
Detecting advanced liver fibrosis using ultrasound shear wave velocity measurement in the general population
Introduction
The chronic liver disease leads to hepatocellular carcinoma (HCC), which is one of the leading causes of cancer death, and liver failure (1). Nonalcoholic fatty liver disease (NAFLD) or metabolic associated fatty liver disease (MAFLD) is one of the causes of chronic liver disease (2-4). Patients with NAFLD or MAFLD have been increasing worldwide, thus chronic liver disease has emerged as an economic and health burden (5-8).
Liver fibrosis is the important predictive factor for HCC and prognosis in patients with chronic liver disease (9-11). Liver biopsy is the gold standard to assess liver fibrosis, but it has several limitations including invasiveness, sampling error, and inter- and intra-observer reproducibility (12). Thus, several noninvasive methods to estimate liver features including liver fibrosis have been developed and used in clinical practice to resolve these limitations (13-20). Transient elastography is the first approved ultrasound-based elastography that measures liver stiffness to estimate liver fibrosis (13). However, one limitation of transient elastography is the absence of a B-mode ultrasound image, whereas shear wave measurement (SWM) is integrated into a conventional B-mode ultrasound with liver stiffness that simultaneously measures a B-mode image using the same machine (21).
Chronic liver disease is widely distributed in the general population, and high-risk patients for HCC (advanced fibrosis) should be detected among a large population. SWM can simultaneously measure liver stiffness following B-mode ultrasound, thus it may be useful for diagnosing liver fibrosis in large populations such as health checkups. SWM generally is used to diagnose liver fibrosis in patients diagnosed with chronic liver disease; however, its usability to detect advanced fibrosis in the general population is unknown. In this prospective study, we measured the SWM in health checkups that represented the general population and examined its diagnostic accuracy for advanced fibrosis to address this knowledge gap.
Methods
Study design
This prospective study was registered with the University Hospital Medical Information Network clinical trial registry (UMIN000041609). A total of 2,685 health checkup subjects presenting to Musashino Red Cross Hospital between September 2020 to August 2021 were registered in the study. Patients diagnosed with chronic liver disease (chronic hepatitis C and B, and primary biliary cholangitis) and subjects who did not agree to have SWM were excluded, thus 2,233 subjects who agreed for SWM were included in the study (Figure 1). Liver stiffness of SWM shear wave velocity (Vs) of ≥1.3 m/s was used as the optimal threshold for any liver fibrosis [fibrosis stage of 0 vs. 1–4 (F0 vs. F1–4)] (21), and magnetic resonance elastography (MRE) was conducted in subjects with SWM Vs of ≥1.3 m/s as a detailed examination. The diagnostic accuracy for advanced fibrosis among the subjects with SWM Vs of ≥1.3 m/s was investigated. Informed consent was obtained using the opt-out method from each patient. This study was approved by the Ethics Review Committee of Musashino Red Cross Hospital (No. 1107). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All authors had access to the study data and reviewed and approved the final manuscript.

Clinical and laboratory evaluation
The patient’s age, sex, height, weight, abdominal circumference, alcohol consumption, and current medication were recorded. Blood count and biochemical tests were simultaneously conducted with a physical examination. An alcohol consumption of ≥15 drinks/week for males and ≥10 drinks/week for females were defined as alcohol use (22).
Ultrasound diagnosis and SWM
Ultrasonography was simultaneously performed using ARIETTA 850 (FUJIFILM Healthcare, Tokyo, Japan) with a physical examination. Technicians were blinded to all clinical and biochemical information. Any ultrasonographic findings of parenchymal brightness, liver-to-kidney contrast, deep beam attenuation, bright vessel walls, and gallbladder wall definition were defined as indication of fatty liver (23). SWM was measured following the conventional B-mode ultrasonography. SWM Vs can be measured using B-mode ultrasound with the region of interest’s exact location (yellow box of Figure 2A). SWM Vs was measured more than five times and its median value (m/s) was calculated.
