMed]
- Wang YX, Wu JT, He GX, Pan ZL. CT of adrenal myelolipoma: report of 7 cases. JBR-BTR 1999;82:231-3. [PubMed]
- Schieda N, Al Dandan O, Kielar AZ, Flood TA, McInnes MD, Siegelman ES. Pitfalls of adrenal imaging with chemical shift MRI. Clin Radiol 2014;69:1186-97. [Crossref] [PubMed]
- Boland GW, Blake MA, Hahn PF, Mayo-Smith WW. Incidental adrenal lesions: principles, techniques, and algorithms for imaging characterization. Radiology 2008;249:756-75. [Crossref] [PubMed]
- Rowe SP, Bishop JA, Prescott JD, Salvatori R, Fishman EK. CT Appearance of Adrenal Cystic Lymphangioma: Radiologic-Pathologic Correlation. AJR Am J Roentgenol 2016;206:81-5. [Crossref] [PubMed]
- Kim KH, Lee JI, Bae JM. Significant growth of adrenal lymphangioma: A case report and review of the literature. Int J Surg Case Rep 2015;17:48-50. [Crossref] [PubMed]
- Ellis CL, Banerjee P, Carney E, Sharma R, Netto GJ. Adrenal lymphangioma: clinicopathologic and immunohistochemical characteristics of a rare lesion. Hum Pathol 2011;42:1013-8. [Crossref] [PubMed]
- Secil M, Demir O, Yorukoglu K. MRI of adrenal lymphangioma: a case report. Quant Imaging Med Surg 2013;3:347-8. [PubMed]
- Liu B, Li Y, Wang S. Adrenal lymphangioma removed by a retroperitoneoscopic procedure. Oncol Lett 2013;5:539-40. [Crossref] [PubMed]
- Niu M, Liu A, Zhao Y, Feng L. Malignant transformation of a mature teratoma of the adrenal gland: A rare case report and literature review. Medicine 2017;96. [Crossref] [PubMed]
- Ramakant P, Rana C, Singh KR, Mishra A. Primary adrenal teratoma: An unusual tumor - Challenges in diagnosis and surgical management. J Postgrad Med 2018;64:112-4. [Crossref] [PubMed]
- Narla SL, Jacob S, Kurian A, Parameswaran A. Primary mature cystic teratoma with carcinoid mimicking an adrenal tumor: Report of a rare association and review of literature. Indian J Pathol Microbiol 2016;59:200-2. [Crossref] [PubMed]
- Mazzaglia PJ. Radiographic evaluation of nonfunctioning adrenal neoplasms. Surg Clin North Am 2014;94:625-42. [Crossref] [PubMed]
- Mylonas KS, Schizas D, Economopoulos KP. Adrenal ganglioneuroma: What you need to know. World J Clin Cases 2017;5:373-7. [Crossref] [PubMed]
- Herr K, Muglia VF, Koff WJ, Westphalen AC. Imaging of the adrenal gland lesions. Radiol Bras 2014;47:228-39. [Crossref] [PubMed]
- Lim W, Ridge CA, Nicholson AG, Mirsadraee S. The 8th lung cancer TNM classification and clinical staging system: review of the changes and clinical implications. Quant Imaging Med Surg 2018;8:709-18. [Crossref] [PubMed]
- Wang YX, Gong JS, Suzuki K, Morcos SK. Evidence based imaging strategies for solitary pulmonary nodule. J Thorac Dis 2014;6:872-87. [PubMed]
- Kerkhofs TM, Verhoeven RH, Van der Zwan JM, Dieleman J, Kerstens MN, Links TP, Van de Poll-Franse LV, Haak HR. Adrenocortical carcinoma: a population-based study on incidence and survival in the Netherlands since 1993. Eur J Cancer 2013;49:2579-86. [Crossref] [PubMed]


