
Predicting recurrent laryngeal nerve invasion by preoperative ultrasonography in patients with thyroid carcinoma
Introduction
The global incidence of thyroid cancer has grown rapidly in the last 3 decades (1,2). Tumors in up to 20% of patients with thyroid cancer invade the recurrent laryngeal nerve (RLN) (3), which can cause nerve palsy manifesting as hoarseness or severe dyspnea (4,5). Such invasion disqualifies patients from robotic surgery, which is increasingly popular because it can lead to milder alterations of neck sensation and discomfort when swallowing than conventional thyroidectomy, while also avoiding scarring (6,7). Except low-risk well differentiated thyroid cancer (WDTC), patients with advanced/metastatic differentiated thyroid cancers (DTCs), anaplastic cancers, and medullary thyroid cancers (MTCs) are also increasing. These patients often present extrathyroidal extension (invasion of the vessels and nerves of the neck, trachea, esophagus, etc.), lymph node metastasis, and sometimes distant metastases (8). For resectable thyroid cancer, the American Thyroid Association (ATA) guidelines (9) require tumor staging with imaging before surgery, because evaluation of extent of local invasion may assist surgical decision-making. However, the current preoperative assessment methods are incomplete, especially for vessels and nerves.
Currently, RLN invasion before thyroidectomy is detected based on laryngoscopy by evaluating vocal cord mobility, but this approach is inadequate. First, vocal cord mobility may still be normal in the presence of unilateral lesions: some studies have reported that only about 30% of patients manifest vocal cord hypomobility during examination (10,11). Second, laryngoscopy is only an indirect indicator of RLN infiltration (12). These considerations highlight the need for a non-invasive way to predict RLN invasion when planning surgical treatment.
High-frequency ultrasonography is widely used to diagnose disorders involving peripheral nerves (13-17), and our previous study showed that preoperative ultrasound can be applied to visualize the RLN reliably (18). In our previous study, we injected the lymph nodes surrounding the RLN with carbon nanoparticles to confirm the same RLNs that had been identified by preoperative ultrasound during surgery. In addition, the concordance of results between preoperative and intraoperative ultrasound parameters [intraclass correlation coefficients (ICC) =0.403] and the statistics for measurements taken independently by 2 sonographers (ICCs ranged from 0.495 to 0.824) showed good reliability (Appendix 1, Method 1) (18). Therefore, we wondered whether preoperative ultrasonography could reliably detect RLN invasion in patients with thyroid carcinoma. In this study, we examined this question using independent patient cohorts at our hospital, leading to the establishment of 2 models that may help to identify those at high risk of RLN invasion in order to guide treatment decisions. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-332/rc).
Methods
Patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of West China Hospital and informed consent was provided by all individual participants. This study was registered in the Chinese Clinical Trial Registry (ChinCTR2100049742). For development of a predictive model, a consecutive sample of patients who underwent thyroidectomy at the Department of Thyroid and Parathyroid Surgery Center at West China Hospital of Sichuan University between August 2020 and December 2021 were enrolled. To validate the model, a separate consecutive sample of patients between January 2022 and March 2022 was also enrolled (Figure 1).
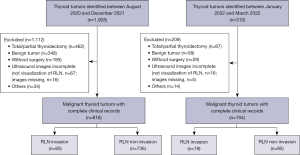
To be randomly enrolled, patients had to meet the following criteria: (I) thyroid lesions detected by preoperative radiologic imaging, with or without enlargement of cervical lymph nodes; (II) undergo thyroid surgery, with pathology analysis of surgical specimens confirming thyroid carcinoma; (III) provide complete clinical information, including sex, age, height, weight, body mass index (BMI), and comorbidities; and (IV) undergo ultrasound evaluation of thyroid lesions and RLN within 1 week before surgery. Patients were excluded if they had a history of thyroidectomy, whether total or partial, or if surgical records or ultrasound imaging of RLN were incomplete or missing.