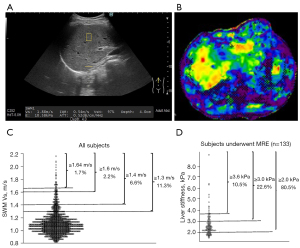
The previous study with SWM and liver biopsy demonstrated that subjects with SWM velocity (Vs) of <1.3 m/s had very low risk of any fibrosis (F0 vs. F1–4) (21), so we defined subjects with SWM Vs of ≥1.3 m/s as those requiring detailed examination of liver fibrosis by MRE. The cutoff values for F1, F2, F3 and F4 for SWM Vs are 1.30, 1.47, 1.81 and 2.0 respectively as previously reported (21,24).
MRE
MRE was performed using Signa HDxt 1.5T (GE Medical Systems, Waukesha, WI, USA) and MR Touch (GE Healthcare), as previously described (25). MRE value was obtained by one image analyst who was blinded to all clinical and biochemical information. Shear waves were generated by external vibration at 60 Hz using a passive driver as a vibration device. All processing steps were automatic, without manual intervention, and yielded quantitative images of tissue shear stiffness in kPa. In this study, the region of interest was placed as large as possible at the right hepatic lobe on each slice of the stiffness map, carefully avoiding the liver surface, liver edge, gallbladder, blood vessels, bile ducts, tumors and artefacts. The mean stiffness value of three circular regions of interest placed at different slices was used for analysis.
MRE has the highest diagnostic accuracy for liver fibrosis than other noninvasive modalities (26) and is permitted to use as inclusion criteria and endpoint in clinical trials instead of liver biopsy (27,28), thus it was used as the gold standard for liver fibrosis in this study. Subjects with a liver stiffness of ≥3.62 kPa by MRE were defined with advanced liver fibrosis based on previous studies. The cutoff values for F1, F2, F3 and F4 for MRE are 2.61, 2.97, 3.62, and 4.69 kPa respectively as previously reported (21,24,29).
Statistical analysis
Subject characteristics were compared using the T-test or Fisher’s exact test. The best threshold of SWM Vs was determined using the receiver operating characteristics curve (ROC) analysis and the Youden index. Correlations between two variables were tested using Pearson’s correlation analysis. he logistic regression analysis was used for the multivariable analysis. Age, gender, sex, body mass index (BMI), hypertension, diabetes mellitus (DM), dyslipidemia, and alcohol use were chosen as a priori factors and used for multivariable analysis. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Shimotsuke, Japan), a graphical user interface for R version 3.2.2 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Subject characteristics
A total of 2,233 subjects who agreed to SWM were included in the study. Subject characteristics are shown in Table 1. The mean ± standard deviation (SD) age and BMI were 56.5±12.1 years and 22.8±3.4 kg/m2, respectively. The mean ± SD SWM was 1.1±0.2 m/s, and SWM Vs of ≥1.3 m/s was observed in 253 subjects (11.3%). The comparison of subjects with SWM Vs of ≥1.3 m/s and those with <1.3 m/s revealed that the presence of fatty liver, hypertension, DM, and dyslipidemia was significantly higher in subjects with SWM Vs of ≥1.3 m/s.
Table 1
| Characteristics | All subjects (n=2,233) | Subject with any fibrosis: SWM Vs ≥1.3 m/s (n=253) |
Subject with no fibrosis: SWM Vs <1.3 m/s (n=1,980) |
P value |
|---|---|---|---|---|
| Age (years) | 56.5±12.1 | 60.2±12.5 | 56.1±11.9 | <0.001 |
| Male gender | 1,234 (55.3) | 176 (69.6) | 1,058 (53.4) | <0.001 |
| BMI (kg/m2) | 22.8±3.4 | 23.4±4.6 | 22.7±3.2 | 0.001 |
| Abdominal circumference (cm) | 85.0±9.6 | 87.2±13 | 84.8±9.1 | <0.001 |
| AST (IU/L) | 23.5±9.2 | 27.4±17 | 23.0±7.5 | <0.001 |
| ALT (IU/L) | 23.6±17 | 30.2±29 | 22.8±14 | <0.001 |
| GGT (IU/L) | 36.3±42 | 46.4±47 | 35.0±41 | <0.001 |
| Albumin (g/dL) | 4.3±0.3 | 4.3±0.3 | 4.3±0.2 | 0.09 |
| Platelet (109/L) | 235±55 | 223±57 | 236±55 | <0.001 |
| Total cholesterol (mg/dL) | 209±34 | 204±34 | 209±34 | 0.04 |
| Triglycerides (mg/dL) | 105±85 | 119±101 | 103±82 | 0.006 |
| SWM Vs (m/s) | 1.1±0.2 | 1.5±0.2 | 1.1±0.1 | <0.001 |
| HbA1c (%) | 5.7±0.5 | 5.9±0.7 | 5.7±0.5 | <0.001 |
| Fatty liver | 871 (39.0) | 176 (69.6) | 1,058 (53.4) | 0.009 |
| Hypertension | 520 (23.3) | 85 (33.6) | 435 (22.0) | <0.001 |
| Diabetes mellitus | 193 (8.6) | 36 (14.2) | 157 (7.9) | 0.002 |
| Dyslipidemia | 498 (22.3) | 83 (32.8) | 415 (21.0) | <0.001 |
| Alcohol use | 108 (4.8) | 14 (5.5) | 94 (4.7) | 0.536 |
Data are shown as mean ± standard deviation and n (%). SWM, shear wave measurement; Vs, shear wave velocity; BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transferase.
