
Dynamic contrast-enhanced ultrasound findings of focal nodular hyperplasia-like nodules in cirrhosis: a description of two cases and literature analysis
Introduction
Focal nodular hyperplasia (FNH) is the second most common benign hepatic tumor, consisting of hyperplastic hepatocytes with a central fibrous scar, and it typically occurs in non-cirrhotic livers. FNH arising from a cirrhotic liver has rarely been reported. Sugihara et al. previously reported a case of hyperplastic nodules resembling FNH that developed in a cirrhotic liver and subsequently proposed the term focal nodular hyperplasia-like nodules (FNH-LNs) (1). FNH-LNs are histologically similar to classic FNHs but occur in chronic underlying liver diseases. FNH-LN pathogenesis may be associated with liver vascular disorders such as liver cirrhosis.
It is thought that liver vascular disorders often lead to overall perfusion disturbances in the liver, increasing hepatic arterial blood perfusion and promoting FNH-LN formation. The incidence of FNH-LNs in cirrhotic livers ranges from 3.4% to 15% (2). FNH-LN imaging characteristics are generally similar to those of typical FNH; however, FNH-LNs may mimic hepatocellular carcinoma (HCC) by displaying hyperenhancement during the arterial phase or washout during the portal venous or delayed phase on contrast-enhanced computed tomography (CECT) or magnetic resonance imaging (MRI) (3,4).
Dynamic contrast-enhanced ultrasound (DCE-US) is a real-time imaging method that uses pure blood pool agents to dynamically characterize the microperfusion features of hepatic lesions and differentiate between benign and malignant liver tumors by displaying nodule enhancement patterns objectively. Nevertheless, the use of DCE-US for detecting FNH-LNs in cirrhotic livers has been rarely reported in the radiological literature. Here, we present 2 rare cases of pathologically confirmed FNH-LNs in cirrhotic livers that were misdiagnosed as HCC based on the radiographic evaluation.
Case presentation
Case 1
A 27-year-old female presented to our hospital with symptoms of upper gastrointestinal bleeding and a history of intermittent hematemesis. The presence of palmar erythema and ascites on physical examination revealed chronic liver disease, leading to the diagnosis of decompensated liver cirrhosis. Laboratory tests revealed elevated liver enzymes, as well as an increased activated partial thromboplastin time (APTT) level of 41.6 s (reference range: 21–37 s) and prothrombin time (PT) level of 16.9 s (reference range: 9.4–12.5 s), indicating impaired blood coagulation. In addition, the patient tested positive for hepatitis B surface antigen (HBsAg), and tumor markers such as alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), and carbohydrate antigen 19-9 (CA19-9) were all within normal limits. Upper gastrointestinal endoscopy revealed severe bleeding and gastroesophageal varices.
Abdominal ultrasound imaging revealed a small liver with an ill-defined border, heterogeneous and coarse parenchyma, and a hypoechoic nodule approximately 2.1 cm in size with an unclear boundary in segment II (Figure 1A). To further evaluate the nodule, contrast-enhanced ultrasound (CEUS) was performed in accordance with the guidelines of the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) (5). A 1.2 mL bolus injection of SonoVue- contrast agent (Bracco, Milan, Italy) was administered via the left cubital vein, followed by a 5 mL saline flush. For 3 minutes, video clips in digital imaging and communications in medicine (DICOM) format were continuously saved. Time-intensity curves (TICs) were automatically analyzed using VueBox quantification software (Bracco Suisse Software Applications, Geneva, Switzerland). CEUS images showed that the nodule was hyperenhanced in the arterial phase with persistent enhancement in the portal venous and delayed phases (Figure 1B-1D). According to the perfusion parameters, the local mean transit time (mTT) of the nodule was significantly prolonged at 458.80 seconds, and wash-in rate (WiR) was higher at 70.8 a.u. (Figure 1E). Subsequently, CECT revealed hyperenhancement in the arterial phase with isoenhancement in the portal venous and delayed phases (Figure 1F-1H).
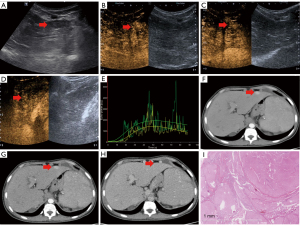
Based on the imaging findings and the patient’s history of decompensated liver cirrhosis, the nodule was initially suspected to be HCC. The patient underwent surgical resection of the hepatic tumor. However, a gross investigation revealed a sharp lesion margin and soft tissues. Histological examination revealed that the nodule consisted of hepatocytes separated by fibrous septa containing abnormally thick-walled arteries (Figure 1I). Ultimately, the nodule was identified as an FNH-LN. The patient underwent radiological follow-up for 4 years, and there were no indications of malignancy.
