
Hounsfield unit as a predictor of symptomatic vasospasm and hydrocephalus in good-grade subarachnoid hemorrhage treated with endovascular coiling
Introduction
In the last three decades, new management strategies, such as endovascular coiling, have been developed for intracranial aneurysms patients. However, subarachnoid hemorrhage (SAH) caused by a ruptured intracranial aneurysm shows high morbidity and mortality (1,2). Functional outcomes after aneurysmal SAH are strongly influenced by the patient’s initial clinical presentation, as evaluated by clinical grading systems such as the Hunt and Hess (H-H) grade or the World Federation of Neurological Surgeons scores (3-5). Good-grade aneurysmal SAH patients are generally considered to have good clinical outcomes. However, approximately 20% of patients have unfavorable outcomes (6,7). Previous studies have reported that old age, hydrocephalus, delayed cerebral ischemia or vasospasm, pneumonia, and meningitis predict poor prognosis in good-grade SAH patients (6,7). Among them, vasospasm and hydrocephalus, which are significant complications of SAH, are known to be closely related to the subarachnoid hematoma volume at the time of admission (8-10). However, it is difficult to quantitatively measure the volume of subarachnoid hematoma, and the subarachnoid space volume differs depending on the individual or age. The Hounsfield unit (HU) value of brain computed tomography (CT) reflects hematoma density (11,12) and might be an appropriate quantitative and objective parameter for predicting vasospasm or hydrocephalus.
This study aimed to evaluate the relationship between the incidence of symptomatic vasospasm or hydrocephalus and the HU value of the subarachnoid space in good-grade SAH patients treated with endovascular coiling. We present this article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-355/rc).
Methods
Patient population
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was reviewed and approved by the Institutional Review Board of Jeonbuk National University Hospital (No. CUH 2023-02-058). Informed consent was not required because of the retrospective nature of the study and, thus, waived by the Ethics Committee of Jeonbuk National University Hospital. We identified 108 eligible patients with SAH admitted to the hospital between January 2010 and December 2019 using the following inclusion criteria: (I) aneurysmal SAH confirmed by CT within 24 h of symptom onset; (II) anterior circulation aneurysm demonstrated by CT angiography (CTA); (III) patients treated with endovascular coiling in the first 48 h after ictus; (IV) patients with H-H grade I or II; and (V) patients with only pure SAH (modified Fisher scale I or III). Patients with intraventricular hemorrhage (IVH) or intracerebral hemorrhage (ICH) were excluded; cases with procedure-related complications (intraoperative rupture, ischemic complication) or patients with previous SAH history were also excluded from the study (Figure 1).
Baseline and imaging data acquisition
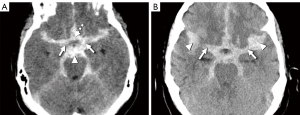
Patient age, sex, neurological status at admission, as measured by the H-H grade, and risk factors were obtained from their medical records. The modified Fisher scale and aneurysmal location were assessed using brain CT and CTA with a multi-slice CT scanner (SOMATOM Sensation 16; Siemens, Erlangen, Germany). The HU value was determined using axial CT imaging with a 5-mm slice thickness. The region of interest (ROI) was set as a circle with a diameter of 1–1.5 mm in the most hyperdense part within the cistern, as described in previous study (13). The HU value of the basal cistern was the average of the ROI values of the bilateral carotid, interpeduncular, and lamina terminalis cisterns on CT axial imaging, with the basal cistern visible (Figure 2A). The HU value of the Sylvian cistern was measured as the average ROI of the bilateral medial and lateral Sylvian cistern (Figure 2B). The site and average ROI value measuring the HU values were assessed via an agreement of the two measurers (JSP, HGK). One measurer (JSP) worked for 16 years and the other measurer (HGK) worked for 15 years.
Outcome assessment
Symptomatic vasospasm was defined as previously described: (I) presence of neurological worsening 4–14 days after SAH, including focal deficit, decline in consciousness, and motor paresis; (II) no other identifiable cause (intracranial disorder and systemic complication) of neurological worsening; and (III) confirmation of vasospasm by medical examinations, including evidence of vasospasm on cerebral angiography, CTA, and magnetic resonance angiography (14). All patients with vasospasm underwent induced hypertension. If necessary, they also received chemical angioplasty by using nicardipine. Hydrocephalus was defined as (I) a higher increase in ventricular size on follow-up CT than on CT at the time of admission; (II) ventriculocranial ratio larger than the 95th percentile for age (15); and (III) patients requiring temporary cerebrospinal fluid (CSF) diversion or permanent ventriculoperitoneal shunt. This study also defined the case where permanent shunt was not performed as hydrocephalus considering that even if temporary CSF diversion was required, acute ventricular enlargement affecting neurological symptoms was present. The clinical outcome was assessed using the modified Rankin scale (mRS) at 1-year follow-up, and any neurological complication after SAH was defined as a mRS score of 1–5.