Subjects with SWM Vs of ≥1.3 m/s were offered with MRE. After excluding the subjects who refused for MRE (n=118) and MRE measurement failure (n=2), 133 subjects underwent MRE (Figure 1).
The distribution of SWM Vs and in all subjects and liver stiffness by MRE
Representative image of SWM measurement and MRE were shown in Figure 2A,2B. The distribution of SWM Vs in all subjects is shown in Figure 2C. The mean ± SD SWM Vs was 1.1±0.2 m/s. With SWM Vs threshold of ≥1.3, ≥1.4, ≥1.5, ≥1.6, ≥1.7, and ≥1.8 m/s, 253 (11.3%), 148 (6.6%), 85 (3.8%), 50 (2.2%), 13 (0.6%), and 7 (0.3%) felled into the threshold. SWM-based fibrosis stage 0, 1, 2, 3, and 4 were 1,980 (88.7%), 151 (6.7%), 89 (4.0%), 9 (0.4%) and 4 (0.2%), respectively. A total of 925 subjects (41.4%) showed Fibrosis-4 (FIB-4) index ≥1.3, the threshold recommended detailed examination of liver asymptomatic fibrosis in Japan Society of Hepatology (JSH) guideline (22).
The distribution of liver stiffness by MRE in 133 subjects is shown in Figure 2D. With liver stiffness threshold of ≥2.0, ≥3.0, ≥4.0, and ≥5.0 kPa, 107 (80.5%), 30 (22.6%), 10 (7.5%), and 5 (3.8%) felled into the threshold. MRE-based fibrosis stage 0, 1, 2, 3, and 4 were 87 (65.4%), 14 (10.5%), 17 (12.8%), 9 (6.8%) and 6 (4.5%), respectively.
In 133 subjects underwent MRE, Pearson’s correlation analysis between SWM Vs and liver stiffness by MRE revealed a significant correlation (r=0.28; P=0.001).
The diagnostic accuracy of SWM for advanced fibrosis
Among the subjects who measured MRE, 14 (10.5%) had advanced fibrosis (MRE of ≥3.62 kPa). With SWM Vs threshold of ≥1.4, ≥1.5, ≥1.6, ≥1.7, ≥1.8, and ≥1.9 m/s, positive predictive values (PPVs) of SWM Vs for advanced fibrosis were 17.7%, 23.7%, 33.3%, 40.0%, 50.0%, and 60.0%, respectively (Figure 3). Using the ROC analysis and the Youden index, the best SWM threshold for advanced fibrosis was 1.64 m/s. With SWM Vs threshold of ≥1.64 m/s, subjects were narrowed down to 37 (1.7%), and sensitivity, specificity, PPV, and negative predictive value (NPV) for advanced fibrosis were 53.3%, 92.4%, 47.1%, and 94.0%, respectively, among these subjects.

