Sonographic appearance of fluid in peripheral joints and bursae of healthy asymptomatic Chinese population
Introduction
High frequency ultrasound has been increasingly used in musculoskeletal diseases, such as rheumatoid arthritis, for diagnostic purposes and to evaluate disease activity or drug response (1-4). Although MRI can detect lesions of minor joint structures, such as triangular fibrocartilage complex (TFCC) tears (5), high resolution ultrasound has become an important complement to musculoskeletal imaging beyond MRI. Ultrasound manifestation of inflammatory joints may include bone erosion, synovial hyperplasia, synovial vascularization and synovial fluid (2,3,6). Synovial fluid can also be found in certain joints under normal conditions, and can be visualized on sonography (7-9).
Assessing the ultrasound appearance of peripheral joints, bursae and tendon sheaths in a healthy asymptomatic population may provide insights into interpreting ultrasound exams in pathologic cases. It is important for clinicians to differentiate between effusion—the excess fluid in joints—and physiologic fluid accumulation, as the latter does not need clinical intervention. Therefore, in this study, we aimed to (I) use ultrasound to measure the thickness of fluid in peripheral joints, bursae and tendon sheaths in regards to situation, gender, age and BMI (US); (II) calculate the upper limit of the 95% reference range, in order to better understand both normal and pathologic conditions of joints and bursae fluids.
Methods
The study was approved by the local Ethics Committee. Informed consent was obtained from the volunteers for the acquisition, analysis and reporting of imaging data at the time of their examinations.
This study was conducted with healthy adult Chinese volunteers between January 2017 and June 2017, who were consecutively included from (I) healthcare medical, paramedical and administrative staff of the local hospital, (II) medical students at the same hospital, (III) healthy relatives visiting or accompanying patients and (IV) volunteers enrolled through online announcement of the study.
Inclusion criteria were age from 18 to 90 years, and free consensus to participate in the study. Exclusion criteria were the following: (I) history of rheumatoid arthritis, diabetes mellitus, hypothyroidism, hemophilia, trauma, surgery, symptomatic osteoarthritis, or septic arthritis in any studied joint; (II) any kind of joint pain experienced during any time in the previous month; (III) pregnant or breast-feeding women. Patient’s age, sex, height and weight data were collected as demographic characteristics and body mass index (BMI) was subsequently calculated later.
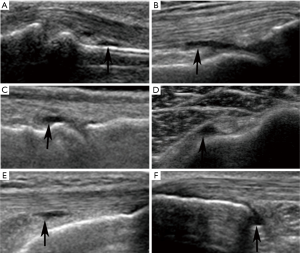
US examinations were performed using Philips IU22 with a 9–12 MHz linear array transducer. Musculoskeletal (MSK) presetting was selected with the standard default mode. The transducer, coupled with several millimeters of ultrasound gel, was smoothly placed perpendicular to the skin to avoid anisotropic artifacts. All examinations were performed by 2 experienced radiologists (QL and TYJ) with more than 8 years of experience in musculoskeletal ultrasonography, and US examination was done by QL or TYJ at random in our study. Schmidt et al. have shown excellent inter- and intra-observer agreement (both lager than 0.8) when using similar ultrasound measurement methods (10). According to the OMERACT definition (11), the presence of fluid was defined as an anechoic displaceable and compressible intracapsular area in B mode, and which does not exhibit Doppler signal (Figure 1). US examination was performed in peripheral joints, bursae and tendon sheaths based on standard scans (12), and displayed in Table 1. Scanning planes showed the maximum amount of joint fluid selected and the three consecutive US measurements were performed for every location to obtain the average value.

Full table
SPSS software was used for statistical analysis (SPSS, version 19.0, Chicago III). Descriptive statistics were expressed as means ± standard deviations. Categorical data were described using counts, percentages and 95% confidence intervals. Chi-squared test was used for comparison in detection rate. Independent-sample’s t test was used for comparison in fluid thickness. We correlated detection rate and fluid thickness with demographic characters using Spearman correlation analysis. Correlation index (r) was interpreted as 0.8–1.0 strong correlation, 0.5–0.8 moderate correlation, 0.3–0.5 low correlation, and <0.3 weak correlation. Two-sided statistical significance was defined as P<0.05. Increase of fluid is considered to be pathological, so we only calculated the upper limit of 95% reference range using the equation: the upper limit = mean + 1.64 SD.
