
Isolated thyroid gland metastasis from bladder urothelial carcinoma: a rare description and literature analysis
Introduction
Autopsies reveal a 2–17% incidence of thyroid metastases, which are rarely detected in clinical practice and easily overlooked, likely on account of the long course of primary cancer and inconspicuous symptoms of thyroid metastases (1). Lin et al. found that the incidence of thyroid metastases was approximately 1.4% in 1,013 thyroid cancer patients, and most of the patients’ primary cancers were in an advanced state, generally involving multiple organs, and had a poor prognosis (2). Primary carcinomas that metastasize to the thyroid are commonly renal cell carcinoma, followed by colorectal cancer, lung cancer, and breast cancer (3). Metastasis to the thyroid gland from primary bladder urothelial cancer is very rare. A MEDLINE database search identified only two cases of urothelial carcinoma of the bladder with metastasis to the thyroid reported in the literature, that we now bring to a total of three cases by including the case reported herein. To better summarize metastasis of bladder urothelial carcinoma to the thyroid gland, we documented this rare case and performed a literature review.
Case presentation
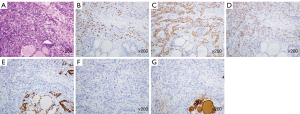
A 60-year-old male patient was admitted to the hospital on account of a bladder mass lesion. Urological ultrasound showed a hypoechoic mass on the left lateral wall of the bladder, with an irregular shape and a strip of blood flow. Radical cystectomy with double ureterostomy was performed directly, which can effectively reduce postoperative complications. Postoperative pathological results showed high-grade invasive urothelial carcinoma of the bladder (Figure 1A). Although chemotherapy was recommended according to postoperative pathological findings, the patient refused, and it was not carried out. Immunohistochemistry (IHC) was positive for urothelial malignancy [CK7(+) (Figure 1B), P63(+) (Figure 1C), GATA-3(+) (Figure 1D)], and the results indicated high-grade invasive urothelial carcinoma (T3bN0M0). Preoperative chest computed tomography (CT) for bladder cancer contained a thyroid bed, suggesting only uneven thyroid echo. The patient underwent ultrasonography 7 months after surgery for the neck mass, revealing a nodule in the right lobe of the thyroid gland, approximately 3.45 cm × 2.42 cm in size, with cystic and solid mixture. The solid part was dominant and hypoechoic, irregular in shape, and the boundary was not clear (Figure 2A); there were visible dotted strips of strong echo, and a visible dotted stripe of colored blood flow (Figure 2B). According to the Chinese reporting and data system, the ultrasound indicated that the nodules belonged to category 3. A month later, ultrasound examination was performed again due to symptoms of compression and significant enlargement of the mass. The right lobe of the thyroid gland displayed a cystic and solid mixed nodule with a size of about 6.64 cm × 4.28 cm, with mainly cystic components (Figure 2C). A fine needle aspiration (FNA) of the thyroid gland nodule was performed, the results of which indicated atypical follicular cells of unclear significance (Figure 3), Bethesda classification III, and Braf V600E negativity. Given that the local compression of thyroid nodules was obvious, swallowing was uncomfortable. The nodule continued to grow, and the patient was readmitted to the hospital 8 months after the operation. Laboratory tests showed the presence of autoimmune thyroiditis. Thyroid enhanced CT scan did not show lymph node metastasis, and “total thyroidectomy” was performed. During the operation, the right thyroid nodule was significantly enlarged and strongly adhered to the surrounding tissue. Pathological examination was performed, and hard yellowish areas and cystic solid areas were observed in the right thyroid node which measured 11 cm × 7.5 cm × 5.5 cm (Figure 4A). The results of thyroid IHC showed P63(+) (Figure 4B), CK7(+) (Figure 4C), GATA-3(+) (Figure 4D), TTF-1(−) (Figure 4E), calcitonin(−) (Figure 4F), and Tg(−) (Figure 4G). The results of special dyeing showed reticular fiber staining (+), which combined with the history and IHC results suggested metastases of urothelial carcinoma. Fluoro18-deoxyglucose positron emission tomography/CT (18F-FDG PET/CT) was performed 4 months after thyroid surgery. Recurrence was observed only in the thyroid bed area after the operation Thus, the patient treated with a 4-week cycle of intravenous gemcitabine on days 1 and 8 and cisplatin on days 2, 3, and 4. No recurrence was observed during the regular follow-up to 18 months after surgery. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.