Factors associated with advanced fibrosis
Factors associated with advanced fibrosis were examined in subjects with MRE measurement. The univariable analysis revealed that SWM Vs of ≥1.64 m/s was associated with advanced fibrosis and the odds ratio (OR) [95% confidence interval (CI)] was 13.8 (4.1–47) (P<0.001, Figure 4). The multivariable analysis, after adjusting the age, sex, BMI, hypertension, DM, dyslipidemia, and alcohol use, revealed that SWM Vs of ≥1.64 m/s was the significant factor for advanced fibrosis with an OR (95% CI) of 14.5 (3.4–62) (P<0.001).

Discussion
Main findings
This prospective study demonstrated that SWM was the significant predictor of advanced fibrosis in health checkups.
The OR of SWM Vs of ≥1.64 m/s was 14.5 even after excluding the low-risk subjects (SWM Vs of <1.3 m/s).
SWM is integrated into conventional B-mode ultrasound and can be simultaneously measured with the B-mode image, thus it may be used as a screening tool for liver fibrosis in large populations.
Context with published literature
Liver biopsy has several limitations, including invasiveness, sampling error, and observer reproducibility (12); therefore, several noninvasive and objective modalities for liver fibrosis have been developed. Serum fibrosis markers are easily and widely available, thus, using them as the first screening tool in the general population is recommended (22). Meanwhile, ultrasound-based elastography has higher diagnostic accuracy for liver fibrosis than serum markers and is recommended as a detailed examination (22). In the present study population, 925 subjects (41.4%) showed FIB-4 index ≥1.3, while using the SWM Vs threshold of ≥1.64 m/s, subjects were narrowed down to 1.7%. The PPV of SWM for advanced fibrosis was 47.1%, suggesting that SWM is more efficient in narrowing down high-risk cases for primary screening. One limitation of ultrasound-based elastography, such as transient elastography, is its blindness to the exact localization of the region of interest and its unsuitable application to large populations (26). Thus, SWM has been developed and used in clinical practice to mitigate the limitation. SWM can simultaneously measure liver stiffness following B-mode ultrasound and has high diagnostic accuracy for liver fibrosis, thus we hypothesized its use as a screening tool for liver fibrosis in large populations such as health checkups.
Several studies demonstrated high diagnostic accuracy of ultrasound-based elastography for liver fibrosis; however, these studies were conducted in patients diagnosed with chronic liver disease (14,30). Additionally, patients with chronic liver disease are distributed in the general population and a method to detect advanced fibrosis in the general population is an unmet need. Studies that measured ultrasound-based elastography in the general population are limited and the utility of ultrasound-based elastography as a first screening tool for liver fibrosis is still unknown. Some studies have investigated population-based screening trials with transient elastography, but the limitation of these studies includes the absence of detailed examination (liver biopsy or MRE) and the unknown actual proportion of advanced fibrosis (31,32). This study measured MRE as a detailed examination in subjects with an SWM Vs of ≥1.3 m/s [the threshold for any fibrosis (F1–4)]. Using the SWM Vs threshold of ≥1.64 m/s, subjects were narrowed down to 1.7%, and PPV for advanced fibrosis in these subjects was 47.1%. SWM can be easily measured following the conventional B-mode examination, thus it may be used as a screening tool for liver fibrosis in large populations.
Strength and limitation
This prospective study performed SWM in over 2,000 subjects. Furthermore, MRE was performed in over 100 subjects as a detailed examination. This study was conducted in a single center, and all protocols, including SWM and MRE measurements, underwent an aligned protocol. The SWM Vs threshold of ≥1.3 m/s was used. SWM Vs of ≥1.3 m/s is the threshold for any fibrosis (F0 vs. F1–4) and MRE was measured in these subjects. MRE was not measured in subjects with an SWM Vs of <1.3 m/s, and liver fibrosis of these subjects were not precisely evaluated. However, a detailed examination is not recommended in subjects with a low risk of advanced fibrosis by the American Association for the Study of Liver Diseases because it is not cost-effective (33). Therefore, we think that this study protocol is reasonable. Furthermore, the study was conducted in a single center in Japan. The prevalence of advanced fibrosis differs among regions and ethnicity (2), thus a further study including other centers and regions is needed. Recently, other viscosity-related elastography parameters were reported as tools for evaluation of liver fibrosis (34). However, these parameters were lacked in this study. Comparison to these parameters may be effective for liver fibrosis screening and a future study is needed.
Future implications
This study demonstrated that SWM has a high diagnostic accuracy for advanced fibrosis in the general population and may be used as a screening tool for liver fibrosis.