Results
A total of 152 healthy asymptomatic Chinese volunteers were included. The mean age was 48.0±14.1 years with a range of 20–75 years. The mean BMI was 22.58±3.15 kg/m2 with a range of 15.82-31.51 kg/m2. Seventy-one participants were male and 81 were female. None of the recruited volunteers were excluded for further analysis. There was detectable ultrasonic fluid in all the 24 kinds of joints, 3 bursae and 1 tendon sheath studied.
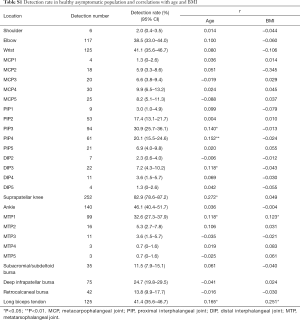
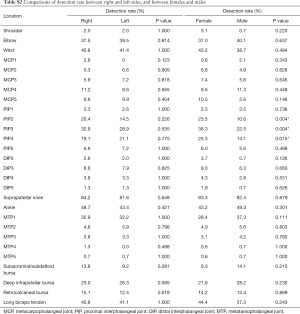
Fluid was found in suprapatellar knees in 252/304 (82.9%), which made it the highest one among all the studied structures. Weak positive correlations (r<0.3) between detection rate and both age and BMI were found in 21.4% and 7.1% of all the 28 structures studied respectively (Table S1). There was no situational (right or left) difference found in detection rate. Gender difference was found in the 2nd, 3rd and 4th PIPs, with the detection rates in these PIPs being higher in female subjects (Table S2).
Full table
Full table
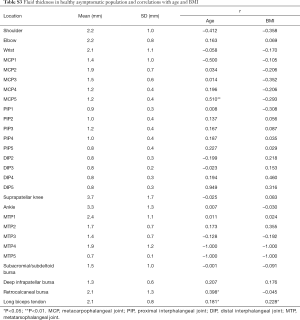
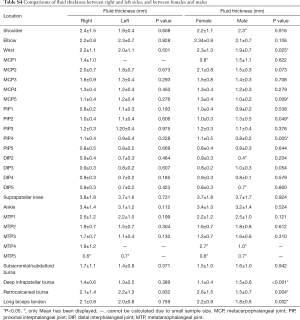
The thickest fluid was also found in the suprapatellar knee, and its average fluid thickness was 3.7±1.7 mm. Moderate positive correlation was found between fluid thickness and age in MCP 5 (r=0.510, P<0.05). Low positive correlations were found between fluid thickness and age in the retrocalcaneal bursae, and between fluid thickness and BMI in the long biceps tendon (r=0.398 and 0.228, respectively, both P<0.05). The positive correlation between fluid thickness and age was weak in long the biceps tendon (r=0.181, P<0.05) (Table S3). There was no significant difference found between the right and left side in fluid thickness (P>0.05) (Table S4). As for gender, fluid thickness was higher in women in wrists, MCP5, PIP 4, retrocalcaneal bursae and long biceps tendon (P<0.05). Conversely, the fluid thickness of PIP 2 and deep infrapatellar bursae was higher in male subjects (P<0.05).