Case 2
A 45-year-old male was admitted to our hospital with upper gastrointestinal bleeding. The patient had a history of hepatitis B virus-related compensated cirrhosis that had been treated with antiviral therapy for 5 years. A physical examination revealed that the patient had mild upper abdominal pain, nausea, and vomiting. Laboratory tests indicated liver dysfunction with an elevated serum alanine aminotransferase (ALT) level of 74 U/L (reference range: 0–40 U/L) and serum aspartate aminotransferase (AST) level of 44 U/L (reference range: 5–34 U/L). The patient tested positive for HBsAg and anti-hepatitis B core (anti-HBc). Tumor markers, including AFP, CEA, and CA19-9, were within normal limits. Upper gastrointestinal endoscopy revealed mild gastroesophageal variceal hemorrhage.
Ultrasound imaging revealed a small liver with an irregular surface and rough parenchyma. A hypoechoic nodule approximately 1.3 cm in size was observed in segment V with an unclear boundary (Figure 2A), consistent with HCC. To further classify the nodule, CEUS was performed in accordance with the guidelines of EFSUMB (5). A 1.2 mL bolus injection of SonoVue contrast agent was administered via the left cubital vein, followed by a 5 mL saline flush. CEUS imaging showed arterial hyperenhancement and early washout during the venous and delayed phases (Figure 2B-2D). Contrast-enhanced MRI further revealed multiple hypervascular nodules, approximately 1.5 cm in size, with arterial hyperenhancement consistent with HCC (Figure 2E-2G).
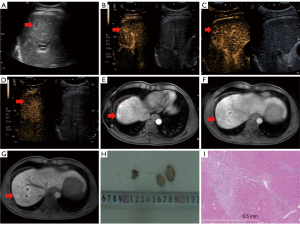
Owing to the patient’s history of hepatitis B virus-related compensated cirrhosis and imaging findings, HCC was suspected, and the patient underwent partial hepatectomy. Gross examination revealed that the tissue was moderately inhomogeneous (Figure 2H). Histologically, the nodule was hyperplastic with normal-appearing hepatocytes, and the periphery of the fibrous septa exhibited the proliferation of biliary structures and arteries. Ultimately, the nodule was diagnosed as an FNH-LN (Figure 2I). The patient underwent radiological follow-up examination for 6 years, and there were no indications of malignancy. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patients for publication of the case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
FNH-LNs are histologically similar to classic FNH found in abnormal livers but have unique features such as diffuse capillarization, iron deposits in Kupffer cells or hepatocytes with sinusoidal dilatation, and pathologic pseudocapsules (6).
Few studies have investigated the CEUS characteristics of FNH-LNs. The CEUS characteristics of FNH-LNs are similar to those of classic FNH, typically exhibiting hyperenhancement with a centrifugal enhancement pattern on CEUS in the arterial phase and sustained enhancement in the portal or late phases. Additional features such as spoke-wheel arteries, feeding arteries, and central scars, can aid in the diagnosis of FNH-LNs (7,8). However, these signs may not always be easily recognized in FNH-LNs on CEUS, especially in small lesions, which can increase the difficulty of diagnosing FNH-LNs. The FNH-LNs in Case 1 showed hyperenhancement in the arterial phase with isoenhancement in the portal and delayed phases, making it challenging to differentiate from HCC. Although typical HCC shows hyperenhancement in the arterial phase and washout in the portal or delayed phase on CEUS, recent studies have shown that the washout pattern in HCC is related to the differentiated types, and well-differentiated HCC may show iso- or hyper-enhancement in the portal or delayed phase (9). Therefore, differentiating HCC from FNH-LNs can be challenging. FNH-LNs on CEUS also exhibit atypical imaging features, such as hyperenhancement in the arterial phase and washout in the portal or delayed phase, which mimics typical HCC (10). The FNH-LNs in Case 2 showed the above atypical CEUS features. This could be due to the direct drainage of blood from the FNH-LNs into the hepatic vein, resulting in a washout in the portal phase. Thus, CEUS incurs diagnostic challenges in differentiating FNH-LNs and HCC with cirrhosis.