Chronic liver diseases, such as NAFLD, have been increasing worldwide and have emerged as a health problem (2). Detecting subjects with advanced fibrosis among the general population is an important issue, but the effective protocol has not been established. The guidelines recommended FIB-4 index as a first screening because of its high NPV for advanced fibrosis and it is associated with prognosis (22,33,35,36). However, FIB-4 value increases in elderly subjects and narrowing-down of high-risk subjects by FIB-4 is inadequate (37,38), especially in regions with many elderly people. Undergoing MRE or biopsy in all subjects is not practical and cost-effective, thus a two-step screening is recommended (22,39,40). With the SWM Vs threshold of ≥1.64 m/s, subjects were narrowed down to 1.7%. MRE application to these limited subjects is easy and cost-effective, thus SWM may be used as a screening tool for liver fibrosis in the general population.
Conclusions
In conclusion, SWM has a high diagnostic accuracy for advanced fibrosis and may be used as a screening tool for liver fibrosis in the general population.
Acknowledgments
The authors would like to extend their gratitude to Shinobu Nagai and all staffs of Musashino Red Cross hospital Medical Examination Center for the assistance.
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-511/coif). NI received lecture fees from Gilead Sciences Inc., and Abbvie. MK received lecture fees from Gilead Sciences Inc., Abbvie, Eisai Co., Ltd., Bayer AG, and Otsuka Holdings Co., Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013;10:686-90. [Crossref] [PubMed]
- Eslam M, Sanyal AJ, George J. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020;158:1999-2014.e1. [Crossref] [PubMed]
- Yoneda M, Yamamoto T, Honda Y, Imajo K, Ogawa Y, Kessoku T, Kobayashi T, Nogami A, Higurashi T, Kato S, Hosono K, Saito S, Nakajima A. Risk of cardiovascular disease in patients with fatty liver disease as defined from the metabolic dysfunction associated fatty liver disease or nonalcoholic fatty liver disease point of view: a retrospective nationwide claims database study in Japan. J Gastroenterol 2021;56:1022-32. [Crossref] [PubMed]
- Tampi RP, Wong VW, Wong GL, Shu SS, Chan HL, Fung J, Stepanova M, Younossi ZM. Modelling the economic and clinical burden of non-alcoholic steatohepatitis in East Asia: Data from Hong Kong. Hepatol Res 2020;50:1024-31. [Crossref] [PubMed]
- Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2021;18:223-38. [Crossref] [PubMed]
- Eguchi Y, Wong G, Lee IH, Akhtar O, Lopes R, Sumida Y. Hepatocellular carcinoma and other complications of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in Japan: A structured review of published works. Hepatol Res 2021;51:19-30. [Crossref] [PubMed]
- Kawaguchi T, Tsutsumi T, Nakano D, Torimura T. MAFLD: Renovation of clinical practice and disease awareness of fatty liver. Hepatol Res 2022;52:422-32. [Crossref] [PubMed]
- Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, Ekstedt M, Hagstrom H, Nasr P, Stal P, Wong VW, Kechagias S, Hultcrantz R, Loomba R. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017;65:1557-65. [Crossref] [PubMed]
- Higuchi M, Tamaki N, Kurosaki M, Inada K, Kirino S, Yamashita K, Hayakawa Y, Osawa L, Takaura K, Maeyashiki C, Kaneko S, Yasui Y, Takahashi Y, Tsuchiya K, Nakanishi H, Itakura J, Loomba R, Enomoto N, Izumi N. Longitudinal association of magnetic resonance elastography-associated liver stiffness with complications and mortality. Aliment Pharmacol Ther 2022;55:292-301. [Crossref] [PubMed]
- Tamaki N, Higuchi M, Kurosaki M, Loomba R, Izumi N. Risk Difference of Liver-Related and Cardiovascular Events by Liver Fibrosis Status in Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol 2022;20:1171-3.e2.