Ultrasonography
Thyroid lesion(s) and the RLN in each patient were analyzed by ultrasonography of the anterior cervical region as described (18), with the patient in the supine position and the neck at maximal extension (Appendix 1, Method 2). Longitudinal and transverse sections were captured with the thyroid lesions and RLN positioned in the middle of the images. In this study, 2 ultrasound systems were used: an Aixplorer system (SuperSonic Imagine, Aix-en-Provence, France) with a 5–14 MHz line probe, and an RS80A system (Samsung Medison, Seoul, South Korea) with a 3–12 MHz linear probe.
Data were collected on the following imaging features: tumor location, categorized as diffuse, isthmic, upper, middle, or lower; tumor size; tumor margin, whether smooth or ill-defined; tumor shape, as regular or irregular; calcification, classified as macro, micro, mixed, or none; adjacency of the tumor to the anterior, posterior, medial, or lateral thyroid capsule; distance 31 or <1 mm between tumor and RLN; as well as loss of the typical fascicular echotexture of the RLN along the long axis, loss of the echotexture of the RLN epineurium, and loss of the echotexture of RLN fibers and perineurium, all categorized as yes or no. In patients with multiple lesions, we collected data only from the one that was categorized as 4b, 4c, or 5 according to Thyroid Imaging Reporting and Data System (TI-RADS) criteria (19,20) and that lay close to the tracheoesophageal groove.
Given that the RLN in healthy individuals resembles peripheral nerves in ultrasound images (typical fascicular echotexture appears as elongated structures with alternating hypo- and hyperechoic bands, Figure 2A,2B), we defined 2 ultrasound signs of RLN invasion (Appendix 1, Method 3; Figure 2C,2D): thyroid tumor <1 mm from the RLN (Figure 2E,2F), and loss of the typical fascicular echotexture of the RLN along the long axis (21,22).

Surgery and definitive diagnosis of RLN invasion
All surgeries were performed by 3 thyroid specialists using standard procedures (23,24) in 1 week (Appendix 1, Method 4). RLN invasion was detected by cryosections confirmation; alternatively, it was detected during the surgery itself in the following situations: (I) the tumor was found to adhere firmly to the RLN and electrical activity of the RLN was lost after resection, or resection also damaged the RLN; or (II) the RLN could not be preserved during surgery because it was intertwined with the tumor.
Statistical analysis
Data were analyzed using SPSS 19.0 (IBM Corp., Armonk, NY, USA) and R (v.3.3.3; R Foundation for Statistical Computing, Vienna, Austria). Continuous data were presented as the mean ± standard deviation. Inter-group differences in continuous data were assessed for significance using Student’s t-test, whereas differences in categorical data were assessed using Pearson’s chi-squared test. Variables that emerged as significantly associated with RLN invasion during univariate analysis were entered into multivariate logistic regression, and independent risk relationships were reported in terms of odds ratios (ORs) with 95% confidence intervals (CIs). Risk factors identified by multiple logistic regression were used to create predictive nomograms, for which cut-offs were determined using Youden’s index. The ability of nomograms to diagnose RLN invasion was assessed in terms of sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV), area under the receiver operating characteristic (ROC) curve (AUC), and concordance index (C-index). Results associated with P<0.05 (2-sided test) were considered statistically significant.
Results
Patient characteristics
Predictive nomograms were developed using data from 816 patients (612 women) whose mean age was 40±12 years, and 80 of whom had RLN invasion. The frequent types of cancers were papillary carcinoma (769/816, 94.24%), medullary carcinoma (28/816, 3.43%), follicular carcinoma (8/816, 0.98%), anaplastic carcinoma (9/816, 1.10%), and squamous carcinoma (2/816, 0.25%). Most patients (614/816, 75.25%) had a single lesion, whereas fewer than 20% had Hashimoto’s thyroiditis. According to the tumor-node-metastasis/American Joint Committee on Cancer (TNM/AJCC) classification, tumor staging was as follows: stage I (491/816, 60.17%), stage II (224/816, 27.45%), stage III (65/816, 7.97%), and stage IV (36/816, 4.41%).