Statistical analysis
Statistical analysis was performed using the SPSS software (version 21.0; IBM SPSS, Chicago, IL, USA). Patients were grouped according to the incidence of symptomatic vasospasm and hydrocephalus, and baseline data were compared. Continuous variables were summarized as median ± standard deviations or means with ranges, and categorical variables were summarized as counts and percentages. We performed a univariate comparison of the groups using an independent t-test or Wilcoxon rank-sum test for continuous variables and Pearson χ2 test or Fisher’s exact test for categorical variables. Receiver operating characteristic (ROC) curve analysis and area under the curve (AUC) were used to determine whether HU values were significant predictors of symptomatic vasospasm or hydrocephalus. Additionally, we used ROC curve analysis to identify the optimal cut-off points using the Youden index [maximal point of (sensitivity + specificity − 1)] to discriminate patients with symptomatic vasospasm. Multivariable logistic regression analyses were performed, including variables with a P value ≤0.2 as independent variables in the univariate analysis and any neurological complications at 1 year (mRS 1–5) as the dependent variable. Statistical significance was defined as a two-tailed P value <0.05.
Results
Patients’ baseline characteristics are summarized in Table 1. Of the 108 eligible patients, 34 (31%) were males [mean age, 60.9±12.3 years; H-H grade I, 46 (43%); modified Fisher scale I, 75 (69%)]. Symptomatic vasospasms developed in 26 patients (24%). There were significantly more H-H grade II patients [20/26 (77%) vs. 42/85 (51%), P=0.021] and more modified Fisher scale III patients [15/26 (58%) vs. 18/82 (22%), P=0.001] in the symptomatic vasospasm group. Hydrocephalus developed in 31 (29%) patients. Patients in the hydrocephalus group were significantly older than those in the non-hydrocephalus group (65.9±12.3 vs. 58.9±11.7, P=0.006). The hydrocephalus group showed a higher proportion of H-H grade II [23/31 (74%) vs. 39/77 (51%), P=0.025] and modified Fisher scale III [14/31 (45%) vs. 19/77 (25%), P=0.037] than did the non-hydrocephalus group (Table 1).
Table 1
| Variables | Overall (n=108) | Symptomatic vasospasm | Hydrocephalus | |||||
|---|---|---|---|---|---|---|---|---|
| Yes (n=26) | No (n=82) | P value | Yes (n=31) | No (n=77) | P value | |||
| Mean age (years) | 60.9±12.3 | 64.4±13.0 | 59.8±11.9 | 0.094 | 65.9±12.3 | 58.9±11.7 | 0.006 | |
| Men | 34 [31] | 8 [31] | 26 [32] | 0.928 | 6 [19] | 28 [36] | 0.085 | |
| H-H grade | 0.021 | 0.025 | ||||||
| I | 46 [43] | 6 [23] | 40 [49] | 8 [26] | 38 [49] | |||
| II | 62 [57] | 20 [77] | 42 [51] | 23 [74] | 39 [51] | |||
| Modified Fisher grade | 0.001 | 0.037 | ||||||
| I | 75 [69] | 11 [42] | 64 [78] | 17 [55] | 58 [75] | |||
| III | 33 [31] | 15 [58] | 18 [22] | 14 [45] | 19 [25] | |||
| Aneurysm location | 0.203 | 0.914 | ||||||
| ICA | 49 [45] | 11 [42] | 38 [46] | 15 [48] | 34 [44] | |||
| ACA | 51 [47] | 11 [42] | 40 [49] | 14 [45] | 37 [48] | |||
| MCA | 8 [8] | 4 [16] | 4 [5] | 2 [7] | 6 [8] | |||
| Risk factors | ||||||||
| Hypertension | 43 [40] | 9 [35] | 34 [41] | 0.534 | 14 [45] | 29 [38] | 0.471 | |
| Smoking | 33 [31] | 4 [15] | 29 [35] | 0.054 | 7 [23] | 26 [34] | 0.254 | |
Values are the number of patients [%] or mean ± standard deviation. H-H, Hunt and Hess; ICA, internal cerebral artery; ACA, anterior cerebral artery; MCA, middle cerebral artery.
