
Superiority of spleen stiffness on two-dimensional magnetic resonance elastography over liver stiffness and serum tests in assessing portal hypertension in chronic liver disease
Introduction
Portal hypertension (PH) is a frequent complication of chronic liver disease (CLD) and is mainly responsible for its serious consequences, which include variceal hemorrhage, hepatic encephalopathy, and ascites (1). The accurate assessment of portal venous pressure is essential the proper treatment to reduce the mortality rate of PH-related complications (2). The current gold standard for assessing portal venous pressure remains the hepatic venous pressure gradient (HVPG) (3). However, HVPG measurement is not widely available due to its invasive nature. The risk of variceal bleeding can also be assessed via esophagogastroduodenoscopy (4), but it has similar drawbacks, including invasiveness, cost, and patient discomfort. Therefore, reliable noninvasive methods that can potentially replace HVPG measurements and endoscopy are being examined.
Over the past years, elastography has shown promising results in assessing PH, and ultrasound elastography, particularly transient elastography (TE), has been studied extensively (5). Magnetic resonance elastography (MRE) is another alternative means to measuring liver stiffness (LS) and spleen stiffness (SS). This technique is theoretically superior to ultrasound elastography since it can scan the entire liver and spleen to obtain a larger measurement area. Several preliminary studies have confirmed the value of MRE in assessing PH and esophageal varices (EVs) (6-13); however, evidence is still scarce. First, there is no clear answer as to which organ is more useful for revealing PH between the liver and the spleen. Some studies have shown that SS has significant superiority (6,7,11), while others have reported that LS is comparable or even better (10,14,15). Second, the reported cutoff values vary considerably across different studies and therefore need to be harmonized. Third, a comparison of MRE with established serum biomarkers is lacking. Fourth, the diagnostic value of MRE for higher HVPG thresholds remains unclear. Previous studies have focused mainly on the thresholds of clinically significant portal hypertension (CSPH) (HVPG ≥10 mmHg) and severe PH (HVPG ≥12 mmHg) (6,7,10,15). However, the risk stratification of patients above these thresholds remains clinically important. There is evidence that an HVPG higher than 16 and 20 mmHg is associated with poor prognosis (higher mortality with HVPG above 16 (16,17), while an HVPG above 20 is associated with a higher failure rate to control bleeding (18).
Therefore, the aim of this study was to assess the predictive power of LS and SS for HVPG and high-risk EVs in a cohort of patients with CLD and a generally high HVPG and to compare them with several established serum biomarkers. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1415/rc).
Methods
Patients
In this prospective study, patients with CLD who underwent HVPG measurement at the Department of Hepatology, Beijing Friendship Hospital, between April 2018 and March 2022 were screened for enrolment. The exclusion criteria for patients were as follows: a history of transjugular intrahepatic portosystemic shunt (TIPS), splenectomy, hepatectomy, liver transplantation or partial splenic embolization, portal system thrombosis, previous or ongoing nonselective beta-blocker (NSBB) treatment, episodes of recent variceal bleeding, hepatic venous-to-venous communications, contraindications to MRE, and refusal to participate. The diagnosis of CLD was confirmed on the basis of the results of liver histology or clinical, biochemical, and radiologic findings. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Institutional Review Board of Beijing Friendship Hospital (no. 2018-P2-142-01). Informed consent was obtained from all patients.
HVPG measurement
All patients, after an overnight fast, underwent HVPG measurement conducted by 2 experienced hepatologists (W.Y. and H.F., with 5 and 10 years of experience with HVPG measurement, respectively). Under local anesthesia, a 6-F venous introducer was inserted into the right internal jugular vein via the Seldinger technique. Under fluoroscopic control, a balloon-tipped catheter was inserted into the right hepatic vein to measure the wedged hepatic venous pressure (WHVP) and free hepatic venous pressure (FHVP). The HVPG was recorded as the difference between the WHVP and FHVP. Permanent pressure tracings were recorded and printed. At least three valid measurements were carried out in each patient, with their average being taken as the final result.
Upper gastrointestinal endoscopy
We collected the endoscopy results of the patients who were admitted for HVPG measurement. Endoscopy was performed by experienced endoscopists. EVs were classified into four groups as follows according to international guidelines (19): (I) F0, lack a varicose appearance; (II) F1 straight, small-caliber varices; (III) F2 moderately enlarged, beady varices; and (IV) F3, markedly enlarged, nodular or tumor-shaped varices. The presence of the red color (RC) sign was also recorded. High-risk EVs were defined as F2 to F3 varices or F1 varices with RC according to the Baveno VI criteria (20).