The nomograms were validated in an independent cohort of 104 patients (85 women), whose mean age was 42±11 years and 19 of whom had RLN invasion. The distribution of types of thyroid carcinoma was similar to that in the cohort used to develop the nomograms.
Development of nomograms to predict RLN invasion
Univariate comparison of the clinicodemographic and ultrasound characteristics between patients with or without RLN invasion in the development cohort of 816 patients identified the following factors associated with invasion (Table S1): sex, age, BMI, lesion location, tumor size, calcification, tumor adjacent to any part of the thyroid capsule, distance between lesion and RLN, loss of typical fascicular echotexture of RLN along the long axis and loss of the echotexture of the RLN epineurium or fibers and perineurium. Forward stepwise multivariate regression with all these variables identified the following as independent predictors of invasion (Table 1): distance <1 mm between lesion and RLN, lesion adjacent to medial, posterior, and lateral thyroid capsules, tumor larger than 2 cm, age older than 60 years, and BMI 324 kg/m2. The presence of high-risk features (distance <1 mm between lesion and RLN, lesion adjacent to medial and posterior thyroid capsules, tumor larger than 2 cm, age older than 60 years, BMI ≥24 kg/m2 and loss of typical fascicular echotexture of RLN along the long axis) was associated with RLN invasion, and lower risk of invasion was observed with lesion adjacent to lateral thyroid capsule.
Table 1
| Predictor (reference condition) | Category | Beta | OR (95% CI) | P |
|---|---|---|---|---|
| Nomogram 1 | ||||
| Age (<30 years) | 30 to <40 | 0.75 | 2.117 (0.334, 13.405) | 0.426 |
| 40 to <50 | 2.034 | 7.645 (0.819, 71.322) | 0.074 | |
| 50 to <60 | 1.308 | 3.699 (0.451, 30.346) | 0.223 | |
| ≥60 | 2.629 | 13.854 (1.315, 145.933) | 0.029 | |
| Body mass index (<18.5 kg/m2) | 18.5 to <24 | 0.992 | 2.696 (0.395, 18.392) | 0.311 |
| ≥24 | 1.296 | 3.654 (1.228, 10.874) | 0.020 | |
| Tumor size (≤10 mm) | >10–20 | 1.04 | 2.829 (0.712, 11.24) | 0.140 |
| >20–40 | 1.903 | 6.708 (1.194, 37.685) | 0.031 | |
| >40 | 4.384 | 80.133 (4.677, 1,373.1) | 0.002 | |
| Tumor adjacent to posterior thyroid capsule (no) | Yes | 4.173 | 64.9 (5.567, 756.583) | 0.001 |
| Tumor adjacent to medial thyroid capsule (no) | Yes | 1.941 | 6.965 (1.816, 26.713) | 0.005 |
| Tumor adjacent to lateral thyroid capsule | Yes | −2.353 | 0.095 (0.016, 0.559) | 0.009 |
| Distance <1 mm between tumor and RLN (no) | Yes | 4.529 | 92.679 (10.389, 826.746) | <0.001 |
| Nomogram 2 | ||||
| Age (<30 years) | 30 to <40 | 2.945 | 19.912 (0.505, 716.747) | 0.112 |
| 40 to <50 | 3.169 | 23.787 (0.486, 1,164.334) | 0.110 | |
| 50 to <60 | 1.684 | 5.386 (0.119, 243.301) | 0.386 | |
| ≥60 | 3.886 | 48.705 (0.634, 3,740.447) | 0.079 | |
| Tumor size (≤10 mm) | >10–20 | 1.838 | 6.285 (0.366, 107.869) | 0.205 |
| >20–40 | 0.408 | 1.504 (0.033, 69.515) | 0.835 | |
| >40 | 2.636 | 13.963 (0.113, 1,719.757) | 0.283 | |
| Adjacent posterior thyroid capsule (no) | Yes | 5.976 | 393.833 (2.922, 53,074.51) | 0.017 |
| Adjacent medial thyroid capsule (no) | Yes | 2.006 | 7.437 (0.326, 169.512) | 0.208 |
| Adjacent lateral thyroid capsule (no) | Yes | −3.066 | 0.047 (0.0008, 2.581) | 0.134 |
| Distance between lesion and RLN (<1 mm) (no) | Yes | 3.758 | 42.845 (1.478, 1,241.646) | 0.029 |
| Loss of the typical fascicular echotexture of RLN in long axis (no) | Yes | 7.221 | 1,367.637 (35.11–53,272.81) | <0.001 |
RLN, recurrent laryngeal nerve; OR, odds ratio; CI, confidence interval.
