
Gorham-Stout disease of spine accompanying chylothorax: a case description
Introduction
Gorham-Stout disease (GSD) is a rare disorder characterized by progressive osteolysis with merely just over 300 cases being reported in the literature. It is also known as “Gorham-Stout syndrome”, “phantom bone disease”, “massive osteolysis”, and “vanishing bone disease”. In 1838, Jackson was first to report this disease as a “boneless arm” case and published it in the Boston Medical and Surgical Journal in 1872 (1). The entire humerus of a 12-year-old boy gradually disappeared over a period of several years. Based on 24 cases, Gorham and Stout presented the disease as a syndrome in 1955. They reported that massive osteolysis was always associated with an angiomatosis of blood and sometimes of lymphatic vessels (2). Despite substantial efforts to understand this disease (3), the etiology and pathogenesis of GSD are still unclear and uncertain. Three hypotheses have been proposed, relating to osteoclasts, angiogenesis/lymphangiogenesis, and osteoblast function, respectively (4). Thus far, approximately 50 cases affecting the spine have been reported (5). Here, we report a case of a GSD patient with involvement of the cervical and thoracic vertebrae, ribs, partial paraspinal soft tissue, and accompanying chylothorax.
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
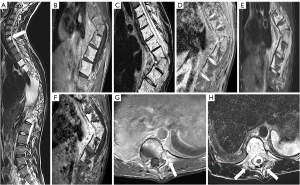
A 23-year-old man, with no previous traumatic history, presented with progressive kyphosis of the thoracic spine and chest-back pain. The symptoms had been ongoing for more than 3 years and had worsened over the previous year. Physical examination revealed tenderness and percussion pain in the thoracic vertebra spinous process and intervertebral space. Neurological examination was normal. The results of laboratory tests (C-reactive protein, antituberculosis antibody, and rheumatoid factor) were within the normal ranges. X-rays showed thoracic spine kyphoscoliosis (Figure 1). Computed tomography (CT) revealed osteolytic lesions in the C7 and T4–T12 vertebrae and adjacent ribs, along with thoracic spine kyphoscoliosis. The maximum angulation angle was observed at the T8–T9 level (Figure 2). The CT scan also indicated bilateral pleural effusion (Figure 2). Subsequently, the patient underwent a magnetic resonance imaging (MRI) examination. The lesions in the C7 and T4–T12 vertebrae appeared as mixed hyperintensity on T1-weighted images (T1WI) and hyperintensity on T2-weighted images (T2WI), while the unaffected segments showed normal signal intensity. The corresponding ribs and paravertebral muscles showed high signal intensities on both T1WI and T2WI. The lesions displayed hyperintensity on T1-weighted spectral presaturation with inversion-recovery (T1 SPIR) sequences and T2 spectral-attenuated inversion-recovery (SPAIR) sequences, and after the injection of a gadolinium-based contrast agent, showed obvious enhancement on T1 SPIR imaging (Figure 3). Whole-body bone scintigraphy was performed, revealing abnormally active metabolism in the T8–T10 vertebrae. The chylous pleural fluid test was positive. The diagnostic puncture of pleural effusion indicated that pleural effusion contained more lymphocytes than neutrophils. After multidisciplinary treatment (MDT) discussion with the doctors from the departments of thoracic surgery, respiratory, spinal surgery, and anesthesiology, the patient was considered to have a vasculature-related disease, likely an invasive hemangioma. During the surgery procedure, the surgeons encountered bone thinning and destruction of the T4–T12 vertebrae and the connection of the pleural cavity, spinal canal, and lamina. Light red chylous fluid gushed out in sync with the respiratory rate from the pleural cavity to lamina trophic foramen, paravertebral soft tissues, bony destruction, and other parts. Eventually, a tissue biopsy of the spine was performed, and the postoperative pathology showed many malformed dilated lymphatic vessels in the bone and surrounding soft tissue, which tested positive for D2-40 staining (Figure 4). Based on the patient’s clinical performance and radiological and pathological findings, a diagnosis of GSD was confirmed. The patient subsequently underwent surgical treatment (Figure 5).