Full table
Full table
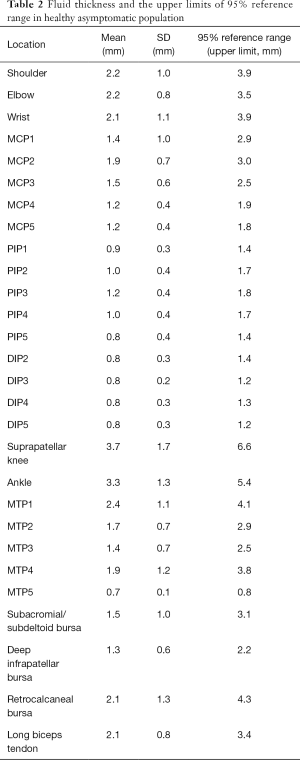
Reference values differed among different joints (Table 2). Overall, the upper limits of the 95% reference range for lower limb joints were larger than upper limb joints. For example, the upper limits in the shoulder (gleno-humeral joint), elbow and wrist are ≤3.9, ≤3.5 and ≤3.9 mm, respectively; whereas the suprapatellar knee and ankle are ≤6.6 and ≤5.4 mm, respectively. As for the hands, the upper limits were over 1mm in PIPs and DIPs with larger fluid thickness found in MCPs (approximately 2 mm). Changeable upper limits were found in the feet, and were ≤0.8 mm for MCP5 to ≤4.1 mm for MTP1.
Full table
Discussion
High-frequency ultrasound has proven its diagnostic power in musculoskeletal diseases; however, identifying differences between normal and pathologic conditions is still difficult. A previous study (7) which included 46 healthy subjects indicated that fluid was present in 20.9% of PIPs, but less frequent in DIPs (3% DIPs). Schmidt et al. reported that fluid in bursae and joints were common findings in healthy people (10). Our results also suggest that all asymptomatic joints, bursae and tendon sheaths examined in our study have ultrasound-detectable accumulation of synovial fluid, of which the detection rate and fluid thickness vary in different structures. The mean thickness of fluid found in asymptomatic joints is listed in the following descending order: suprapatellar knee (3.7 mm), ankle (3.3 mm), shoulder (2.2 mm), elbow (2.2 mm), wrist (2.1 mm), MTPs (0.7–2.4 mm), MCPs (1.2–1.9 mm), PIPs (0.8–1.2 mm), DIPs (0.8 mm).
Similar to previous studies (7,10,13) which reported no significant difference between the dominant and non-dominant side, we similarly found no difference between the left and right side in both detection rate and fluid rate. These results may indicate that symmetrical parts of healthy subjects have the same anatomical structure, and that there would not be significant difference between the left and right side of a structure under normal conditions. Therefore, through examining and comparing joints on both sides, we can recognize unilateral lesion and assess its severity.
We observed that females had a higher detection rate and fluid thickness than males in most examined structures, especially in upper-limb joints, although many of them did not show statistical difference. According to studies about gender impact on ultrasound measurements, Ellegaard et al. suggested that women obtained higher pathological scores than men in healthy small hand joints (14). Poncelet et al. determined different deep joint space distance of the acromioclavicular joint between men and women (13). These studies indicate physiological and anatomical differences between men and women, which might be caused by different kinds of labor or exercise. When considering gender influence, it is better to choose the same sex as the normal reference in US examinations of certain structures, especially in the retrocalcaneal bursa, which had a difference between means larger than 1 mm.
Among the studied demographic parameters, the greatest number of examined structures was found to be affected by age, and all of the correlations were positive. We attribute this to the lasting and irreversible degeneration of joints caused by aging which can induce bone changes including spurs and irregularities in the elderly (15). Higher quantitative joint recess measured sonographically has also been reported in older groups (9). Additionally, we found positive correlations with age, especially in small joints like PIP, DIP and MTP, which are similar to results reported by Machado et al. whose study detailed a higher percentage of synovial effusion in the hand and foot joints of healthy individuals (9). In our study, we did not exclude the older people with asymptomatic osteoarthritis, because asymptomatic osteoarthritis is of less clinical importance and there is usually no need for intervention. Therefore, when attempting to detect joint fluid in aged but asymptomatic people, we should consider age effects especially in the hand and foot joints.
Positive correlations with BMI were found in the long biceps tendon and MTP1. The long biceps tendon is closely related to motion, and MTP1 is an important weight-bearing joint. Therefore, height and weight might have a greater impact on these two structures. Excessive increases in weight-bearing forces caused by obesity may be detrimental to the lower limbs and feet (16), lead to musculoskeletal pain in the legs, and contribute to overall difficulty of daily movements (17). Obesity can also lead to musculoskeletal disorders in children by promoting biomechanical changes in the lumbar spine and lower extremities (18). Conversely, in patients with rheumatoid arthritis, a higher BMI is associated with a less severe disease outcome (19), and disease activity might also be overestimated in obese patients (20). The above results indicate a close relationship between BMI and joint disease. In our study, the mean BMI was 22.58±3.15 kg/m2 which was almost within the normal range. Thus, whether an overweight condition affects joint fluid still remains unclear and open for further investigation.