Discussion
Epidemiology and etiology
Metastases to the thyroid gland are very rare clinically. Approximately 1.4–3% of patients with thyroid cancer have other malignant tumors that metastasize to the thyroid (3). However, the incidence of tumor metastasis to the thyroid has increased in recent years. This situation may be related to the prolonged median survival of patients with malignant tumors and the large number of thyroid cytology puncture examinations (4). Bladder cancer is one of the top 10 most common cancers in the world, and the prevalence and mortality rates of male patients are significantly higher than those of female patients (5). The pathological types of bladder cancer include urothelial carcinoma, squamous cell carcinoma, adenocarcinoma, and small cell carcinoma, among which urothelium carcinoma is the most common, accounting for approximately 90–95% of bladder cancers. Approximately 10–15% of newly diagnosed bladder cancers have metastasized to distant sites, and the most common sites of metastasis are generally the bone, lungs, liver, brain, and lymph nodes (6); thyroid metastases rarely occur. Our search in the MEDLINE database identified two cases of thyroid metastases of bladder urothelial carcinoma. The patient experienced chest pain following transurethral resection of the bladder, multiple metastases were discovered during PET/CT, rib biopsy, and FNA of thyroid nodules confirmed the presence of urothelial metastases, but no further treatment was given, and the patient passed away 6 months later. The first instance of bladder urothelial carcinoma spreading to the thyroid gland was proposed in 2014 (7). The second case, which initially discovered thyroid nodules, was proposed in 2016; subtotal thyroidectomy was performed, and postoperative pathological results suggested undifferentiated carcinoma of the thyroid gland. A CT scan of the chest and abdomen was then performed, revealing that the bladder mass had metastasized to various parts of the body, such as the liver and bones. The postoperative pathological and IHC results suggested that the thyroid tumor originated from the same bladder mass (8). The 3 cases of bladder cancer mentioned in this study were non-muscle-invasive urothelial carcinoma, muscle invasive urothelial carcinoma, and high-grade urinary urothelial carcinoma, respectively.
The thyroid has an abundant blood flow signal of about 560 mL/100 g tissue/minutes, and blood supply is second only to that of the adrenal gland, but other tumors rarely undergo blood-borne metastasis to the thyroid gland. In 1931, Willis proposed that the cause may be the rapid flow of blood, high oxygen saturation, and elevated iodine concentrations that prevent the planting and growth of malignant tumor cells (9). In patients with thyroid diseases, such as thyroid nodules, goiter, and Hashimoto thyroiditis, the chances increase for tumor cells from other tissues or organs metastasizing to the thyroid gland (10,11).
Ultrasound features
Ultrasound is the preferred imaging method for thyroid examination (12). Metastatic thyroid carcinoma can be divided into nodular and diffuse infiltrating types according to its ultrasonographic manifestations. Nodular nonthyroidal malignancies generally present with multiple uneven low echoes, unclear boundaries with surrounding tissues, and irregular edges, and may be accompanied by microcalcification or gravel calcification. Some lesions can invade the envelope, for which color Doppler flow imaging shows rich color blood flow signals, and some patients have metastases from cervical lymph nodes. Ultrasound features in diffuse infiltrating patients suggest diffuse thyroid volume enlargement, uneven low echo or iso-echo echo, and some patients may have cervical lymph node metastases (13). However, such metastases are difficult to distinguish from the primary malignant tumor of the thyroid gland from the image alone, and the fine needle biopsy of the thyroid nodule can be used as an auxiliary examination method for diagnosis.
FNA and IHC
FNA has a high sensitivity and specificity and does not affect patient long-term survival (14,15). The FNA results in this patient suggested atypical follicular cells of unclear significance, Bethesda III. If the pathological results of puncture are unclear, the use of FNA to diagnose metastatic malignancy to the thyroid gland is challenging, and 18F-FDG PET/CT may be used to aid diagnosis (16). IHC can also help to distinguish between primary and secondary tumors, particularly those with histopathological uncertainty (3). In this patient, CK7(+), P63(+), and GATA-3(+) were expressed in the bladder high-grade urothelial carcinoma and thyroid tumor, as revealed by IHC. TTF-1(−), Tg(−), and calcitonin(−) were not expressed in the thyroid tumor. CK7, P63, and GATA-3 have highly specific citations in bladder urothelial carcinoma (17). TTF-1, Tg, and calcitonin are generally expressed in primary thyroid tumors, helping to diagnose thyroid tumors that metastasize from bladder-urinary urothelial carcinoma (18,19). Cytokeratin is usually expressed in the glandular epithelium and urinary urothelium. However, the thyroid gland expresses not only CK7, but also p63 and GATA-3. P63 is a member of the P53 family and is expressed only in multilayer epithelial cells, the bladder, the skin, and the esophagus. GATA binding protein 3 (GATA-3) is a member of the transcription factor GATA family. In addition to being widely expressed in epithelial cells on the lumen surface of the ductal ducts of the mammary glands, GATA-3 is also a new marker of bladder cancer. In the case of this patient, the combination of the history and IHC results indicated a high probability that the tumor of the thyroid gland was the bladder metamorphic epithelial cancer that had metastasized (17,20).