Table 2 presents the distribution of mean HU values for basal and Sylvian cisterns. The symptomatic vasospasm group showed a significantly higher HU value on basal (47.04±10.62 vs. 40.18±9.83, P=0.003) and Sylvian cisterns (53.23±11.00 vs. 43.99±10.81, P<0.001) than the non-symptomatic vasospasm group. Based on the development of hydrocephalus, only the HU value of the basal cistern was significantly higher (45.60±10.61 vs. 40.32±9.99, P=0.016) in the hydrocephalus group (Table 2).
Table 2
| Variables | Overall (n=108) | Symptomatic vasospasm | Hydrocephalus | |||||
|---|---|---|---|---|---|---|---|---|
| Yes (n=26) | No (n=82) | P value | Yes (n=31) | No (n=77) | P value | |||
| Basal cistern | 41.83±10.40 | 47.04±10.62 | 40.18±9.83 | 0.003 | 45.60±10.61 | 40.32±9.99 | 0.016 | |
| Sylvian cistern | 46.22±11.51 | 53.23±11.00 | 43.99±10.81 | <0.001 | 49.44±9.47 | 44.92±12.04 | 0.064 | |
Values are the number of patients (%) or mean ± standard deviation.
AUC-ROC curve analysis revealed a slightly higher predictive value of symptomatic vasospasm for the HU value of the Sylvian cistern (AUC =0.757; 95% CI: 0.63–0.88; P<0.001) than for the HU value of the basal cistern (AUC =0.716; 95% CI: 0.60–0.74; P=0.001). We determined that the best cut-off point was 44.875 and 50.375 for the basal and Sylvian cistern HU values, respectively, to maximize the sensitivity and specificity for discriminating patients with symptomatic vasospasm (Table 3). Using these HU cut-off values, we investigated the baseline variables as independent prognostic factors for any neurological complication at 1 year (1-year mRS 1–5). Multivariable logistic analysis showed that age >70 years [odds ratio (OR) =10.85; 95% CI: 1.95–60.53; P=0.007] and HU value of Sylvian fissure ≥50.375 (OR =10.98; 95% CI: 1.02–118.49; P=0.048) were independent predictors of any neurological complications at 1 year in good-grade patients with ruptured aneurysm of anterior circulation treated with endovascular coiling (Table 4).
Table 3
| Variables (HU) | AUC (95% CI) | P value | Best cutoff value | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Basal cistern | 0.716 (0.60–0.84) | 0.001 | 44.875 | 0.615 | 0.659 |
| Sylvian cistern | 0.757 (0.63–0.88) | <0.001 | 50.375 | 0.692 | 0.683 |
ROC, receiver operating characteristic; HU, Hounsfield unit; AUC, area under the curve: CI, confidence interval.
Table 4
| Variables | Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| Age >70 years | 5.79 (1.94–17.32) | 0.002 | 10.85 (1.95–60.53) | 0.007 | |
| Hypertension | 1.89 (0.67–5.35) | 0.233 | – | – | |
| Smoking | 0.44 (0.12–1.63) | 0.218 | – | – | |
| H-H grade II | 15.65 (1.99–123.01) | 0.009 | 11.75 (0.96–143.30) | 0.054 | |
| Modified Fisher grade III | 4.21 (2.15–8.24) | <0.001 | 1.76 (0.74–4.17) | 0.198 | |
| Mean HU of Sylvian cistern ≥50.375 | 16.88 (3.61–78.80) | <0.001 | 10.98 (1.02–118.49) | 0.048 | |
| Mena HU of basal cistern ≥44.875 | 6.61 (1.99–22.00) | 0.002 | 1.30 (0.22–7.55) | 0.773 | |
mRS, modified Rankin Scale; CI, confidence interval; H-H, Hunt and Hess; HU, Hounsfield unit.
Discussion
In the present study, we evaluated the relationship between symptomatic vasospasm or hydrocephalus incidence and the HU value of the subarachnoid space in good-grade SAH patients (H-H grade I or II) treated with endovascular coiling. The mean HU value of the basal and Sylvian cisterns on brain CT at admission was significantly correlated with the development of symptomatic vasospasm. Hydrocephalus development was significantly associated with the HU value of the basal cistern.