DCE-US parameters with quantitative TICs can provide useful information when hypervascular lesions are difficult to accurately characterize based on the enhancement patterns observed on CEUS. We further hypothesized that WiR and mTT is useful in distinguishing between FNH-LNs and HCC by DCE-US with quantitative TICs. Pei et al. suggested that the rising slope (RS) (the definition of RS in other imaging software is the same as WiR in VueBox) in the arterial phase may have a higher sensitivity and specificity for differentiating FNH from HCC (11). They determined that the RS cut-off value for FNH was 12.94. In our case, the RS of the FNH-LNs was 70.8, which is consistent with the findings of Pei et al. RS reflects the filling-in speed of the contrast agent. Although both HCC and FNH-LNs exhibited hyperenhancement in the arterial phase, FNH-LNs had a higher hyperenhancement ratio on dynamic CEUS than that of HCC. This difference may be due to the distinct pathogeneses of FNH-LNs and HCC. FNH-LNs arise from vascular abnormalities and are characterized by increased arterial blood flow, whereas HCC develops with increased arterial neovascularization. Furthermore, Zheng et al. suggested that mTT was helpful to differentiate FNH from HCC and found that the mTT parameter of FNH was significantly longer than those of HCC (12). Their study found that the cut off value of mTT at 107.93 seconds exhibited better diagnostic performance in differentiating FNH from HCC with a sensitivity of 96.7%. In our case, the mTT of FNH-LNs was 458.80 seconds. which is in accordance with the findings of Zheng et al. The mTT is associated with the speed of contrast agent washout. FNH-LNs showed longer contrast agent washout time compared to that of HCC. This difference may be attributed to the distinct pathological characteristics of HCC and FNH-LNs. In HCC, the normal hepatic sinusoids are partially destroyed, whereas in FNH, the neat trabecular structures with normal hepatic sinusoids are still intact. Combining multiple quantitative parameters to differentiate FNH-LNs from HCC, such as peak time, time to peak, and the area under the curve could be an interesting topic for future research.
Furthermore, in Case 2, FNH-LNs in the patient with cirrhosis demonstrated atypical characteristics using CEUS, such as hyperenhancement in the arterial phase and washout in the portal phase, which could mimic HCC. It is important to differentiate between these 2 entities. Sonazoid (Dalichi-Sankyo Co., Tokyo, Japan) is an ultrasound contrast agent that is taken up by Kupffer cells in the reticuloendothelial system of the liver, resulting in 2 phases of contrast enhancement: vascular and Kupffer imaging. Studies have shown that CEUS with Sonazoid can differentiate between benign and malignant tumors (13). Malignant lesions, such as HCC, which lack Kupffer cells, typically show hypoenhancement in the Kupffer phase, whereas FNH-LNs, which contain Kupffer cells in the reticuloendothelial system, can exhibit hyperenhancement or isoenhancement in the Kupffer phase. However, Lee et al. reported that FNH-LNs exhibit a defect in the Kupffer phase, which is attributed to the presence of a central scar. CEUS with Sonazoid can aid in the diagnosis of FNH-LNs in cases with atypical vascular patterns, such as FNH-LNs in patients with liver cirrhosis (14). It is hoped that in the future, Sonazoid can be utilized for post-vascular phase assessment to better differentiate between FNH-LNs and HCC. This could potentially assist in improving the accuracy of diagnosis.
Moreover, several studies have described FNH-LN CT and MRI findings (6,15). FNH-LN imaging characteristics resemble those of typical FNH in the normal liver. However, owing to alterations in the overall background liver hemodynamics or histological features, FNH-LNs may exhibit atypical imaging features, particularly in dynamic contrast-enhanced imaging. On contrast-enhanced MRI or CT, FNH-LNs exhibit hyperenhancement in the arterial phase and hyperenhancement or isoenhancement in the venous and delayed phases. Some studies have also reported the presence of washout in the venous and delayed phases, mimicking HCC (16). The hepatobiliary phase (HBP) signal intensity distribution of gadoxetic acid-enhanced MRI is critical for distinguishing FNH-LNs from HCC in the cirrhotic liver. FNH-LNs in cirrhotic livers exhibit features similar to those of FNH on HBP imaging, typically appearing hyperintense or isointense. Whereas most HCC lesions exhibit hypointensity on HBP (17). Our cases of FNH-LNs in cirrhotic patients revealed hyperenhancement in the arterial phase and isoenhancement in the portal and late phases, comparable to those of typical FNH on contrast-enhanced MRI and CT.