These 5 variables were incorporated into predictive nomogram 1, in which each variable was scored according to the ORs from multivariate logistic regression. As a result, score increased in larger lesion and older age. BMI 324 kg/m2 was assigned a score of 29; distance <1 mm between lesion and RLN, 100; tumor adjacent to medial thyroid capsule, 43; and tumor adjacent to posterior thyroid capsule, 92. The total possible score ranged from 160 to 420, and Youden’s index identified 260 as the ideal cut-off for predicting RLN invasion (Figure 3A).

Given that the nomogram 1 did not contain loss of typical fascicular echotexture of RLN along the long axis, which is widely applied in diagnosis of RLN invasion and recommended in several consensus guidelines (21,22), we decided to create nomogram 2 including this feature. Repeating multivariate logistic regression identified 3 independent risk factors of invasion (Table 1): loss of typical fascicular echotexture of RLN along the long axis, distance <1 mm between the tumor and RLN, and lesion adjacent to thyroid posterior capsule. The nomogram 2 with these 3 variables was defined as follows: [66 ´ tumor adjacent posterior capsule (no = 0, yes = 1)] + [59 ´ distance <1 mm (no = 0, yes = 1)] + [100 ´ loss of typical structure of RLN (no = 0, yes = 1)]. Youden’s index identified 135 as the optimal cut-off (Figure 3B).
Calibration plots of both nomograms against the data from the development cohort of 816 patients showed good agreement between predicted and actual results (Figure 3C,3D). The C-index was 0.96 (95% CI: 0.94 to 0.98) for nomogram 1 and 0.99 (95% CI: 0.98 to 1.00) for nomogram 2. The corresponding AUCs were 0.89 (95% CI: 0.85 to 0.94) and 0.85 (95% CI: 0.77 to 0.92) (Table 2).
Table 2
| Models | C-index (95% CI) | AUC (95% CI) |
|---|---|---|
| Development cohort | ||
| Nomogram 1 | 0.96 (0.94–0.98) | 0.89 (0.85–0.94) |
| Nomogram 2 | 0.99 (0.98–1.00) | 0.85 (0.77–0.92) |
| Validation cohort | ||
| Nomogram 1 | 0.86 (0.78–0.94) | 0.85 (0.77–0.91) |
| Nomogram 2 | 0.89 (0.80–0.97) | 0.76 (0.33–0.79) |
CI, confidence interval; AUC, area under curve.
Nomogram validation
Both nomograms also performed well when we validated them against the second cohort of 104 patients. Nomogram 1 showed a C-index of 0.86 (95% CI: 0.78 to 0.94), AUC of 0.85 (95% CI: 0.77 to 0.91), and the following diagnostic performance indicators based on a cut-off of 260: sensitivity, 94.74%; specificity, 74.12%; PPV, 45.00%; NPV, 98.43%; and accuracy, 76.92% (Table 3). Nomogram 2 showed a C-index of 0.89 (95% CI: 0.80 to 0.97), AUC of 0.76 (95% CI: 0.33 to 0.79), and the following performance indicators based on a cut-off of 135: sensitivity, 57.89%; specificity, 95.29%; PPV, 73.26%; NPV, 91.03%; and accuracy, 88.46% (Table 3).