- Davison BA, Harrison SA, Cotter G, Alkhouri N, Sanyal A, Edwards C, Colca JR, Iwashita J, Koch GG, Dittrich HC. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol 2020;73:1322-32. [Crossref] [PubMed]
- Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019;156:1264-1281.e4. [Crossref] [PubMed]
- Kakegawa T, Sugimoto K, Kuroda H, Suzuki Y, Imajo K, Toyoda HGITHY Liver Study Group. Diagnostic Accuracy of Two-Dimensional Shear Wave Elastography for Liver Fibrosis: A Multicenter Prospective Study. Clin Gastroenterol Hepatol 2022;20:e1478-82.
- Tamaki N, Kurosaki M, Loomba R, Izumi N. Clinical Utility of Mac-2 Binding Protein Glycosylation Isomer in Chronic Liver Diseases. Ann Lab Med 2021;41:16-24. [Crossref] [PubMed]
- Tamaki N, Ajmera V, Loomba R. Non-invasive methods for imaging hepatic steatosis and their clinical importance in NAFLD. Nat Rev Endocrinol 2022;18:55-66. [Crossref] [PubMed]
- Tamaki N, Kurosaki M, Yasui Y, Tsuchiya K, Izumi N. Attenuation coefficient (ATT) measurement for liver fat quantification in chronic liver disease. J Med Ultrason (2001) 2021;48:481-7.
- Okanoue T, Shima T, Mitsumoto Y, Umemura A, Yamaguchi K, Itoh Y, Yoneda M, Nakajima A, Mizukoshi E, Kaneko S, Harada K. Artificial intelligence/neural network system for the screening of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatol Res 2021;51:554-69. [Crossref] [PubMed]
- Nakamura A, Yamamoto K, Takeda R, Yamada R, Kubo A, Morikawa K, et al. The potential of soluble CD14 in discriminating nonalcoholic steatohepatitis from nonalcoholic fatty liver disease. Hepatol Res 2022;52:508-21. [Crossref] [PubMed]
- Tamaki N, Kurosaki M, Huang DQ, Loomba R. Noninvasive assessment of liver fibrosis and its clinical significance in nonalcoholic fatty liver disease. Hepatol Res 2022;52:497-507. [Crossref] [PubMed]
- Yada N, Tamaki N, Koizumi Y, Hirooka M, Nakashima O, Hiasa Y, Izumi N, Kudo M. Diagnosis of Fibrosis and Activity by a Combined Use of Strain and Shear Wave Imaging in Patients with Liver Disease. Dig Dis 2017;35:515-20. [Crossref] [PubMed]
- Tokushige K, Ikejima K, Ono M, Eguchi Y, Kamada Y, Itoh Y, Akuta N, Yoneda M, Iwasa M, Yoneda M, Otsuka M, Tamaki N, Kogiso T, Miwa H, Chayama K, Enomoto N, Shimosegawa T, Takehara T, Koike K. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. J Gastroenterol 2021;56:951-63. [Crossref] [PubMed]
- Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, Clark JM. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 2011;54:1082-90. [Crossref] [PubMed]
- Yada N, Sakurai T, Minami T, Arizumi T, Takita M, Hagiwara S, Ueshima K, Ida H, Nishida N, Kudo M. A Newly Developed Shear Wave Elastography Modality: With a Unique Reliability Index. Oncology 2015;89:53-9. [Crossref] [PubMed]
- Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, Fujita K, Yoneda M, Taguri M, Hyogo H, Sumida Y, Ono M, Eguchi Y, Inoue T, Yamanaka T, Wada K, Saito S, Nakajima A. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology 2016;150:626-37.e7. [Crossref] [PubMed]
- Tapper EB, Loomba R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat Rev Gastroenterol Hepatol 2018;15:274-82. [Crossref] [PubMed]
- Nakajima A, Eguchi Y, Yoneda M, Imajo K, Tamaki N, Suganami H, Nojima T, Tanigawa R, Iizuka M, Iida Y, Loomba R. Randomised clinical trial: Pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), versus placebo in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2021;54:1263-77. [Crossref] [PubMed]
