
Mixed-reality and computer-aided design/computer-aided manufacturing technology for mandibular reconstruction: a case description
Introduction
Ameloblastomas are odontogenic tumors that account for approximately 1% of tumors and cysts occurring in the jawbone and 11% to 59% of all odontogenic tumors (1). Various digital technologies have been recently applied in reconstructive surgery for jawbone tumors (2,3); a notable example is the use of patient-specific implants (PSI), in which patient-specific medical images are used to create a reconstruction plate and apply it to surgery. PSI can facilitate surgery with high precision and contribute to shortening of the surgery time (4).
The conventional method involves an intraoperative free-hand approach to tumor resection, titanium plate alignment, donor bone shaping, and reconstructive orientation in which the surgeon estimates measurements of free flap size and osteotomy angles to appropriately fill a defect and contour the donor tissue (5). Another method developed using computer-aided design/computer-aided manufacturing (CAD/CAM) technology computed tomography (CT) data and intraoral scans. By making three-dimensional (3D) models of the donor and recipient sites, it is no more a freehand approach. Virtual resections and reconstructions may be performed for a better understanding of the intraoperative condition. Based on the virtual planning, surgical positioning, cutting guides, and/or patient-specific implants are designed (4). This method is effective to reflect the virtual operation results in actual surgery.
Mixed-reality (MR) is also being used in various fields as an application of digital technology to surgery. MR with Microsoft® HoloLens has been used to confirm the penetrating branch of blood vessels for flap operation (6). Although maxillary tumor resection has been reported in oral and maxillofacial surgery (7), MR is rarely applied to reconstructive surgery. Moreover, reports describing the combined use of PSI and MR in surgery are scarce. We report a case of the mandible reconstruction for ameloblastomas by using PSI and MR for segmental resection of the mandible and iliac bone graft.
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A 49-year-old woman presented to the hospital in July 2020 with a complaint of bulging of the right ramus of the mandible. This patient had a history of appendicitis but no comorbidities. The patient underwent right mandibular segmental resection and left nonvascularized iliac bone graft for ameloblastoma of the right mandible in 2004, but right mandibular recurrence was noted and segmental resection and right nonvascularized iliac bone graft was re-performed in 2009. The panoramic radiograph showed a transmission image with a clear boundary and marginalization from the mandibular branch to the posterior acetabular region (Figure 1A).
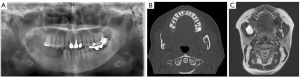
CT imaging revealed low-density lesions in the anterior margin of the ramus and the mandibular molars, and thinning of the cortical bone due to the buccal bulge of the mandible (Figure 1B). Magnetic resonance imaging also showed discontinuous unilocular areas at the anterior margin of the ramus and the mandibular molars (Figure 1C). On the basis of the biopsy findings, right mandibular segment resection and iliac block graft was planned under the diagnosis of ameloblastoma. The excision range was the line from the right mandibular premolar equivalent to the posterior mandibular notch to the anterior horn notch. However, since the patient had undergone two segmental resections and graft of both side iliac blocks, a detailed preoperative plan to take the iliac bone necessary for graft was essential. Therefore, PSI was used in this case (TRUMATCH CMF, DePuy Synthes). The excision range and graft range were determined before surgery, and osteotomy guides were prepared by a 3D printer and a titanium plates for mandibular fixation were prepared by milled. There is a method using surgical navigation to check whether the jawbone was cut as planned during surgery and whether the reconstruction position was accurate, but it is expensive and not available at all facilities (8). Also, the iliac osteotomy guide shows the osteotomy line of the superficial layer but cannot guide the angle and the deep part with the osteotomy guide design. Therefore, in this case, we applied MR as a new method to compensate it.
Preoperative preparation (virtual operation and Microsoft HoloLens applications)
For using TRUMATCH CMF, preoperative CT data (SOMATOM Definition AS: Siemens, Forchheim, Germany) of the head and neck and ilium were sent to an engineer employed by Materialise, and the data was prepared for surgical planning in ProPlan CMF (Materialise, Leuven, Belgium).