MRE imaging and processing
Within a week before HVPG measurement (mean, 2.4 days; range, 1–6 days), MRE examinations were performed on a 3.0-T MRI machine (750 W; GE HealthCare, Chicago, IL, USA). After fasting for at least 6 hours, patients were scanned in the supine position, and an elastic band was used to secure a 19-cm-diameter passive driver against the upper abdomen (over the right upper abdomen at the level of the xiphoid process). The MRE scan was consistent with that described in Yin et al.’s study (21), with Figure 1 showing the schematic. A 60-Hz vibration was generated by the active driver (Resoundant, Inc., Rochester, MN, USA) and delivered by the plastic hose connected to the passive driver, which transmitted the vibrations into the liver and spleen. MRE data were acquired using the breath-hold two-dimensional spin echo-echo planar imaging (SE-EPI) sequence. The MRE sequence parameters were as follows: axis position; repetition time/echo time (TR/TE) 1,000 ms/Min Full; matrix, 64×64; field of view (FOV), 42 cm ×42 cm; slice thickness, 10 mm; slice gap, 5 mm; number of layers, 7; excitation times, 3; bandwidth, 250 Hz; driver frequency, 60 Hz; amplitude, 70%; and time to complete the scan with three breath-holds, 51 s.
MRE data were analyzed by an experienced radiologist (with more than 5 years of experience in MRE) using the Volume Viewer postprocessing software (version 13.0, GE HealthCare) who was blinded to the HVPG and clinical data. An inversion algorithm (22) was used to postprocess the wave information, generating wave, magnitude, and elastogram images. Regions of interest (ROIs) were drawn on the anatomical images and copied to the elastogram for reading. The ROI needed to be drawn as large as possible on the liver and spleen parenchyma, with large vessels, organ edges, and areas with crosshatch (a confidence level of less than 0.95) or with poor wave propagation in the corresponding wave images being avoided. Measurements were performed on the center three slices of the liver and spleen, and the average value of the ROI for three slice locations was used as the stiffness value.
Clinical and laboratory data collection
Demographic data, including age, sex, BMI, and cause of CLD, were collected from all patients. Additionally, laboratory parameters, including platelet (PLT) count, biochemical data, and coagulation profiles, were obtained within the week prior to MRE, upper gastrointestinal endoscopy, and HVPG. The Child-Pugh score (23) and the model for end stage liver disease (MELD) score (24) were calculated.
The following published serum biomarkers were evaluated in all patients: aspartate aminotransferase (AST)-to-PLT ratio index (APRI), fibrosis 4, and King’s score (25). The formulae for each were as follows:
Statistical analysis
Depending on the normality of the data, continuous variables are expressed as the mean ± standard deviation (SD) or as the median and interquartile range. Qualitative variables are expressed as absolute values and relative frequencies. The correlations between all the variables (including LS, SS, and clinical and imaging parameters) and HVPG were computed using the Pearson correlation coefficient. Factors that significantly correlated with HVPG in the univariable analyses were included in a multivariable stepwise linear regression analysis. Binary logistic regression analysis was performed to determine the factors associated with the presence of an HVPG ≥16 mmHg, HVPG ≥20 mmHg, and high-risk EVs. Variables with a P value less than 0.1 in univariable analysis were entered into multivariable forward stepwise analysis. To avoid the effect of collinearity, composite parameters, Child-Pugh score, MELD score, fibrosis 4, APRI, and King’s score were not included in the multivariable model either in linear or in logistic regression analysis.
The diagnostic performance of the different noninvasive methods for HVPG ≥16 mmHg, HVPG ≥20 mmHg, and high-risk EVs was assessed via receiver operator characteristic (ROC) curves analysis. The performance was compared using the DeLong method (26). The optimal cutoff points for identifying HVPG ≥16 mmHg and ≥20 mmHg were determined using the highest Youden index. The cutoff for ruling out high-risk EVs was selected by optimizing the percentage of endoscopies spared and by keeping the risk of high-risk EVs below the 5% threshold, as recommended by the Baveno VI consensus (20). The sensitivity, specificity, positive likelihood ratio (+LR), and negative likelihood ratio (−LR) were calculated. All tests were two-sided, and the α value was set at 0.05. Data analysis was performed with SPSS software version 26.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics (Figure 2, Table 1)