Discussion
In the case described here, GSD was characterized by progressive osteolysis and proliferation of lymphatic vessels, leading to the destruction and absorption of bone. GSD can occur at any age, but it usually affects children and young adults, with the average age of diagnosis being 25 years. There is no gender or race predilection for GSD (6,7). While every bone in the body may be potentially involved, it commonly involves the maxilla, mandible, clavicle, ribs, pelvis, and femur (3). Johnson and McClure classified the disease into an early stage characterized by intramedullary and cortical lucencies and a later stage characterized by destruction and resorption of bone (8). Primary involvement of the spine is less common (7). The first comprehensive study of spinal GSD demonstrated that the cervical and thoracic vertebrae are the most frequently involved regions. The early clinical manifestations of GSD are often subtle and nonspecific. The most common symptoms experienced by patients with GSD include pain, dysfunction, and swelling of the affected area or a long history of chronic dull pain (9). As osteolysis progresses, it can lead to various spinal deformities, such as scoliosis, kyphosis, subluxation, or even spinal dislocation. When the ribs, scapula, or thoracic vertebrae are involved, the extension of lymphangiectasia into the pleural cavity or via invasion of the thoracic duct results in chylothorax (10), which is a severe complication and carries a high mortality rate (11). Spinal GSD is frequently misdiagnosed or missed due to limited awareness of this rare disease. The results of blood routine tests are often normal in patients with GSD, with the possible situation of increased levels of alkaline phosphatase. Diagnosing GSD requires imaging and pathological examination, followed by the exclusion of all other potential causes of osteolysis, such as hereditary, metabolic, oncologic, immune, and infectious diseases, which is quite challenging. X-rays usually show bone absorption and pathological fractures, without periosteal reaction. CT scanning and 3-dimensional reconstruction can show the range of bone destruction more clearly than can X-rays. MRI is useful for ascertaining disease extension and soft tissue involvement, with contrast enhancement at the region of active osteolysis (4,12). Lymphography can be used to evaluate the thoracic duct in the case of chylothorax. Histopathological examination is considered the gold standard. In 1983, Heffez et al. suggested 8 criteria for the diagnosis of GSD: (I) positive biopsy (angiomatous tissue with abnormal lymphatic channels and numerous osteoclasts); (II) absence of cellular atypia; (III) minimal or no osteoblastic response and absence of dystrophic calcifications; (IV) evidence of progressive local bone resorption; (V) a lesion that is not ulcerative and does not provoke cortical expansion; (VI) absence of visceral involvement; (VII) osteolytic radiographic pattern; and (VIII) negative hereditary, metabolic, neoplastic, immunological, and infectious etiology (13). In our case, all criteria were met (Table 1). Due to the rarity of GSD, there is no standard treatment (14). Primary strategies include surgery, radiotherapy, and pharmaceutical treatment. Surgery aims to reduce or prevent fluid accumulation in the pleural cavity and stabilize the affected areas of the skeleton (7,14). Surgical intervention may be the preferred option for severe and unstable deformities (9). Radiotherapy has been used in situations where surgery is not possible or in combination with surgery (15). Drug therapy includes the use of bisphosphonates and interferon alfa-2b, androgens, calcium fluoride, vitamins, somatotropic hormone, calcitonin, and cytotoxic drugs such as actinomycin D or cisplatin.
Table 1
| Criteria | Clinical case fulfillment |
|---|---|
| (I) Positive biopsy (angiomatous tissue with abnormal lymphatic channels and numerous osteoclasts) | Abnormal lymphatic channels |
| (II) Absence of cellular atypia | √ |
| (III) Minimal or no osteoblastic response and absence of dystrophic calcifications | √ |
| (IV) Evidence of progressive local bone resorption | √ |
| (V) A lesion that is not ulcerative and does not provoke cortical expansion | √ |
| (VI) Absence of visceral involvement | √ |
| (VII) Osteolytic radiographic pattern | √ |
| (VIII) Negative hereditary, metabolic, neoplastic, immunological, and infectious etiology | √ |
Conclusions
This report describes a case of GSD involving the cervical and thoracic spine, accompanied by chylothorax. The characteristic features include cortical resorption, progressive osteolysis, changes in adjacent soft tissue, and spinal deformity. Although GSD is extremely rare, it should be considered in patients presenting with osteolytic spinal deformity and chylothorax, particularly in the absence of a mass or infection. GSD is a rare disorder of the musculoskeletal system that lacks clear guidelines for therapy. Therefore, physicians need to develop appropriate treatment plans based on the patient’s condition. Through studying this rare case, physicians may have a higher awareness of this condition, which is critical, as early detection and treatment can help in improving the outcome. When confronted with seemingly unrelated multisystem symptoms, a search for the underlying connections among various symptoms is recommended in conjunction with an extensive literature review to locate relevant information and enhance understanding of rare diseases. At last, fostering multidisciplinary collaboration is paramount in managing such cases.