Both cases of FNH-LNs occurred in a hepatitis B virus-related cirrhosis background. Laboratory tests indicated that the tumor markers were within normal limits. The size of the lesions was less than 3 cm. Contrast-enhanced MRI or CT imaging showed hyperenhancement in the arterial phase and isoenhancement in the portal and late phases. Both FNH-LNs in Case 1 and Case 2 exhibited hyperenhancement during the arterial phase on CEUS. However, there was a difference in the washout pattern between 2 cases on CEUS. In Case 1, the FNH-LNs did not demonstrate washout, whereas in Case 2, the FNH-LNs displayed washout during the portal or delayed phase on CEUS. The appearance of washout may be associated with the distinct histological features. The Case 2 of FNH-LNs revealed dilated capillaries, resulting in accelerated outflow of contrast agent. Both cases were followed up through imaging and did not show any indications of malignant tumors. In addition to HCC, liver cirrhosis can give rise to various benign and malignant lesions, including regenerative nodules (RNs), hemangiomas, and intrahepatic cholangiocarcinoma (ICC), which can also be differentiated from FNH-LNs. RNs are common in cirrhosis and on CEUS exhibit hypoenhancement in the arterial phase and isoenhancement or hyperenhancement in the portal or late phase, owing to a portal venous supply with decreased arterial blood. In contrast, FNH-LNs show hyperenhancement in the arterial phase, making the enhancement pattern of the arterial phase useful for distinguishing between FNH-LNs and RNs.
Although hemangiomas are rare in liver cirrhosis, they typically exhibit peripheral hyperenhancement in the arterial phase on CEUS, which aids in their diagnosis. However, Brancatelli et al. reported that hemangiomas in patients with cirrhosis may decrease in size with the progression of cirrhosis, resulting in atypical enhancement patterns that display hyperenhancement in the arterial phase and sustained enhancement in the portal and late phases, making it difficult to distinguish FNH-LNs from hemangiomas (18). Furthermore, a biopsy is important for differential diagnosis. ICC is the second most common primary malignant tumor and is found in both non-cirrhotic and cirrhotic livers. On CEUS, ICC shows rim enhancement in the arterial phase, and early (<60 s) or marked washout accompanied by peripheral bile duct dilatation and elevated serum CA19-9 levels. FNH-LNs demonstrate a late or mild washout appearance, and serum CA19-9 levels are normal. These features facilitate a differential diagnosis.
In summary, CEUS provides valuable imaging information and is critical in diagnosing FNH-LNs. However, in cases where the lesion exhibits atypical features, biopsy and laboratory serum examination remain crucial for establishing a definitive diagnosis.
Conclusions
FNH occurs in cirrhosis, and its atypical imaging characteristics on CEUS mimic those of HCC. Understanding the quantitative assessment provided by DCE-US with perfusion parameters and viewing the enhancement pattern of the post-vascular phase on CEUS may help the diagnosis of FNH-LNs. In addition, gadoxetic acid-enhanced MRI of HBP manifestations, combined with laboratory serum AFP and CA19-9 testing, can reduce the risk of misdiagnosing FNH-LNs as HCC. An ultrasound-guided biopsy may also be required in some cases to guide appropriate treatment.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-907/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patients for publication of the case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sugihara S, Nakashima O, Kiyomatsu K, Ijiri M, Edamitsu O, Kojiro M. A case of liver cirrhosis with a hyperplastic nodular lesion. Acta Pathol Jpn 1990;40:699-703. [Crossref] [PubMed]
- Fujita N, Nishie A, Asayama Y, Ishigami K, Ushijima Y, Kakihara D, Nakayama T, Morita K, Ishimatsu K, Honda H. Hyperintense Liver Masses at Hepatobiliary Phase Gadoxetic Acid-enhanced MRI: Imaging Appearances and Clinical Importance. Radiographics 2020;40:72-94. [Crossref] [PubMed]