Takeda et al. reported that the CSF space volume began increasing exponentially with age in patients after their 30s and increased by approximately six times among patients in their 80s compared to those in their 30s (16-19). Hence, the volume of subarachnoid hematoma seen on brain CT may differ according to age. We measured the HU value of the subarachnoid space (basal and Sylvian cisterns), following the quantitative evaluation of the subarachnoid hematoma volume, to compensate for these problems. Blood clot contraction increases hematocrit within the hematoma, resulting in increased HU value on the CT scan (20). Therefore, the HU value for the subarachnoid clot can reflect actual hematoma concentration by supplementing the volume of various subarachnoid spaces by age or the mixing problem of CSF and hematoma. Additionally, HU value analysis provided sufficient objectivity and good inter-rater reliability.
The relationship between symptomatic vasospasm and the density of subarachnoid clots in SAH patients has been investigated. Kanazawa et al. found that a mean CT value ≥49.64 HU predicted cerebral vasospasm with acceptable sensitivity and specificity (21). Ishihara et al. reported a 2.0 OR per 5 HU increase in symptomatic vasospasm (22). Suzuki et al. reported that an HU value >60 for the highest density area was significantly correlated with the development of cerebral infarction (23). However, these studies included all SAH patients regardless of their initial neurological status. In addition, patients with IVH were included. The initial severity of clinical presentation is the strongest prognostic indicator in SAH patients, and a poor H-H grade is strongly associated with the development of vasospasm (24,25). Notably, IVH is closely related to the development of hydrocephalus and vasospasm in SAH patients (10,26). Therefore, to reduce variables that could influence the outcome, we limited the study population to patients with good grades (H-H grade I or II) and those without IVH (modified Fisher scale I or III). Additionally, to minimize the variables depending on the treatment method or location of the ruptured aneurysm, this study was conducted only on patients with anterior circulation aneurysms who had undergone endovascular treatment. In contrast to previous studies, we measured the HU value by dividing the basal and Sylvian cistern values. Measured values from several sites were averaged for each measurement to reduce the bias caused by selecting a specific area. This study confirmed that the high HU values of the Sylvian and basal cisterns observed on initial brain CT at admission significantly correlated with the incidence of symptomatic vasospasm. By contrast, only the HU value of the basal cistern was significantly correlated with the occurrence of hydrocephalus.
In our study, similar to previous research, the hydrocephalus group had a higher mean age, and there were more patients with modified Fisher grade III. However, as mentioned earlier, the increase in age can affect the overestimation of the hematoma volume seen in the initial brain CT by expanding the subarachnoid space. The measurement of HU values is more objective and not affected by patient-specific factors according to age. Mijderwijk et al. reported that the HU difference in the basal cisterns was more significant than in the peripheral subarachnoid space in the shunt-dependent hydrocephalus group of SAH patients and accelerating the clearance of blood in the basal cisterns might potentially prevent the development of chronic hydrocephalus after SAH (27-29). We can confirm that only the HU value of the basal cistern had a significant correlation with the incidence of hydrocephalus. This is thought to be due to basal hematoma directly affecting the normal CSF pathway, consistent with a previous study’s finding.
We set an appropriate cut-off HU value that can predict symptomatic vasospasm through AUC-ROC curve analysis. Subsequently, multivariate logistic analysis was performed, including this cut-off value. Modified fissure grade III or H-H grade II showed no statistical significance as prognostic factors, whereas the HU value of the Sylvian cistern could be a predictor of the occurrence of neurological complications after 1 year.
In SAH patients, the HU value of the subarachnoid space measured by initial brain CT can be an objective, fast, and effective method compared to the somewhat subjective clinical or radiological grading system. In particular, good-grade SAH patients with a good initial neurological status will help predict, prepare, and rapidly treat symptomatic vasospasm and hydrocephalus. These complications can abruptly worsen the patient's condition. Furthermore, even in patients with initially good grade SAH, if the HU value of subarachnoid space hematoma is high, more aggressive induced hypertension can be performed to prevent vasospasm, and more preemptive CSF diversion to prevent hydrocephalus may be considered.