Table 1
| Variable | Data |
|---|---|
| Age, years | 56.0±17.0 [20–73] |
| Gender, M/F | 21/27 |
| BMI, kg/m2 | 24.5±4.3 [16.9–36.2] |
| Etiology | |
| Hepatitis B | 6 (12.2) |
| Alcohol | 8 (16.3) |
| NAFLD | 8 (16.3) |
| Autoimmune liver disease | 7 (14.3) |
| Primary biliary cholangitis | 11 (22.4) |
| Other | 4 (8.2) |
| Cryptogenic | 4 (8.2) |
| Albumin, g/L | 32.4±8.8 [21.2–64.5] |
| Total bilirubin, μmol/L | 31.9±35.3 [10.7–363.7] |
| AST, U/L | 46.1±86.1 [18.3–547.7] |
| ALT, U/L | 36±74 [11–377] |
| Prothrombin time, s | 12.1±0 [11.2–17.5] |
| International normalized ratio | 1.3±0.3 [0.88–2.07] |
| Platelet count, ×109/L | 74.5±6.5 [19–303] |
| Serum creatinine, μmol/L | 59.5±25.9 [32.6–103.2] |
| Child–Pugh score | 7.0±3.0 [5–12] |
| Child–Pugh classification | |
| A | 17 (34.7) |
| B | 19 (38.8) |
| C | 12 (24.5) |
| MELD score | 9.6±4.5 [0.5–18.7] |
| Liver stiffness, kPa | 6.7±3.3 [3.4–12.2] |
| Spleen stiffness, kPa | 11.8±2.9 [6.1–18.2] |
| HVPG, mmHg | 16.8±5.8 [4–28] |
| HVPG ≥5, mmHg | 47 (97.9) |
| HVPG ≥10, mmHg | 43 (87.8) |
| Underwent GI endoscopy | 36 (75.0) |
| Presence of EVs | 28 (77.8) |
| High-risk EVs | 24 (66.7) |
The total number of participants analyzed was 48. Data are expressed the mean ± standard deviation or median and IQR with range in square brackets, or numbers of patients with percentages in parentheses. IQR, interquartile range; BMI, body mass index; NAFLD, nonalcoholic fatty liver disease; AST, aspartate transaminase; ALT, alanine transaminase; MELD, model for end-stage liver disease; HVPG, hepatic venous pressure gradient; GI, gastrointestinal; EVs, esophageal varices.
A total of 49 patients with CLD underwent HVPG and MRE. One patient was excluded due to a failure of MRE. The process of patient enrolment is shown in Figure 2. The clinical, biochemical, endoscopic, and MRE characteristics of the 48 patients are presented in Table 1. The median age was 56.0 years, 21 (43.8%) patients were male, and the mean HVPG was 16.8±5.8 mmHg (range, 4–28 mmHg). Among the patients, 47 (97.9%) had PH, and 43 (87.8%) had CSPH. Moreover, 36 of the 48 patients underwent upper gastrointestinal endoscopy, 28 patients had EVs (77.8%), and 24 had high-risk EVs (66.7%).
Correlations between LS, SS, and HVPG (Figure 3)
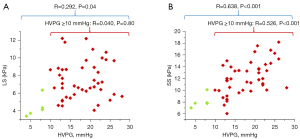
There was a weak correlation between LS and HVPG (r=0.292; P=0.04), and a strong correlation between SS and HVPG (r=0.638; P<0.001). In patients with CSPH at HVPG ≥10 mmHg (n=43), SS was moderately linearly correlated with HVPG (r=0.526; P<0.001), while no correlation was found between LS and HVPG (r=0.040; P=0.80).
Factors associated with HVPG (Table 2)
Table 2
| Variable | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| Correlation coefficient | P | β coefficient | P | ||
| Age | 0.314 | 0.03 | 0.295 | 0.002 | |
| BMI | −0.037 | 0.80 | |||
| Albumin | −0.269 | 0.06 | |||
| Total bilirubin | 0.172 | 0.24 | |||
| Serum creatinine | −0.136 | 0.36 | |||
| AST | 0.183 | 0.21 | |||
| ALT | −0.085 | 0.57 | |||
| Platelet count | −0.550 | <0.001 | |||
| Prothrombin time | −0.074 | 0.62 | |||
| INR | 0.408 | 0.004 | 0.375 | <0.001 | |
| MRE-LS | 0.292 | 0.04 | |||
| MRE-SS | 0.638 | <0.001 | 0.61 | <0.001 | |
| Child-Pugh score | 0.422 | 0.003 | |||
| MELD score | 0.150 | 0.31 | |||
| Fibrosis 4 | 0.560 | <0.001 | |||
| APRI | 0.327 | 0.02 | |||
| King’s score | 0.363 | 0.01 | |||
Bolded type indicates variables included in the multivariable analysis. BMI, body mass index; AST, aspartate transaminase; ALT, alanine transaminase; INR, international normalized ratio; MRE-LS, magnetic resonance elastography–liver stiffness; MRE-SS, magnetic resonance elastography–spleen stiffness; MELD, model for end-stage liver disease; APRI, aspartate aminotransferase-to-platelet ratio index.
In the univariable linear regression analysis, HVPG was significantly associated with age, PLT count, international normalized ratio (INR), MRE-LS, MRE-SS, Child-Pugh score, fibrosis 4, APRI, and King’s score. Among all the parameters, MRE-SS had the highest correlation coefficient (r=0.638; P<0.001). When analyzed by multivariable linear regression, age (β coefficient =0.295; P=0.002), INR (β coefficient =0.375; P<0.001), and MRE-SS (β coefficients =0.61; P<0.001) remained independently associated with HVPG. Neither the PLT count nor MRE-LS retained statistical significance (Table 2).