Acknowledgments
Funding: This work was supported in part by the President Foundation of the Third Affiliated Hospital of Southern Medical University (No. YM2021012).
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-160/coif). The authors report that this work was in part supported by the President Foundation of the Third Affiliated Hospital of Southern Medical University (No. YM2021012). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the relevant institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Absorption of the humerus after fracture. Boston Med J 1872;87:245-7.
- Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J Bone Joint Surg Am 1955;37-A:985-1004.
- Dellinger MT, Garg N, Olsen BR. Viewpoints on vessels and vanishing bones in Gorham-Stout disease. Bone 2014;63:47-52. [Crossref] [PubMed]
- Angelini A, Mosele N, Pagliarini E, Ruggieri P. Current concepts from diagnosis to management in Gorham-Stout disease: a systematic narrative review of about 350 cases. EFORT Open Rev 2022;7:35-48. [Crossref] [PubMed]
- Schell A, Rhee JM, Allen A, Andras L, Zhou F. Surgical management of Gorham disease involving the upper cervical spine with occipito-cervical-thoracic fusion: a case report. Spine J 2016;16:e467-72. [Crossref] [PubMed]
- Jagtap R, Gupta S, Lamfon A, Ruprecht A, Schlott B, Hardeman J, Kashtwari D. Gorham-Stout disease of the mandible: case report and review of literature of a rare type of osteolysis. Oral Radiol 2020;36:389-94. [Crossref] [PubMed]
- Ruggieri P, Montalti M, Angelini A, Alberghini M, Mercuri M. Gorham-Stout disease: the experience of the Rizzoli Institute and review of the literature. Skeletal Radiol 2011;40:1391-7. [Crossref] [PubMed]
- JOHNSON PM. McCLURE JG. Observations on massive osteolysis; a review of the literature and report of a case. Radiology 1958;71:28-42. [Crossref] [PubMed]
- Du CZ, Li S, Xu L, Zhou QS, Zhu ZZ, Sun X, Qiu Y. Spinal Gorham-Stout syndrome: radiological changes and spinal deformities. Quant Imaging Med Surg 2019;9:565-78. [Crossref] [PubMed]
- Rossi M, Buonuomo PS, Battafarano G, Conforti A, Mariani E, Algeri M, Pelle S, D'Agostini M, Macchiaiolo M, De Vito R, Gonfiantini MV, Jenkner A, Rana I, Bartuli A, Del Fattore A. Dissecting the mechanisms of bone loss in Gorham-Stout disease. Bone 2020;130:115068. [Crossref] [PubMed]
- Tie ML, Poland GA, Rosenow EC 3rd. Chylothorax in Gorham's syndrome. A common complication of a rare disease. Chest 1994;105:208-13. [Crossref] [PubMed]
- Ceroni D, De Coulon G, Regusci M, Kaelin A. Gorham-Stout disease of costo-vertebral localization: radiographic, scintigraphic, computed tomography, and magnetic resonance imaging findings. Acta Radiol 2004;45:464-8. [Crossref] [PubMed]
- Heffez L, Doku HC, Carter BL, Feeney JE. Perspectives on massive osteolysis. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol 1983;55:331-43. [Crossref] [PubMed]
- Patel DV. Gorham's disease or massive osteolysis. Clin Med Res 2005;3:65-74.
- Heyd R, Micke O, Surholt C, Berger B, Martini C, Füller J, Schimpke T, Seegenschmiedt MHGerman Cooperative Group on Radiotherapy for Benign Diseases (GCG-BD). Radiation therapy for Gorham-Stout syndrome: results of a national patterns-of-care study and literature review. Int J Radiat Oncol Biol Phys 2011;81:e179-85. [Crossref] [PubMed]