Table 3
| Nomogram | Se, % | Sp, % | PPV, % | NPV, % | Accuracy, % |
|---|---|---|---|---|---|
| 1 | 94.74 | 74.12 | 45.00 | 98.43 | 76.92 |
| 2 | 57.89 | 95.29 | 73.26 | 91.03 | 88.46 |
All values except the concordance index are %. RLN, recurrent laryngeal nerve; Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value.
Discussion
Here we established 2 scoring models for predicting RLN invasion before surgery to treat thyroid carcinoma. Nomogram 1, which included clinicodemographic and ultrasound features, showed excellent sensitivity (94.74%) and NPV (98.43%). Nomogram 2, which included only ultrasound features, showed excellent specificity (95.29%) and accuracy (88.60%). Both models showed a C-index >0.86, suggesting good agreement with observations. Nomogram 1 may be useful for clinical screening because these features are relatively easy to obtain. Nomogram 2 may help refine clinical decision-making because of the added specificity of neurological ultrasound characteristics.
The variables in our sample that were significantly associated with RLN invasion overlapped with several of those identified in previous work (25), including older age, larger tumor, and tumor location near the posterior capsule. The differences in risk factors between that work and ours may reflect our larger RLN sample and the fact that, because of our focus on ultrasonography, we did not consider certain clinical features such as laryngoscopy.
Among the diverse variables that we examined, ultrasound features showed the strongest association with RLN invasion: the OR for distance <1 mm between tumor and RLN was 92.67 in nomogram 1, while the OR for loss of typical fascicular echotexture of the RLN was 1367.63 in nomogram 2. Our study provides the first comprehensive evidence that ultrasonography can be used to diagnose RLN invasion. Our results are consistent with previous work suggesting that among the most frequent ultrasound findings associated with invasion is the loss of fascicular echostructure (21,22,26). However, loss of fascicular echostructure correlates strongly with other factors from a statistical aspect in nomogram 1, which may explain why it was excluded from that model. To take into account the efficacy of ultrasonic features for diagnosis in previous research, we included loss of fascicular echostructure into nomogram 2, which proved to be more specific and accurate than nomogram 1.
Although both nomograms showed good diagnostic performance, they failed to detect RLN invasion in 8 patients in the validation cohort. There may be several reasons for this. One is that we relied on conventional ultrasonography, which does not provide coronal views, and the combination of transverse and sagittal sections can miss certain tumor features (27,28). In 3 patients, we diagnosed RLN infiltration on the basis of loss of typical fascicular echotexture along the long axis, yet invasion was not detected in any of the 3 patients during surgery. We might have correctly determined no invasion from ultrasound images if we had visualized the entire RLN in a coronal image. Other contributors to missed diagnoses may include macro calcification and peripheral calcification, acoustic shadows caused by calcification, as well as architectural distortions that blur the margin between tumor and RLN.
There are some limitations in our study. First, RLN invaded by metastatic central cervical lymph nodes was not discussed in this study. There were 12 cases with RLN invaded by metastatic central cervical lymph nodes that ultrasound failed to detect due to the lack of background parenchymal contrast below thyroid and hyperechoic of the surrounding connective tissues. The characteristics of infiltrated RLN could not be analyzed because of the limited sample (29). Second, we excluded patients in whom ultrasonography failed to visualize the RLN clearly, which may have biased our development and validation cohorts. Third, the percentage of patients with advanced thyroid carcinoma was relatively higher in our institution, which may contribute to a higher incidence of RLN invasion (10%) in this trail. Fourth, this was a single-center prospective analysis, as this design conducted a new method for evaluating RLN invasion in thyroid carcinoma. Our data was derived from a single institution; the generalizability can be improved if data from additional institutions is added to the training set. Our research supplied more information of RLNs to help surgeons evaluate the surgical options for patients with resectable thyroid cancer. Patients with advanced/metastatic DTCs, anaplastic cancers, and MTCs who had the rare opportunity to receive surgery treatment after assessment of local invasion might accept multidisciplinary options including primary chemoradiation, palliative radiotherapy, and systemic therapy as early as possible to approach a better supportive care.