Our study had several limitations, including a relatively small sample size. The single-center retrospective study design may have acted as a selection bias in the patient population setting. Although HU value measurement is objective and has good inter-rater reliability, subjectivity may be involved depending on the ROI’s location. To analyze the prognostic factors of clinical outcomes, we investigated the predictive value of the variable for any neurological sequelae (mRS 1–5) after 1 year. However, an mRS of 1–5 does not indicate a poor prognosis. This was because a relatively small number of patients showed poor outcomes in the statistical analysis. However, it would be meaningful to identify predictors of neurological sequelae, especially in good-grade SAH patients. The mRS on admission was not assessed. However, since our study included only patients with H-H grade I or II, most patients with an initial mRS ≥1 would have been excluded from the study. And also, it is difficult to directly apply HU as a predictor of long-term prognosis. However, our study evaluated whether HU measurement could predict vasospasm or hydrocephalus in the acute stage. We believe that this prediction and preemptive response will help us treat patients better.
Conclusions
Using the HU value, subarachnoid blood clot density measurement can be an objective predictor of symptomatic vasospasm and hydrocephalus in good-grade SAH patients. In addition, an appropriate cut-off value for the HU value can be used as a prognostic factor for long-term clinical outcomes. It is thought that a large-scale, systematic prospective study is needed based on our findings.
Acknowledgments
This study was presented as an abstract of ePoster in WFITN in 2022.
Funding: This study was supported by the Fund of
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-355/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-355/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was reviewed and approved by the Institutional Review Board of Jeonbuk National University Hospital (No. CUH 2023-02-058). Informed consent was not required because of the retrospective nature of the study and, thus, waived by the Ethics Committee of Jeonbuk National University Hospital.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke 1997;28:660-4. [Crossref] [PubMed]
- Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology 1998;50:1413-8. [Crossref] [PubMed]
- Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med 2006;355:928-39. [Crossref] [PubMed]
- Helbok R, Kurtz P, Vibbert M, Schmidt MJ, Fernandez L, Lantigua H, Ostapkovich ND, Connolly SE, Lee K, Claassen J, Mayer SA, Badjatia N. Early neurological deterioration after subarachnoid haemorrhage: risk factors and impact on outcome. J Neurol Neurosurg Psychiatry 2013;84:266-70. [Crossref] [PubMed]
- Rosengart AJ, Schultheiss KE, Tolentino J, Macdonald RL. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke 2007;38:2315-21. [Crossref] [PubMed]
- Beneš V. Rd, Jurák L, Brabec R, Nechanická N, Šercl M, Endrych L, Buchvald P, Suchomel P. Causes of poor outcome in patients admitted with good-grade subarachnoid haemorrhage. Acta Neurochir (Wien) 2017;159:559-65. [Crossref] [PubMed]
- Zijlmans JL, Coert BA, van den Berg R, Sprengers MES, Majoie CBLM, Vandertop WP, Verbaan D. Unfavorable Outcome in Patients with Aneurysmal Subarachnoid Hemorrhage WFNS Grade I. World Neurosurg 2018;118:e217-22.
- Erixon HO, Sorteberg A, Sorteberg W, Eide PK. Predictors of shunt dependency after aneurysmal subarachnoid hemorrhage: results of a single-center clinical trial. Acta Neurochir (Wien) 2014;156:2059-69. [Crossref] [PubMed]
- Friedman JA, Goerss SJ, Meyer FB, Piepgras DG, Pichelmann MA, McIver JI, Toussaint LG 3rd, McClelland RL, Nichols DA, Atkinson JL, Wijdicks EF. Volumetric quantification of Fisher Grade 3 aneurysmal subarachnoid hemorrhage: a novel method to predict symptomatic vasospasm on admission computerized tomography scans. J Neurosurg 2002;97:401-7. [Crossref] [PubMed]
- Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES Jr, MacDonald RL, Mayer SA. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery 2006;59:21-7; discussion 21-7. [Crossref] [PubMed]
- Chakeres DW, Bryan RN. Acute subarachnoid hemorrhage: in vitro comparison of magnetic resonance and computed tomography. AJNR Am J Neuroradiol 1986;7:223-8.