Treatment
Among patients with bladder cancer that has metastasized, chemotherapy is the best way to treat those with bladder urothelial carcinoma, adenocarcinoma, and ≤2 metastases, and surgery plus chemotherapy is the most effective treatment for those with squamous cell carcinoma, >2 metastatic sites, or lymph node metastasis, and is critical for improving prognosis (6). However, if only thyroid metastases are present, the median survival of surgical resection will be prolonged compared with chemotherapy or radiation therapy. The extent of surgical removal of the thyroid gland depends on the extent of the metastatic tumor (21), and the most commonly used surgical method is thyroid lesion glandular lobar plus isthmus resection. The scope of surgery has little impact on survival and prognosis. A crucial consideration is that the cut edge must be negative (22,23). The nodule of the right lobe of the thyroid gland mentioned in this paper adhered to the surrounding tissue, and it was difficult to guarantee a negative incisal margin. In addition to thyroid metastases, in patients with metastases from other organs, surgery is considered a palliative treatment to alleviate symptoms of dyspnea, and combined chemotherapy is an effective treatment measure (21,24). Chemotherapy may kill cancer cells hidden in the circulatory system. The last two cases of bladder urothelial carcinoma mentioned in this study developed thyroid metastases and had undergone thyroidectomy. In the second case, due to the metastasis of various organs throughout the body, subtotal thyroidectomy was performed only to relieve symptoms. The third patient had a local recurrence of the thyroid bed after total thyroidectomy, supplemented by postoperative systemic chemotherapy, with a good prognosis (25,26). The degree of management and surgery of thyroid metastases has not yet received international consensus. Treatment depends on the location of the primary tumor, presence of other metastases, and symptoms caused by thyroid tumors. Generally, thyroidectomy can be performed if only thyroid metastases occur; local recurrence can be supplemented with chemotherapy. Patients with thyroid metastases in organs other than the thyroid gland have a poor prognosis (26).
Conclusions
Thyroid metastasis of bladder urothelial carcinoma is very rare. Although the imaging lacks specificity, patients with a history of bladder urothelial carcinoma and new nodules on the thyroid gland, that in the short term have displayed rapid growth without degression, should consider the possibility of metastasis. Thyroid nodule FNA biopsy can be performed, but the final diagnosis depends on the results of IHC. For tumors that only develop thyroid metastases, aggressive surgery should be performed to improve prognosis. For patients with metastases other than thyroid, if the patient is in good physical condition, the general choice of treatment is surgery combined with chemotherapy.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-397/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shimaoka K, Sokal JE, Pickren JW. Metastatic neoplasms in the thyroid gland. Pathological and clinical findings. Cancer 1962;15:557-65. [Crossref] [PubMed]
- Lin JD, Weng HF, Ho YS. Clinical and pathological characteristics of secondary thyroid cancer. Thyroid 1998;8:149-53. [Crossref] [PubMed]
- Chung AY, Tran TB, Brumund KT, Weisman RA, Bouvet M. Metastases to the thyroid: a review of the literature from the last decade. Thyroid 2012;22:258-68. [Crossref] [PubMed]
- Wada N, Hirakawa S, Rino Y, Hasuo K, Kawachi K, Nakatani Y, Inui K, Takanashi Y. Solitary metachronous metastasis to the thyroid from renal clear cell carcinoma 19 years after nephrectomy: report of a case. Surg Today 2005;35:483-7. [Crossref] [PubMed]
- Richters A, Aben KKH, Kiemeney LALM. The global burden of urinary bladder cancer: an update. World J Urol 2020;38:1895-904. [Crossref] [PubMed]
- Wang P, Zang S, Li G, Qu W, Li S, Qiao Q, Jiang Y. The role of surgery on the primary tumor site in bladder cancer with distant metastasis: significance of histology type and metastatic pattern. Cancer Med 2020;9:9293-302. [Crossref] [PubMed]
- Tuncer M, Faydaci G, Altin G, Erdogan BA, Kibar S, Sanli A, Bilgici D. Metastasis of non-muscle-invasive bladder cancer into the thyroid gland: a literature review accompanied by a rare case. Korean J Urol 2014;55:222-5. [Crossref] [PubMed]
- Mirjalili SM, Hashemipour S, Salehi S, Kazemifar AM, Madani PS. Thyroid metastasis of bladder transitional cell carcinoma. Malays J Pathol 2016;38:65-70.