We have found some difference in reference values compared with Schmidt’s study (10). The most important influencing factor is that we use mean + 1.64 SD to define the upper limit of the 95% reference range, while they use mean ± 2 SD to calculate the standard reference values. As we supposed that only excessive accumulation of fluid in joints is pathological, it is suitable for us to calculate the upper limit. Another reason for this phenomenon might be different the study populations included. As we have calculated the reference values for healthy asymptomatic people, we might be able to help radiologists and clinicians to better distinguish between normal and abnormal conditions, which is of great importance in clinical practice because excessive fluid accumulation may lead to further examinations or treatments. Our study may also provide normal controls for future comparative studied in patients with rheumatoid arthritis or other diseases. There are also some limitations in our study. First, we did not study all the ultrasound-detectable peripheral joints and their sonographic findings, such as bone erosion, bursa effusion or tendinopathy. Second, by design, this study lacked Doppler analysis. The strengths of our study include a relatively large number of patients.
Conclusions
Fluid in the peripheral joints, bursae and tendon sheaths of healthy asymptomatic populations can be frequently found by US. The detection rate and fluid thickness vary in different structures. Some of the fluid thickness and detection rates are associated with gender, age or BMI, but no difference has been found between the left and right side joints. While making diagnoses of joint diseases, it is important to choose suitable control groups regarding the relevant factors. Additionally, reference values provided in this study might be helpful to recognize effusion in the healthy asymptomatic Chinese population. Further multi-center studies are still necessary to determine more generally applicable standard reference values.
Acknowledgements
Funding: This study was supported by the National Natural Science Foundation of China (81671696).
Footnote
Conflicts of Interest: The authors have no conflicts of interests to declare.
Ethical Statement: The study was approved by the West China Hospital of Sichuan University Ethics Committee. Informed consent was obtained from all volunteers.
References
- Dougados M, Jousse-Joulin S, Mistretta F, d'Agostino MA, Backhaus M, Bentin J, Chalès G, Chary-Valckenaere I, Conaghan P, Etchepare F, Gaudin P, Grassi W, van der Heijde D, Sellam J, Naredo E, Szkudlarek M, Wakefield R, Saraux A. Evaluation of several ultrasonography scoring systems for synovitis and comparison to clinical examination: results from a prospective multicentre study of rheumatoid arthritis. Ann Rheum Dis 2010;69:828-33. [Crossref] [PubMed]
- Ziswiler HR, Aeberli D, Villiger PM, Möller B. High-resolution ultrasound confirms reduced synovial hyperplasia following rituximab treatment in rheumatoid arthritis. Rheumatology (Oxford) 2009;48:939-43. [Crossref] [PubMed]
- Weidekamm C, Köller M, Weber M, Kainberger F. Diagnostic value of high-resolution B-mode and doppler sonography for imaging of hand and finger joints in rheumatoid arthritis. Arthritis Rheum 2003;48:325-33. [Crossref] [PubMed]
- Iagnocco A, Perella C, Naredo E, Meenagh G, Ceccarelli F, Tripodo E, Basili S, Valesini G. Etanercept in the treatment of rheumatoid arthritis: clinical follow-up over one year by ultrasonography. Clin Rheumatol 2008;27:491-6. [Crossref] [PubMed]