Parameters for identifying elevated HVPG and high-risk EVs (Tables S1-S3)
PLT count, INR, MRE-SS, Child-Pugh score, and fibrosis 4 were associated with the presence of HVPG ≥16 mmHg according to univariable logistic regression analysis (P=0.01, 0.08, 0.001, 0.02, and 0.003, respectively) (Table S1). Only MRE-SS was selected as an independent factor in the multivariable logistic regression analysis (odds ratio =1.614; 95% CI: 1.202–2.166; P=0.001) (Table S1).
Age, PLT count, INR, MRE-SS, Child-Pugh score, fibrosis 4, and King’s score were associated with the presence of HVPG ≥20 mmHg according to the univariable logistic regression analysis (P=0.08, 0.03, 0.07, 0.001, 0.05, 0.01, and 0.10, respectively) (Table S2). MRE-SS was determined to be an independent factor associated with HVPG ≥20 mmHg after adjustment for INR and age in multivariable logistic regression analysis (P=0.002) (Table S2).
Total bilirubin, ALT, PLT count, MRE-SS, and MELD score were associated with the presence of high-risk EVs according to univariable logistic regression analysis (P=0.02, 0.09, 0.01, 0.003, and 0.04, respectively) (Table S3). Only MRE-SS was selected as an independent factor in multivariable logistic regression analysis (odds ratio =2.076; 95% CI: 1.282–3.362; P=0.003) (Table S3).
Areas under the curve (AUC) of LS, SS, and other noninvasive parameters for identifying elevated HVPG and high-risk EVs (Table 3, Tables S4-S6)
Table 3
| Parameter | HVPG ≥16 mmHg | HVPG ≥20 mmHg | High-risk EVs |
|---|---|---|---|
| MRE-LS | 0.642 (0.490–0.775) | 0.593 (0.441–0.732) | 0.632 (0.455–0.786) |
| MRE-SS | 0.790 (0.662–0.918) | 0.822 (0.707–0.938) | 0.886 (0.764–1.000) |
| Albumin | 0.695 (0.543–0.846) | 0.707 (0.550–0.865) | 0.705 (0.522–0.887) |
| Total bilirubin | – | – | 0.743 (0.574–0.911) |
| Platelet count | 0.771 (0.632–0.910) | 0.741 (0.594–0.888) | 0.803 (0.650–0.956) |
| Child-Pugh score | 0.708 (0.559,0.857) | – | – |
| MELD score | – | – | 0.721 (0.550–0.892) |
| Fibrosis 4 | 0.844 (0.729–0.960) | 0.778 (0.642–0.913) | – |
| APRI | 0.745 (0.598–0.891) | 0.685 (0.533–0.837) | – |
| King’s score | 0.797 (0.656–0.899) | 0.743 (0.600–0.886) | – |
Data are AUCs with 95% confidence intervals in parentheses. AUC, area under curve; HVPG, hepatic venous pressure gradient; EVs, esophageal varices; MRE-LS, magnetic resonance elastography–liver stiffness; MRE-SS, magnetic resonance elastography–spleen stiffness; MELD, model for end-stage liver disease; APRI, aspartate aminotransferase-to-platelet ratio index.
MRE-LS was not a significant factor in the diagnosis of HVPG ≥16 mmHg, HVPG ≥20 mmHg, or high-risk EVs, with AUCs of 0.642, 0.593, and 0.632, respectively. MRE-SS, albumin, PLT count, Child-Pugh score, fibrosis 4, APRI, and King’s score were significant factors for predicting the presence of HVPG ≥16 mmHg. Among these parameters, the predictive performance of the MRE-SS (AUC =0.790; 95% CI: 0.662–0.918) was inferior to that of fibrosis 4 (AUC =0.844; 95% CI: 0.729–0.960), and King’s score (AUC =0.797; 95% CI: 0.656–0.899) but superior to that of the other factors (Table 3).
MRE-SS, albumin, PLT count, fibrosis 4, APRI, and King’s score were significant factors for predicting the presence of HVPG ≥20 mmHg. Among these parameters, MRE-SS was the most accurate predictive factor, as reflected by the highest AUC of 0.822 (Table 3).
For identifying high-risk EVs, MRE-SS, albumin, total bilirubin, PLT count, and MELD score were significant factors. Among these parameters, the AUC of MRE-SS (AUC =0.886; 95% CI: 0.764–1.000) was the highest. However, the difference in AUCs between each group did not reach statistical significance in the above three scenarios (Tables S4-S6).