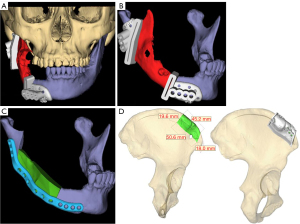
An oral surgeon and an engineer conducted a web-based meeting to determine the extent of tumor resection and designed an osteotomy guide (Figure 2A,2B). Next, the reconstruction plate and screw positions after tumor resection were designed (Figure 2C). Osteotomy guides for the ilium were designed after determining the extent of reconstructed iliac bone graft for the tumor resection range. The extent of the transplant was then determined to avoid iliac defects from previous surgery (Figure 2D). Standard triangle language (STL) data were obtained for each segment, and an application for Microsoft HoloLens was created using Holoeyes XR (Holoeyes Inc, Tokyo, Japan), which is a cloud service for creating applications.
Surgical procedure
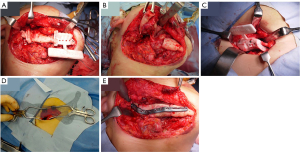
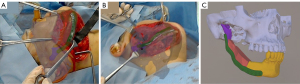
In November 2020, right mandibular segment resection and right iliac bone graft were performed under general anesthesia. After making an incision in the mandible according to the usual method, an osteotomy guide was inserted and fixed with a screw for temporary fixing (Figure 3A), and an ultrasonic cutting instrument was used to perform segmental resection (Figure 3B). After incision of the iliac part as usual, the osteotomy guide was inserted and temporarily fixed with a screw, and the bone was cut with an ultrasonic cutting instrument (Figure 3C). For deeper iliac bone cutting, the iliac part was overlaid in MR and the angle during excision was checked (Figure 3D). The iliac bone was fitted to the mandible with a custom-made metal plate and screw (Figure 3E). The inferior alveolar nerve was preserved because it was separated from the tumor. Reconstruction at the planned position was confirmed using MR, and the positions were confirmed three-dimensionally from various directions (Figure 4A-4C). The operation time was 5 hours and 42 minutes, and the amount of bleeding was 388 mL.
Accuracy of surgery
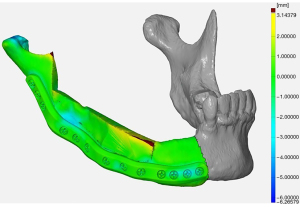
Surgical accuracy was verified using STL data of the mandible that had undergone preoperative virtual operation and STL data created from mandibular CT data 3 months after surgery. The surface deviation value was calculated using the iterative closest point (ICP) method with GOM Inspect (GOM, Braunschweig, Germany) Data superposition was performed automatically by selecting any three screw heads, and regions of interest were set on the temporomandibular joint, the reconstructed ilium, and the left mandible. For the reconstructed iliac bone, the cortical bone surface was targeted to account for postoperative changes in bone resorption. The average error of the temporomandibular joint was −1.13 mm (range, −4.97 to 4.80 mm), and the average error of the reconstructed iliac bone was −0.76 mm (range, −5.82 to 4.52 mm). The ratio of a superposition error of 2 mm or less was 84.5% in the temporomandibular joint, 81.1% in the reconstructed iliac bone, and 80.3% in total (Figure 5). In this case, the postoperative course was uneventful, there was no opening of the wound, and no recurrence was observed on radiographs. Postoperative dental prosthesis has not been applied to date, but the occlusal state is stable and the opening amount is 30 mm or more, so feeding can be performed without problems.