- Ng AW, Griffith JF, Fung CS, Lee RK, Tong CS, Wong CW, Tse WL, Ho PC. MR imaging of the traumatic triangular fibrocartilaginous complex tear. Quant Imaging Med Surg 2017;7:443-60. [Crossref] [PubMed]
- Machado FS, Furtado RN, Takahashi RD, de Buosi AL, Natour J. Sonographic cutoff values for detection of abnormalities in small, medium and large joints: a comparative study between patients with rheumatoid arthritis and healthy volunteers. Ultrasound Med Biol 2015;41:989-98. [Crossref] [PubMed]
- Rosenberg C, Arrestier S, Etchepare F, Fautrel B, Rozenberg S, Bourgeois P. High frequency of ultrasonographic effusion in interphalangeal joints of healthy subjects: a descriptive study. Joint Bone Spine 2009;76:265-7. [Crossref] [PubMed]
- Luukkainen R, Ekman P, Luukkainen P, Koski JM. Ultrasonographic findings in metatarsophalangeal and talocrural joints in healthy persons. Clin Rheumatol 2009;28:311-3. [Crossref] [PubMed]
- Machado FS, Natour J, Takahashi RD, de Buosi AL, Furtado RN. Sonographic assessment of healthy peripheral joints: evaluation according to demographic parameters. J Ultrasound Med 2014;33:2087-98. [Crossref] [PubMed]
- Schmidt WA, Schmidt H, Schicke B, Gromnica-Ihle E. Standard reference values for musculoskeletal ultrasonography. Ann Rheum Dis 2004;63:988-94. [Crossref] [PubMed]
- Wakefield RJ, Balint PV, Szkudlarek M, Filippucci E, Backhaus M, D'Agostino MA, Sanchez EN, Iagnocco A, Schmidt WA, Bruyn GA, Bruyn G, Kane D, O'Connor PJ, Manger B, Joshua F, Koski J, Grassi W, Lassere MN, Swen N, Kainberger F, Klauser A, Ostergaard M, Brown AK, Machold KP, Conaghan PG. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol 2005;32:2485-7. [PubMed]
- Backhaus M, Burmester GR, Gerber T, Grassi W, Machold KP, Swen WA, Wakefield RJ, Manger B. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis 2001;60:641-9. [Crossref] [PubMed]
- Poncelet E, Demondion X, Lapègue F, Drizenko A, Cotten A, Francke JP. Anatomic and biometric study of the acromioclavicular joint by ultrasound. Surg Radiol Anat 2003;25:439-45. [Crossref] [PubMed]
- Ellegaard K, Torp-Pedersen S, Holm CC, Danneskiold-Samsøe B, Bliddal H. Ultrasound in finger joints: findings in normal subjects and pitfalls in the diagnosis of synovial disease. Ultraschall Med 2007;28:401-8. [Crossref] [PubMed]
- Ustuner E, Toprak U, Baskan B, Oztuna D. Sonographic examination of the common extensor tendon of the forearm at three different locations in the normal asymptomatic population. Surg Radiol Anat 2013;35:547-52. [Crossref] [PubMed]
- Hills AP, Hennig EM, McDonald M, Bar-Or O. Plantar pressure differences between obese and non-obese adults: a biomechanical analysis. Int J Obes Relat Metab Disord 2001;25:1674-9. [Crossref] [PubMed]
- Tsuritani I, Honda R, Noborisaka Y, Ishida M, Ishizaki M, Yamada Y. Impact of obesity on musculoskeletal pain and difficulty of daily movements in Japanese middle-aged women. Maturitas 2002;42:23-30. [Crossref] [PubMed]
- de Sá Pinto AL, de Barros Holanda PM, Radu AS, Villares SM, Lima FR. Musculoskeletal findings in obese children. J Paediatr Child Health 2006;42:341-4. [Crossref] [PubMed]
- Caplan L, Davis LA, Bright CM, Kerr GS, Lazaro DM, Khan NA, Richards JS, Johnson DS, Cannon GW, Reimold AM, Mikuls TR. Body mass index and the rheumatoid arthritis swollen joint count: an observational study. Arthritis Care Res (Hoboken) 2013;65:101-6. [Crossref] [PubMed]
- Bauer EM, Ben-Artzi A, Duffy EL, Elashoff DA, Vangala SS, Fitzgerald J, Ranganath VK. Joint-specific assessment of swelling and power Doppler in obese rheumatoid arthritis patients. BMC Musculoskelet Disord 2017;18:99. [Crossref] [PubMed]