In summary, MRE-SS had the best performance for the diagnosis of HVPG ≥20 mmHg and high-risk EVs but demonstrated no advantage over fibrosis 4 in identifying HVPG ≥16 mmHg (Table 3).
Diagnostic performance of LS and SS for identifying elevated HVPG and high-risk EVs (Table 4)
Table 4
| Cutoff value | Sensitivity (%) | Specificity (%) | +LR | −LR |
|---|---|---|---|---|
| HVPG ≥16 mmHg | ||||
| SS >9.5 kPa | 100 | 45.5 | 1.83 | 0 |
| SS >10.6 kPa | 80.8 | 63.6 | 2.22 | 0.3 |
| SS >12.5 kPa | 57.7 | 81.8 | 3.17 | 0.52 |
| SS >14.3 kPa | 34.6 | 95.5 | 7.62 | 0.68 |
| LS >5.6 kPa | 88.5 | 45.5 | 1.62 | 0.25 |
| LS >7.1 kPa | 50 | 63.6 | 1.37 | 0.79 |
| LS >8.7 kPa | 34.6 | 86.4 | 2.54 | 0.76 |
| HVPG ≥20 mmHg | ||||
| SS >10.05 kPa | 100 | 50 | 2 | 0 |
| SS >11.0 kPa | 83.3 | 56.7 | 1.92 | 0.29 |
| SS >13.5 kPa | 55.6 | 86.7 | 4.17 | 0.51 |
| SS >14.3 kPa | 44.4 | 93.3 | 6.67 | 0.6 |
| LS >5.6 kPa | 83.3 | 33.3 | 1.25 | 0.5 |
| LS >6.7 kPa | 61.1 | 56.7 | 1.41 | 0.69 |
| LS >8.9 kPa | 33.3 | 86.7 | 2.50 | 0.77 |
| High-risk EVs | ||||
| SS >9.9 kPa | 100 | 60 | 2.5 | 0 |
| SS >10.6 kPa | 90.5 | 80 | 4.52 | 0.12 |
| SS >11.5 kPa | 81.0 | 86.7 | 6.07 | 0.22 |
| SS >13.3 kPa | 57.1 | 93.3 | 8.57 | 0.46 |
| SS >14.3 kPa | 33.3 | 93.3 | 5 | 0.71 |
| LS ≤5.6 kPa | 33.3 | 80 | 1.67 | 0.83 |
| LS ≤6.3 kPa | 47.6 | 73.3 | 1.79 | 0.71 |
| LS ≤6.8 kPa | 76.2 | 66.7 | 2.29 | 0.36 |
| LS ≤7.5 kPa | 85.7 | 53.3 | 1.84 | 0.27 |
LS, liver stiffness; SS, spleen stiffness; HVPG, hepatic venous pressure gradient; EVs, esophageal varices; +LR, positive result likelihood ratio; −LR, negative likelihood ratio.
The MRE-SS cutoff values of 9.5 and 10.05 kPa were selected to predict the presence of HVPG ≥16 mmHg and HVPG ≥20 mmHg, respectively (HVPG ≥16 mmHg: sensitivity 100%, specificity 45.5%, +LR 1.83, −LR 0; HVPG ≥20 mmHg: sensitivity 100%, specificity 50%, +LR 2, −LR 0) (Table 4).
The cutoff value of MRE-SS was set to 9.9 kPa to rule out high-risk EVs, which could spare 9 of 15 (60%) patients from unnecessary endoscopy, with a 0% risk of missing high-risk EVs (0/21). The sensitivity for the identification of patients with high-risk EVs was 100%, the specificity was 60%, the +LR was 2.5, and the −LR was 0. Table 4 shows the diagnostic ability of LS and SS at different thresholds.
Discussion
The main findings of this study can be summarized as follows: (I) SS measured by two-dimensional MRE has a closer linear relationship with HVPG than does LS and routinely used serum tests. (II) SS demonstrated the best performance in predicting HVPG ≥20 mmHg and high-risk EV but was less accurate than was fibrosis 4 in detecting HVPG ≥16 mmHg. (III) We recommend that despite its low specificity, SS be used to rule out the use of the proposed thresholds owing to its excellent sensitivity.
Literature on MRE in PH using HVPG as a reference standard are scarce. We found a total of six studies with human participants, five of which assessed both the liver and spleen (6,7,10,11,15) and one of which assessed only LS (14). The correlation coefficient of hepatic viscoelastic parameters with HVPG reported in these studies ranged from 0.407 to 0.92, which was higher than the result of our study (r=0.292). We attribute this discrepancy to the study population, which in our study consisted mainly of patients with advanced PH, who typically have higher HVPG values. It is believed that the correlation of LS and HVPG decreases significantly in the higher HVPG range (27-29). Our data support and add to the evidence that LS is not a valuable indicator of HVPG in advanced PH.