Discussion
A meta-analysis by Powcharoen et al. reported that computer-assisted surgery improved surgical time and precision compared to freehand reconstruction (9). Regarding the operation time, the average total operation time was 270 to 526 minutes. In addition, the accuracy of computer-assisted surgery is said to have an error of 1.36–8.9 mm. Microvascular bone flaps have proven to be the gold standard for mandibular reconstruction, but recent report indicates that graft surgery is a safe and effective treatment option (10). In addition, graft surgery was selected because the reconstruction range was about 50 mm, which was less than 60 mm, which is the cutoff value for which a flap is required. In Japan, coverage of PSI in reconstruction was confirmed from December 2020. Therefore, the operation time of 342 minutes is related to the fact that this case was an early case at our institution. Activation and use of MR navigation during surgery is smooth because the surgeon starts the surgery wearing the head mounted display (HMD). In the future, if the HMD itself can be sterilized, it may be possible to perform surgery more smoothly. Furthermore, reconstruction using a cutting guide reported that the accuracy of superimposing the preoperative virtual operation and postoperative CT was 58.73%±8.96%, and the measured distance error was 4.98±1.46 mm (2). These results suggest that the accuracy of this method is high. In contrast, placement of mandible in the planned position could not be confirmed by the conventional method. In the report by Tepper et al., MR was used for jawbone reconstruction, but only information was confirmed during surgery (11). Zhu et al. reported the accuracy of mandibular angle osteotomy using augmented reality (AR)-navigation surgery. They showed that osteotomy was performed more accurately in the AR group than in the freehand group (12). A study of mandibular reconstruction in a cadaver using a 3D-AR device reported an average accuracy deviation of 2 mm but a diagonal deviation of about 7 mm, an error of this size that is incompatible with many clinical procedures (13). This study showed that the ability to view complex surgical information during surgery is a major advantage of using 3D-AR devices, but a drawback is that visual misinterpretation can cause virtual objects to appear on top of real objects, making it difficult to recognize the objects. Our method improves this problem by adding a function to the application that allows the transparency of the object to be changed by gesture manipulation. Various approaches have been explored to improve the accuracy of 3D-AR registration to date, including manual registrations, computer vision-based registrations, and registrations that incorporate external tracking systems to increase accuracy, but evaluations of user accuracy when performing clinically relevant tasks suggest that accuracies of around 2 mm are feasible (14). Although the surgical accuracy of this method is largely dependent on the PSI, it has been difficult to achieve 100% accuracy even with PSI. Schulz et al. reported that high accuracy in reposition of temporomandibular joint in reconstruction surgery using a surgical guide. The median Euclidean distance was 2.07 mm for the left condyle and 2.11 mm for the right condyle. However, there were some cases where an error of 10 mm or more was recognized, and there is room for improvement in accuracy. In order to improve the accuracy of PSI, MR was applied in this case (15). In our study, it was possible to confirm with a hologram whether the 3D characteristics of the reconstructed mandible were as planned by superimposing the MR on the mandible reconstructed during the operation. The position of the temporomandibular joint at the time of reconstruction was originally invisible, and it was necessary to operate while imagining the position. But in this method, the position can be confirmed more accurately with a hologram. Notably, fixation was possible while confirming the temporomandibular joint, which led to an improvement in the accuracy of the surgery. In addition, since the ilium osteotomy guide does not guide the deep part and angle of the ultrasonic cutting instrument, the ilium could be treated more accurately by performing osteotomy while checking the angle with MR, improving the accuracy of the reconstructed ilium. AR-guided navigation was used for the iliac graft harvest.
The future potential of AR-guided navigation seems promising due to the method’s lower costs, reduced logistical efforts, and intraoperative flexibility in comparison with CAD/CAM and cutting-guide technologies (16). However, a limitation of this method is that aligning the surgical field and the MR is performed manually, and the accuracy is expected to improve further by alignment using a registration marker. Koyachi et al. reported that the accuracy of LeFort I osteotomy improved when a marker was used for superimposing the surgical field and the hologram (17). This superposition technique will be applied to cases of tumor resection in the future.