Conclusions
Our work indicates that preoperative ultrasound is a feasible approach to evaluate RLN invasion in patients with thyroid carcinoma. We developed nomogram 1 that may be useful for easy, rapid screening of patients for risk of invasion. The results from nomogram 1 can be refined using nomogram 2, which is more specific and accurate yet requires skillful interpretation of neck anatomy in ultrasound images. These findings suggest that preoperative ultrasound evaluation of RLN could supply additional information for individual surgical treatment decision.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-332/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-332/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of West China Hospital and informed consent was provided by all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim JI, Kim SJ, Xu Z, Kwak J, Ahn JH, Yu HW, Chai YJ, Choi JY, Lee KE. Efficacy of Intraoperative Neuromonitoring in Reoperation for Recurrent Thyroid Cancer Patients. Endocrinol Metab (Seoul) 2020;35:918-24. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Chiang FY, Wang LF, Huang YF, Lee KW, Kuo WR. Recurrent laryngeal nerve palsy after thyroidectomy with routine identification of the recurrent laryngeal nerve. Surgery 2005;137:342-7. [Crossref] [PubMed]
- Jeannon JP, Orabi AA, Bruch GA, Abdalsalam HA, Simo R. Diagnosis of recurrent laryngeal nerve palsy after thyroidectomy: a systematic review. Int J Clin Pract 2009;63:624-9. [Crossref] [PubMed]
- Erbil Y, Barbaros U, Işsever H, Borucu I, Salmaslioğlu A, Mete O, Bozbora A, Ozarmağan S. Predictive factors for recurrent laryngeal nerve palsy and hypoparathyroidism after thyroid surgery. Clin Otolaryngol 2007;32:32-7. [Crossref] [PubMed]
- Lee J, Chung WY. Robotic surgery for thyroid disease. Eur Thyroid J 2013;2:93-101.
- Lee J, Yun JH, Nam KH, Choi UJ, Chung WY, Soh EY. Perioperative clinical outcomes after robotic thyroidectomy for thyroid carcinoma: a multicenter study. Surg Endosc 2011;25:906-12. [Crossref] [PubMed]
- Laha D, Nilubol N, Boufraqech M. New Therapies for Advanced Thyroid Cancer. Front Endocrinol (Lausanne) 2020;11:82. [Crossref] [PubMed]
- Bible KC, Kebebew E, Brierley J, Brito JP, Cabanillas ME, Clark TJ Jr, Di Cristofano A, Foote R, Giordano T, Kasperbauer J, Newbold K, Nikiforov YE, Randolph G, Rosenthal MS, Sawka AM, Shah M, Shaha A, Smallridge R, Wong-Clark CK. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid 2021;31:337-86. [Crossref] [PubMed]
- Rosato L, Carlevato MT, De Toma G, Avenia N. Recurrent laryngeal nerve damage and phonetic modifications after total thyroidectomy: surgical malpractice only or predictable sequence? World J Surg 2005;29:780-4. [Crossref] [PubMed]
- Kay-Rivest E, Mitmaker E, Payne RJ, Hier MP, Mlynarek AM, Young J, Forest VI. Preoperative vocal cord paralysis and its association with malignant thyroid disease and other pathological features. J Otolaryngol Head Neck Surg 2015;44:35. [Crossref] [PubMed]
- Dionigi G, Boni L, Rovera F, Rausei S, Castelnuovo P, Dionigi R. Postoperative laryngoscopy in thyroid surgery: proper timing to detect recurrent laryngeal nerve injury. Langenbecks Arch Surg 2010;395:327-31. [Crossref] [PubMed]
- Abraham A, Izenberg A, Dodig D, Bril V, Breiner A. Peripheral Nerve Ultrasound Imaging Shows Enlargement of Peripheral Nerves Outside the Brachial Plexus in Neuralgic Amyotrophy. J Clin Neurophysiol 2016;33:e31-3.