In our series of patients with HVPG measurements, we found that the stiffness of the spleen is better at predicting PH severity than is the liver. These results are in line with published data regarding ultrasound and MRE methods (6,7,11,15,30,31). The superior performance of SS over LS may be related to the pathophysiological changes in the development of PH. As liver fibrosis progresses in CLD, the subsequent morphological changes cause elevated intrahepatic resistance and concomitant portal pressure increase (32). However, as PH progresses, extrahepatic factors such as hyperdynamic circulation begin to play a role in maintaining elevated portal pressure (33). Increased SS is produced by temporary upturns in hydrostatic pressure, stemming from tissue congestion, whereas LS reflects both the variations of PH-induced congestion and liver fibrosis (34). This may partly explain why SS is more directly linked to HVPG. However, this finding was not consistently described across other studies. In the study of Wagner et al. (10), no significant correlation was observed between HVPG and SS with MRE. It is reasonable to suspect that the diagnostic ability of liver and SS for PH is strongly influenced by the characteristics of the patient population, such as etiology, stage of disease, and distribution of HVPG. More studies are needed to determine their respective values in different clinical settings.
Identification of the progression of PH beyond the threshold of CSPH has gained considerable attention in recent years. However, evidence for a higher HVPG thresholds is scarce. We believe this is also clinically important because HVPG increases on a continuous scale and retains prognostic value for further decompensation and patient survival even if it exceeds the threshold of CSPH (35). Only two studies have reported the noninvasive prediction of HVPG at higher cutoff values. Gouya et al. (36) evaluated the hemodynamic parameters of phase contrast MRI and demonstrated that azygos flow was a good predictor of HVPG ≥16 mmHg. Frankova et al. (37) found that LS assessed using two-dimensional shear-wave elastography was a reliable predictor of HVPG above 16 and 20 mmHg. In contrast to the SWE study, our results indicate that the measurement of LS is of little value for these two thresholds. We suspect that this discrepancy may be due to the varying liver disease etiology. Unfortunately, Frankova et al. (37) did not assess SS, which was proven to be a better predictor in our study. Notably, although our results indicated SS to have good diagnostic value overall, it should only be used to rule out elevated HVPG and high-risk EVs at the proposed thresholds. In addition, we found that fibrosis 4, an inexpensive and widely used indicator of liver fibrosis, may be a useful tool for HVPG prediction, and it even outperformed SS in detecting the presence or absence of HVPG ≥16 mmHg. If our results are corroborated by future studies, fibrosis 4 could potentially be used as an alternative to more advanced methods.
Although there are some technical concerns that may hinder the applicability of MRE, mainly the cost and the accessibility compared to ultrasound-based methods, MRE has several advantages, including enabling the viscoelastic assessment of whole organs and a higher technical success rate. Patients with CLD often require contrast-enhanced MRI examinations for liver cancer screening. Therefore, we believe that MRE can be integrated into a conventional liver MRI examination without increasing patient cost.
Our study has some limitations. First, the sample size was relatively small because, in addition to the limited number of HVPG measurements, the exclusion criteria of this study were quite restrictive. For example, we excluded patients with hepatic venous-to-venous communications, a condition that could lead to an underestimation of portal venous pressure by the HVPG value (38), which has been overlooked in most previous studies. We believe that the narrow selection of patients may yield stronger scientific data. Nevertheless, the performance of our criteria for SS by MRE in larger cohorts and other settings remains to be confirmed. The second limitation is that not all included patients underwent endoscopy; however, we do not believe this introduced bias, as the only reason for not performing endoscopy in some patients was that the time was too close to their last examination and was thus driven entirely by random chance. Third, in this study, we performed two-dimensional MRE scans and therefore assessed only the stiffness or elasticity characteristics of the organs. The introduction of three-dimensional MRE will allow us to fully assess the role of viscoelastic characteristics in a future study.
Conclusions
In this prospective study, we found that SS measured by two-dimensional MRE may be superior to LS and serum tests in revealing PH in patients with generally high HVPG values. However, when used alone, SS used with the recommended thresholds is more suitable for ruling out elevated HVPG and high-risk EVs.