- Loomba R, Ratziu V, Harrison SA. Expert Panel Review to Compare FDA and EMA Guidance on Drug Development and Endpoints in Nonalcoholic Steatohepatitis. Gastroenterology 2022;162:680-8. [Crossref] [PubMed]
- Hsu C, Caussy C, Imajo K, Chen J, Singh S, Kaulback K, Le MD, Hooker J, Tu X, Bettencourt R, Yin M, Sirlin CB, Ehman RL, Nakajima A, Loomba R. Magnetic Resonance vs Transient Elastography Analysis of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Pooled Analysis of Individual Participants. Clin Gastroenterol Hepatol 2019;17:630-7.e8. [Crossref] [PubMed]
- Sharpton SR, Tamaki N, Bettencourt R, Madamba E, Jung J, Liu A, Behling C, Valasek MA, Loomba R. Diagnostic accuracy of two-dimensional shear wave elastography and transient elastography in nonalcoholic fatty liver disease. Therap Adv Gastroenterol 2021;14:17562848211050436. [Crossref] [PubMed]
- Roulot D, Costes JL, Buyck JF, Warzocha U, Gambier N, Czernichow S, Le Clesiau H, Beaugrand M. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut 2011;60:977-84. [Crossref] [PubMed]
- Caballería L, Pera G, Arteaga I, Rodríguez L, Alumà A, Morillas RM, et al. High Prevalence of Liver Fibrosis Among European Adults With Unknown Liver Disease: A Population-Based Study. Clin Gastroenterol Hepatol 2018;16:1138-45.e5. [Crossref] [PubMed]
- Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328-57. [Crossref] [PubMed]
- Reiter R, Shahryari M, Tzschätzsch H, Haas M, Bayerl C, Siegmund B, Hamm B, Asbach P, Braun J, Sack I. Influence of fibrosis progression on the viscous properties of in vivo liver tissue elucidated by shear wave dispersion in multifrequency MR elastography. J Mech Behav Biomed Mater 2021;121:104645. [Crossref] [PubMed]
- Tamaki N, Kurosaki M, Yasui Y, Mori N, Tsuji K, Hasebe C, Joko K, Akahane T, Furuta K, Kobashi H, Kimura H, Yagisawa H, Marusawa H, Kondo M, Kojima Y, Yoshida H, Uchida Y, Loomba R, Izumi N. Change in Fibrosis 4 Index as Predictor of High Risk of Incident Hepatocellular Carcinoma After Eradication of Hepatitis C Virus. Clin Infect Dis 2021;73:e3349-54. [Crossref] [PubMed]
- Mao X, Liu Z, Shi O, Yu K, Jiang Y, Jin L, Zhang T, Chen X. Non-invasive fibrosis markers are associated with mortality risk in both general populations and non-alcoholic fatty liver disease patients. Hepatol Res 2021;51:90-101. [Crossref] [PubMed]
- Ishiba H, Sumida Y, Tanaka S, Yoneda M, Hyogo H, Ono M, et al. The novel cutoff points for the FIB4 index categorized by age increase the diagnostic accuracy in NAFLD: a multi-center study. J Gastroenterol 2018;53:1216-24. [Crossref] [PubMed]
- Tamaki N, Higuchi M, Kurosaki M, Kirino S, Osawa L, Watakabe K, Wang W, Okada M, Shimizu T, Takaura K, Takada H, Kaneko S, Yasui Y, Tsuchiya K, Nakanishi H, Itakura J, Takahashi Y, Enomoto N, Izumi N. Wisteria floribunda agglutinin-positive mac-2 binding protein as an age-independent fibrosis marker in nonalcoholic fatty liver disease. Sci Rep 2019;9:10109. [Crossref] [PubMed]
- Tamaki N, Imajo K, Sharpton SR, Jung J, Sutter N, Kawamura N, Yoneda M, Valasek MA, Behling C, Sirlin CB, Kurosaki M, Izumi N, Nakajima A, Loomba R. Two-Step Strategy, FIB-4 Followed by Magnetic Resonance Elastography, for Detecting Advanced Fibrosis in NAFLD. Clin Gastroenterol Hepatol 2023;21:380-7.e3. [Crossref] [PubMed]
- Tamaki N, Imajo K, Sharpton S, Jung J, Kawamura N, Yoneda M, Valasek MA, Behling C, Sirlin CB, Nakajima A, Loomba R. Magnetic resonance elastography plus Fibrosis-4 versus FibroScan-aspartate aminotransferase in detection of candidates for pharmacological treatment of NASH-related fibrosis. Hepatology 2022;75:661-72. [Crossref] [PubMed]