- Samarawickrama D, Therimadasamy AK, Chan YC, Vijayan J, Wilder-Smith EP. Nerve ultrasound in electrophysiologically verified tarsal tunnel syndrome. Muscle Nerve 2016;53:906-12. [Crossref] [PubMed]
- Gruber H, Glodny B, Bendix N, Tzankov A, Peer S. High-resolution ultrasound of peripheral neurogenic tumors. Eur Radiol 2007;17:2880-8. [Crossref] [PubMed]
- Goedee HS, Brekelmans GJ, van Asseldonk JT, Beekman R, Mess WH, Visser LH. High resolution sonography in the evaluation of the peripheral nervous system in polyneuropathy--a review of the literature. Eur J Neurol 2013;20:1342-51. [Crossref] [PubMed]
- Huang A, Jiang L, Zhang J, Wang Q. Attention-VGG16-UNet: a novel deep learning approach for automatic segmentation of the median nerve in ultrasound images. Quant Imaging Med Surg 2022;12:3138-50. [Crossref] [PubMed]
- He Y, Li Z, Yang Y, Lei J, Peng Y. Preoperative Visualized Ultrasound Assessment of the Recurrent Laryngeal Nerve in Thyroid Cancer Surgery: Reliability and Risk Features by Imaging. Cancer Manag Res 2021;13:7057-66. [Crossref] [PubMed]
- Zhou J, Yin L, Wei X, Zhang S, Song Y, Luo B, et al. 2020 Chinese guidelines for ultrasound malignancy risk stratification of thyroid nodules: the C-TIRADS. Endocrine 2020;70:256-79. [Crossref] [PubMed]
- Liang F, Li X, Ji Q, He D, Yang M, Xu Z. Revised Thyroid Imaging Reporting and Data System (TIRADS): imitating the American College of Radiology TIRADS, a single-center retrospective study. Quant Imaging Med Surg 2023;13:3862-72. [Crossref] [PubMed]
- Kerasnoudis A, Tsivgoulis G. Nerve Ultrasound in Peripheral Neuropathies: A Review. J Neuroimaging 2015;25:528-38. [Crossref] [PubMed]
- Telleman JA, Herraets IJ, Goedee HS, van Asseldonk JT, Visser LH. Ultrasound scanning in the diagnosis of peripheral neuropathies. Pract Neurol 2021;21:186-95. [Crossref] [PubMed]
- Gao M, Ge M, Ji Q, Cheng R, Lu H, Guan H, et al. 2016 Chinese expert consensus and guidelines for the diagnosis and treatment of papillary thyroid microcarcinoma. Cancer Biol Med 2017;14:203-11. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Chen W, Lei J, You J, Lei Y, Li Z, Gong R, Tang H, Zhu J. Predictive factors and prognosis for recurrent laryngeal nerve invasion in papillary thyroid carcinoma. Onco Targets Ther 2017;10:4485-91. [Crossref] [PubMed]
- Beekman R, Visser LH. High-resolution sonography of the peripheral nervous system -- a review of the literature. Eur J Neurol 2004;11:305-14. [Crossref] [PubMed]
- Tang G, An X, Xiang H, Liu L, Li A, Lin X. Automated Breast Ultrasound: Interobserver Agreement, Diagnostic Value, and Associated Clinical Factors of Coronal-Plane Image Features. Korean J Radiol 2020;21:550-60. [Crossref] [PubMed]
- Timor-Tritsch IE, Monteagudo A, Ramos J, Kupchinska S, Mastriciani F, Spier M. Three-Dimensional Coronal Plane of the Uterus: A Critical View for Diagnostic Accuracy. J Ultrasound Med 2021;40:607-19. [Crossref] [PubMed]
- Xing Z, Qiu Y, Yang Q, Yu Y, Liu J, Fei Y, Su A, Zhu J. Thyroid cancer neck lymph nodes metastasis: Meta-analysis of US and CT diagnosis. Eur J Radiol 2020;129:109103. [Crossref] [PubMed]