Acknowledgments
We would like to express our enormous appreciation and gratitude to Fuliang He, Guanhua Zhang, Yuerong Li, and Chunyu Wang.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-1415/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1415/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Review Board of Beijing Friendship Hospital (no. 2018-P2-142-01). Written consent forms were obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sanyal AJ, Bosch J, Blei A, Arroyo V. Portal hypertension and its complications. Gastroenterology 2008;134:1715-28. [Crossref] [PubMed]
- Bosch J, Garcia-Pagán JC, Berzigotti A, Abraldes JG. Measurement of portal pressure and its role in the management of chronic liver disease. Semin Liver Dis 2006;26:348-62. [Crossref] [PubMed]
- Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol 2009;6:573-82. [Crossref] [PubMed]
- de Franchis R, Baveno V. Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2010;53:762-8. [Crossref] [PubMed]
- Qi X, Berzigotti A, Cardenas A, Sarin SK. Emerging non-invasive approaches for diagnosis and monitoring of portal hypertension. Lancet Gastroenterol Hepatol 2018;3:708-19. [Crossref] [PubMed]
- Ronot M, Lambert S, Elkrief L, Doblas S, Rautou PE, Castera L, Vilgrain V, Sinkus R, Van Beers BE, Garteiser P. Assessment of portal hypertension and high-risk oesophageal varices with liver and spleen three-dimensional multifrequency MR elastography in liver cirrhosis. Eur Radiol 2014;24:1394-402. [Crossref] [PubMed]
- Kennedy P, Stocker D, Carbonell G, Said D, Bane O, Hectors S, Abboud G, Cuevas J, Bolster BD Jr, Friedman SL, Lewis S, Schiano T, Bhattacharya D, Fischman A, Thung S, Taouli B. MR elastography outperforms shear wave elastography for the diagnosis of clinically significant portal hypertension. Eur Radiol 2022;32:8339-49. [Crossref] [PubMed]
- Abe H, Midorikawa Y, Matsumoto N, Moriyama M, Shibutani K, Okada M, Udagawa S, Tsuji S, Takayama T. Prediction of esophageal varices by liver and spleen MR elastography. Eur Radiol 2019;29:6611-9. [Crossref] [PubMed]
- Shin SU, Lee JM, Yu MH, Yoon JH, Han JK, Choi BI, Glaser KJ, Ehman RL. Prediction of esophageal varices in patients with cirrhosis: usefulness of three-dimensional MR elastography with echo-planar imaging technique. Radiology 2014;272:143-53. [Crossref] [PubMed]
- Wagner M, Hectors S, Bane O, Gordic S, Kennedy P, Besa C, Schiano TD, Thung S, Fischman A, Taouli B. Noninvasive prediction of portal pressure with MR elastography and DCE-MRI of the liver and spleen: Preliminary results. J Magn Reson Imaging 2018;48:1091-103. [Crossref] [PubMed]
- Shi Y, Qi YF, Lan GY, Wu Q, Ma B, Zhang XY, Ji RY, Ma YJ, Hong Y. Three-dimensional MR Elastography Depicts Liver Inflammation, Fibrosis, and Portal Hypertension in Chronic Hepatitis B or C. Radiology 2021;301:154-62. [Crossref] [PubMed]
- Yoon H, Shin HJ, Kim MJ, Han SJ, Koh H, Kim S, Lee MJ. Predicting gastroesophageal varices through spleen magnetic resonance elastography in pediatric liver fibrosis. World J Gastroenterol 2019;25:367-77. [Crossref] [PubMed]
- Morisaka H, Motosugi U, Ichikawa S, Sano K, Ichikawa T, Enomoto N. Association of splenic MR elastographic findings with gastroesophageal varices in patients with chronic liver disease. J Magn Reson Imaging 2015;41:117-24. [Crossref] [PubMed]
- Gharib AM, Han MAT, Meissner EG, Kleiner DE, Zhao X, McLaughlin M, Matthews L, Rizvi B, Abd-Elmoniem KZ, Sinkus R, Levy E, Koh C, Myers RP, Subramanian GM, Kottilil S, Heller T, Kovacs JA, Morse CG. Magnetic Resonance Elastography Shear Wave Velocity Correlates with Liver Fibrosis and Hepatic Venous Pressure Gradient in Adults with Advanced Liver Disease. Biomed Res Int 2017;2017:2067479. [Crossref] [PubMed]
- Danielsen KV, Hove JD, Nabilou P, Yin M, Chen J, Zhao M, Kallemose T, Teisner AS, Siebner HR, Ehman RL, Møller S, Bendtsen F. Using MR elastography to assess portal hypertension and response to beta-blockers in patients with cirrhosis. Liver Int 2021;41:2149-58. [Crossref] [PubMed]
- Berzigotti A, Rossi V, Tiani C, Pierpaoli L, Zappoli P, Riili A, Serra C, Andreone P, Morelli MC, Golfieri R, Rossi C, Magalotti D, Zoli M. Prognostic value of a single HVPG measurement and Doppler-ultrasound evaluation in patients with cirrhosis and portal hypertension. J Gastroenterol 2011;46:687-95. [Crossref] [PubMed]
- Stanley AJ, Robinson I, Forrest EH, Jones AL, Hayes PC. Haemodynamic parameters predicting variceal haemorrhage and survival in alcoholic cirrhosis. QJM 1998;91:19-25. [Crossref] [PubMed]
- Jepsen P, Ott P, Andersen PK, Sørensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology 2010;51:1675-82. [Crossref] [PubMed]
- Tajiri T, Yoshida H, Obara K, Onji M, Kage M, Kitano S, et al. General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Dig Endosc 2010;22:1-9.