- Tang M, Li Y, Lin Z, Shen B, Huang M, Li ZP, Li X, Feng ST. Hepatic nodules with arterial phase hyperenhancement and washout on enhanced computed tomography/magnetic resonance imaging: how to avoid pitfalls. Abdom Radiol (NY) 2020;45:3730-42. [Crossref] [PubMed]
- Caraiani C, Boca B, Bura V, Sparchez Z, Dong Y, Dietrich C. CT/MRI LI-RADS v2018 vs. CEUS LI-RADS v2017-Can Things Be Put Together? Biology (Basel) 2021;10:412. [Crossref] [PubMed]
- Dietrich CF, Nolsøe CP, Barr RG, Berzigotti A, Burns PN, Cantisani V, et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med Biol 2020;46:2579-604. [Crossref] [PubMed]
- Lee YH, Kim SH, Cho MY, Shim KY, Kim MS. Focal nodular hyperplasia-like nodules in alcoholic liver cirrhosis: radiologic-pathologic correlation. AJR Am J Roentgenol 2007;188:W459-63. [Crossref] [PubMed]
- LeGout JD, Bolan CW, Bowman AW, Caserta MP, Chen FK, Cox KL, Sanyal R, Toskich BB, Lewis JT, Alexander LF. Focal Nodular Hyperplasia and Focal Nodular Hyperplasia-like Lesions. Radiographics 2022;42:1043-61. [Crossref] [PubMed]
- Myers L, Ahn J. Focal Nodular Hyperplasia and Hepatic Adenoma: Evaluation and Management. Clin Liver Dis 2020;24:389-403. [Crossref] [PubMed]
- Yang D, Li R, Zhang XH, Tang CL, Ma KS, Guo DY, Yan XC. Perfusion Characteristics of Hepatocellular Carcinoma at Contrast-enhanced Ultrasound: Influence of the Cellular differentiation, the Tumor Size and the Underlying Hepatic Condition. Sci Rep 2018;8:4713. [Crossref] [PubMed]
- Kim SR, Kondo F, Otono Y, Imoto S, Ando K, Hirakawa M, Fukuda K, Sasaki M, Kim SK, Komaki T, Tsuchida S, Kobayashi S, Matsuoka T, Kudo M. Serum amyloid A and C-reactive protein positive nodule in alcoholic liver cirrhosis, hard to make definite diagnosis. Hepatol Res 2014;44:584-90. [Crossref] [PubMed]
- Pei XQ, Liu LZ, Xiong YH, Zou RH, Chen MS, Li AH, Cai MY. Quantitative analysis of contrast-enhanced ultrasonography: differentiating focal nodular hyperplasia from hepatocellular carcinoma. Br J Radiol 2013;86:20120536. [Crossref] [PubMed]
- Zheng SG, Xu HX, Liu LN, Wang Y, Zhang YF, Guo LH, Liu C, Xu JM, Sun LP, Wu J. Parametric imaging with contrast-enhanced ultrasound: usefulness for characterization of dynamic effects of microvascularization for hepatocellular carcinoma and focal nodular hyperplasia. Clin Hemorheol Microcirc 2013;55:375-89. [Crossref] [PubMed]
- Dietrich CF, Tana C, Caraiani C, Dong Y. Contrast enhanced ultrasound (CEUS) imaging of solid benign focal liver lesions. Expert Rev Gastroenterol Hepatol 2018;12:479-89. [Crossref] [PubMed]
- Lee J, Jeong WK, Lim HK, Kim AY. Focal Nodular Hyperplasia of the Liver: Contrast-Enhanced Ultrasonographic Features With Sonazoid. J Ultrasound Med 2018;37:1473-80. [Crossref] [PubMed]
- Nolan PE, Catania R, Vendrami CL, Borhani AA, Miller FH. Large Regenerative Nodules and Focal Nodular Hyperplasia-Like Lesions: Definition, Pathogenesis, and Imaging Findings. Radiol Clin North Am 2022;60:795-808. [Crossref] [PubMed]
- Van Wettere M, Purcell Y, Bruno O, Payancé A, Plessier A, Rautou PE, Cazals-Hatem D, Valla D, Vilgrain V, Ronot M. Low specificity of washout to diagnose hepatocellular carcinoma in nodules showing arterial hyperenhancement in patients with Budd-Chiari syndrome. J Hepatol 2019;70:1123-32. [Crossref] [PubMed]
- van Kessel CS, de Boer E, ten Kate FJ, Brosens LA, Veldhuis WB, van Leeuwen MS. Focal nodular hyperplasia: hepatobiliary enhancement patterns on gadoxetic-acid contrast-enhanced MRI. Abdom Imaging 2013;38:490-501. [Crossref] [PubMed]
- Brancatelli G, Federle MP, Grazioli L, Golfieri R, Lencioni R. Large regenerative nodules in Budd-Chiari syndrome and other vascular disorders of the liver: CT and MR imaging findings with clinicopathologic correlation. AJR Am J Roentgenol 2002;178:877-83. [Crossref] [PubMed]