- Scott WR, New PF, Davis KR, Schnur JA. Computerized axial tomography of intracerebral and intraventricular hemorrhage. Radiology 1974;112:73-80. [Crossref] [PubMed]
- Woo PYM, Tse TPK, Chan RSK, Leung LNY, Liu SKK, Leung AYT, Wong HT, Chan KY. Computed tomography interobserver agreement in the assessment of aneurysmal subarachnoid hemorrhage and predictors for clinical outcome. J Neurointerv Surg 2017;9:1118-24. [Crossref] [PubMed]
- Shirao S, Yoneda H, Ishihara H, Kajiwara K, Suzuki M. A proposed definition of symptomatic vasospasm based on treatment of cerebral vasospasm after subarachnoid hemorrhage in Japan: Consensus 2009, a project of the 25 Spasm Symposium. Surg Neurol Int 2011;2:74. [Crossref] [PubMed]
- van Gijn J, Hijdra A, Wijdicks EF, Vermeulen M, van Crevel H. Acute hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurosurg 1985;63:355-62. [Crossref] [PubMed]
- Inagawa T. Risk Factors for Cerebral Vasospasm Following Aneurysmal Subarachnoid Hemorrhage: A Review of the Literature. World Neurosurg 2016;85:56-76.
- Ko SB, Choi HA, Carpenter AM, Helbok R, Schmidt JM, Badjatia N, Claassen J, Connolly ES, Mayer SA, Lee K. Quantitative analysis of hemorrhage volume for predicting delayed cerebral ischemia after subarachnoid hemorrhage. Stroke 2011;42:669-74. [Crossref] [PubMed]
- Reilly C, Amidei C, Tolentino J, Jahromi BS, Macdonald RL. Clot volume and clearance rate as independent predictors of vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg 2004;101:255-61. [Crossref] [PubMed]
- Takeda S, Matsuzawa T. Brain atrophy during aging: a quantitative study using computed tomography. J Am Geriatr Soc 1984;32:520-4. [Crossref] [PubMed]
- Jeong HG, Bang JS, Kim BJ, Bae HJ, Han MK. Hematoma Hounsfield units and expansion of intracerebral hemorrhage: A potential marker of hemostatic clot contraction. Int J Stroke 2021;16:163-71. [Crossref] [PubMed]
- Kanazawa T, Takahashi S, Minami Y, Jinzaki M, Toda M, Yoshida K. Early prediction of clinical outcomes in patients with aneurysmal subarachnoid hemorrhage using computed tomography texture analysis. J Clin Neurosci 2020;71:144-9. [Crossref] [PubMed]
- Ishihara H, Oka F, Kawano R, Shinoyama M, Nishimoto T, Kudomi S, Suzuki M. Hounsfield Unit Value of Interpeduncular Cistern Hematomas Can Predict Symptomatic Vasospasm. Stroke 2020;51:143-8. [Crossref] [PubMed]
- Suzuki J, Komatsu S, Sato T, Sakurai Y. Correlation between CT findings and subsequent development of cerebral infarction due to vasospasm in subarachnoid haemorrhage. Acta Neurochir (Wien) 1980;55:63-70. [Crossref] [PubMed]
- Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, Patel AB, Thompson BG, Vespa PAmerican Heart Association Stroke Council. Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 2012;43:1711-37. [Crossref] [PubMed]
- Li K, Barras CD, Chandra RV, Kok HK, Maingard JT, Carter NS, Russell JH, Lai L, Brooks M, Asadi H. A Review of the Management of Cerebral Vasospasm After Aneurysmal Subarachnoid Hemorrhage. World Neurosurg 2019;126:513-27. [Crossref] [PubMed]
- Wilson TJ, Stetler WR Jr, Davis MC, Giles DA, Khan A, Chaudhary N, Gemmete JJ, Xi G, Thompson BG, Pandey AS. Intraventricular hemorrhage is associated with early hydrocephalus, symptomatic vasospasm, and poor outcome in aneurysmal subarachnoid hemorrhage. J Neurol Surg A Cent Eur Neurosurg 2015;76:126-32. [Crossref] [PubMed]
- Graff-Radford NR, Torner J, Adams HP Jr, Kassell NF. Factors associated with hydrocephalus after subarachnoid hemorrhage. A report of the Cooperative Aneurysm Study. Arch Neurol 1989;46:744-52. [Crossref] [PubMed]
- Mijderwijk HJ, Fischer I, Zhivotovskaya A, Bostelmann R, Steiger HJ, Cornelius JF, Petridis AK. Prognostic Model for Chronic Shunt-Dependent Hydrocephalus After Aneurysmal Subarachnoid Hemorrhage. World Neurosurg 2019;124:e572-9.
- Wang GX, Liu LL, Yang Y, Wen L, Duan CM, Yin JB, Zhang D. Risk factors for the progression of unruptured intracranial aneurysms in patients followed by CT/MR angiography. Quant Imaging Med Surg 2021;11:4115-24. [Crossref] [PubMed]