- de Franchis R, Baveno VI. Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63:743-52. [Crossref] [PubMed]
- Yin M, Talwalkar JA, Glaser KJ, Manduca A, Grimm RC, Rossman PJ, Fidler JL, Ehman RL. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol 2007;5:1207-1213.e2. [Crossref] [PubMed]
- Silva AM, Grimm RC, Glaser KJ, Fu Y, Wu T, Ehman RL, Silva AC. Magnetic resonance elastography: evaluation of new inversion algorithm and quantitative analysis method. Abdom Imaging 2015;40:810-7. [Crossref] [PubMed]
- Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646-9. [Crossref] [PubMed]
- Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK, Krom RA, Kim WR. MELD and PELD: application of survival models to liver allocation. Liver Transpl 2001;7:567-80. [Crossref] [PubMed]
- Wang L, Feng Y, Ma X, Wang G, Wu H, Xie X, Zhang C, Zhu Q. Diagnostic efficacy of noninvasive liver fibrosis indexes in predicting portal hypertension in patients with cirrhosis. PLoS One 2017;12:e0182969. [Crossref] [PubMed]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837-45.
- Procopet B, Berzigotti A, Abraldes JG, Turon F, Hernandez-Gea V, García-Pagán JC, Bosch J. Real-time shear-wave elastography: applicability, reliability and accuracy for clinically significant portal hypertension. J Hepatol 2015;62:1068-75. [Crossref] [PubMed]
- Palaniyappan N, Cox E, Bradley C, Scott R, Austin A, O'Neill R, Ramjas G, Travis S, White H, Singh R, Thurley P, Guha IN, Francis S, Aithal GP. Non-invasive assessment of portal hypertension using quantitative magnetic resonance imaging. J Hepatol 2016;65:1131-9. [Crossref] [PubMed]
- Vizzutti F, Arena U, Romanelli RG, Rega L, Foschi M, Colagrande S, Petrarca A, Moscarella S, Belli G, Zignego AL, Marra F, Laffi G, Pinzani M. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology 2007;45:1290-7. [Crossref] [PubMed]
- Takuma Y, Nouso K, Morimoto Y, Tomokuni J, Sahara A, Toshikuni N, Takabatake H, Shimomura H, Doi A, Sakakibara I, Matsueda K, Yamamoto H. Measurement of spleen stiffness by acoustic radiation force impulse imaging identifies cirrhotic patients with esophageal varices. Gastroenterology 2013;144:92-101.e2. [Crossref] [PubMed]
- Hirooka M, Ochi H, Koizumi Y, Kisaka Y, Abe M, Ikeda Y, Matsuura B, Hiasa Y, Onji M. Splenic elasticity measured with real-time tissue elastography is a marker of portal hypertension. Radiology 2011;261:960-8. [Crossref] [PubMed]
- Lim JK, Groszmann RJ. Transient elastography for diagnosis of portal hypertension in liver cirrhosis: is there still a role for hepatic venous pressure gradient measurement? Hepatology 2007;45:1087-90. [Crossref] [PubMed]
- Colecchia A, Montrone L, Scaioli E, Bacchi-Reggiani ML, Colli A, Casazza G, Schiumerini R, Turco L, Di Biase AR, Mazzella G, Marzi L, Arena U, Pinzani M, Festi D. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology 2012;143:646-54. [Crossref] [PubMed]
- Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, Peeters F, ter Beek LC, Rahier J, Sinkus R, Horsmans Y, Van Beers BE. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008;135:32-40. [Crossref] [PubMed]
- Garcia-Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology 2010;51:1445-9. [Crossref] [PubMed]
- Gouya H, Grabar S, Vignaux O, Saade A, Pol S, Legmann P, Sogni P. Portal hypertension in patients with cirrhosis: indirect assessment of hepatic venous pressure gradient by measuring azygos flow with 2D-cine phase-contrast magnetic resonance imaging. Eur Radiol 2016;26:1981-90. [Crossref] [PubMed]
- Frankova S, Lunova M, Gottfriedova H, Senkerikova R, Neroldova M, Kovac J, Kieslichova E, Lanska V, Urbanek P, Spicak J, Jirsa M, Sperl J. Liver stiffness measured by two-dimensional shear-wave elastography predicts hepatic vein pressure gradient at high values in liver transplant candidates with advanced liver cirrhosis. PLoS One 2021;16:e0244934. [Crossref] [PubMed]
- de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C, Baveno V. II Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol 2022;76:959-74. [Crossref] [PubMed]