- Willis RA. Metastatic Tumours in the Thyreoid Gland. Am J Pathol 1931;7:187-208.3.
- Heffess CS, Wenig BM, Thompson LD. Metastatic renal cell carcinoma to the thyroid gland: a clinicopathologic study of 36 cases. Cancer 2002;95:1869-78. [Crossref] [PubMed]
- Luo M, Huang Y, Li Y, Zhang Y. Metastatic rectal cancer to papillary thyroid carcinoma: a case report and review of literature. BMC Gastroenterol 2020;20:136. [Crossref] [PubMed]
- Azadi JR, Hoang JK. Increasing Confidence in Detecting Metastatic Thyroid Cancer With Neck Ultrasonography. JAMA Otolaryngol Head Neck Surg 2020; Epub ahead of print. [Crossref]
- Kim HK, Kim SS, Oak CY, Kim SJ, Yoon JH, Kang HC. Diffuse metastasis to the thyroid: unique ultrasonographic finding and clinical correlation. J Korean Med Sci 2014;29:818-24. [Crossref] [PubMed]
- Smith SA, Gharib H, Goellner JR. Fine-needle aspiration. Usefulness for diagnosis and management of metastatic carcinoma to the thyroid. Arch Intern Med 1987;147:311-2. [Crossref] [PubMed]
- Pusztaszeri M, Wang H, Cibas ES, Powers CN, Bongiovanni M, Ali S, Khurana KK, Michaels PJ, Faquin WC. Fine-needle aspiration biopsy of secondary neoplasms of the thyroid gland: a multi-institutional study of 62 cases. Cancer Cytopathol 2015;123:19-29. [Crossref] [PubMed]
- Malani AK, Gupta C, Rangineni S, Gupta V. Thyroid metastasis from colorectal cancer: role of [18F]-fluoro-2-deoxy-D-glucose positron emission tomography. Clin Colorectal Cancer 2005;5:287-91. [Crossref] [PubMed]
- Agarwal H, Babu S, Rana C, Kumar M, Singhai A, Shankhwar SN, Singh V, Sinha RJ. Diagnostic utility of GATA3 immunohistochemical expression in urothelial carcinoma. Indian J Pathol Microbiol 2019;62:244-50. [Crossref] [PubMed]
- Giovanella L, Fontana M, Keller F, Verburg FA, Ceriani L. Clinical performance of calcitonin and procalcitonin Elecsys(®) immunoassays in patients with medullary thyroid carcinoma. Clin Chem Lab Med 2021;59:743-7. [Crossref] [PubMed]
- Dupain C, Ali HM, Mouhoub TA, Urbinati G, Massaad-Massade L. Induction of TTF-1 or PAX-8 expression on proliferation and tumorigenicity in thyroid carcinomas. Int J Oncol 2016;49:1248-58. [Crossref] [PubMed]
- Liang Y, Heitzman J, Kamat AM, Dinney CP, Czerniak B, Guo CC. Differential expression of GATA-3 in urothelial carcinoma variants. Hum Pathol 2014;45:1466-72. [Crossref] [PubMed]
- Battistella E, Pomba L, Mattara G, Franzato B, Toniato A. Metastases to the thyroid gland: review of incidence, clinical presentation, diagnostic problems and surgery, our experience. J Endocrinol Invest 2020;43:1555-60. [Crossref] [PubMed]
- Ivy HK. Cancer metastatic to the thyroid: a diagnostic problem. Mayo Clin Proc 1984;59:856-9.
- Straccia P, Mosseri C, Brunelli C, Rossi ED, Lombardi CP, Pontecorvi A, Fadda G. Diagnosis and Treatment of Metastases to the Thyroid Gland: a Meta-Analysis. Endocr Pathol 2017;28:112-20. [Crossref] [PubMed]
- Treadwell T, Alexander BB, Owen M, McConnell TH, Ashworth CT. Clear cell renal carcinoma masquerading as a thyroid nodule. South Med J 1981;74:878-9. [Crossref] [PubMed]
- Kim TY, Kim WB, Gong G, Hong SJ, Shong YK. Metastasis to the thyroid diagnosed by fine-needle aspiration biopsy. Clin Endocrinol (Oxf) 2005;62:236-41. [Crossref] [PubMed]
- Vardar E, Erkan N, Bayol U, Yılmaz C, Dogan M. Metastatic tumours to the thyroid gland: report of 3 cases and brief review of the literature. Radiol Oncol 2011;45:53-8. [Crossref] [PubMed]