To further improve the accuracy, it is necessary to improve not only the marker accuracy but also the hardware. Pose-Díez-de-la-Lastra et al. reported that HoloLens 2 is more accurate than HoloLens 1 in surgery in the orthopedic field (18). In addition, this method is cheaper and lighter than the conventional navigation system, and it is useful in that the hologram can be viewed three-dimensionally rather than on a two-dimensional monitor. Furthermore, it is hygienic because it can be operated by gesture or voice. This method is considered to be particularly effective for surgery involving hard tissue such as bone, since the object may be deformed during surgery. However, MR can be shared by HMD wearers, and by using MR for preoperative discussions, the parts that should be treated with caution when performing surgery can be shared three-dimensionally with all surgeons.
The 3D hologram obtained through the HMD assisted the surgeon in comprehending the spatial relationship between crucial structures and the pathological lesion during the operation (19). Therefore, we believe that MR is highly effective even in operations that involve soft tissue if it is used in such procedures. Recently, the hurdles for the medical application of HMD have been reduced by the creation of patient-specific VR and MR models using the webservice. Pre- and intra-operative usages of HMD indicated the potential of innovative adjunctive surgical instrument (20).
In conclusion, on the basis of preoperative discussions using MR technology and the combined use of CAD/CAM and MR during the surgery, we were able to perform highly accurate mandibular reconstruction.
Acknowledgments
We would like to thank Holoeyes Inc. for technical support during the creation of the MR application and Prof. Masayuki Takano, Department of Oral and Maxillofacial Surgery, Tokyo Dental College, Tokyo, Japan involved in the surgery.
Funding: This work was supported by JSPS KAKENHI (No. JP21K17123, to M Koyachi).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1118/coif). MK reports that this work was supported by JSPS KAKENHI (No. JP21K17123). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: biological profile of 3677 cases. Eur J Cancer B Oral Oncol 1995;31B:86-99. [Crossref] [PubMed]
- Roser SM, Ramachandra S, Blair H, et al. The accuracy of virtual surgical planning in free fibula mandibular reconstruction: comparison of planned and final results. J Oral Maxillofac Surg 2010;68:2824-32. [Crossref] [PubMed]
- Sugahara K, Katsumi Y, Koyachi M, Koyama Y, Matsunaga S, Odaka K, Abe S, Takano M, Katakura A. Novel condylar repositioning method for 3D-printed models. Maxillofac Plast Reconstr Surg 2018;40:4. [Crossref] [PubMed]
- Tarsitano A, Mazzoni S, Cipriani R, et al. The CAD-CAM technique for mandibular reconstruction: an 18 patients oncological case-series. J Craniomaxillofac Surg 2014;42:1460-4. [Crossref] [PubMed]
- Naros A, Weise H, Tilsen F, Hoefert S, Naros G, Krimmel M, Reinert S, Polligkeit J. Three-dimensional accuracy of mandibular reconstruction by patient-specific pre-bent reconstruction plates using an "in-house" 3D-printer. J Craniomaxillofac Surg 2018;46:1645-51. [Crossref] [PubMed]
- Pratt P, Ives M, Lawton G, Simmons J, Radev N, Spyropoulou L, Amiras D. Through the HoloLens™ looking glass: augmented reality for extremity reconstruction surgery using 3D vascular models with perforating vessels. Eur Radiol Exp 2018;2:2. [Crossref] [PubMed]
- Sugahara K, Koyachi M, Koyama Y, Sugimoto M, Matsunaga S, Odaka K, Abe S, Katakura A. Mixed reality and three dimensional printed models for resection of maxillary tumor: a case report. Quant Imaging Med Surg 2021;11:2187-94. [Crossref] [PubMed]
- Badiali G, Roncari A, Bianchi A, et al. Navigation in Orthognathic Surgery: 3D Accuracy. Facial Plast Surg 2015;31:463-73. [Crossref] [PubMed]
- Powcharoen W, Yang WF, Yan Li K, et al. Computer-Assisted versus Conventional Freehand Mandibular Reconstruction with Fibula Free Flap: A Systematic Review and Meta-Analysis. Plast Reconstr Surg 2019;144:1417-28. [Crossref] [PubMed]
- Marechek A, AlShare A, Pack S, et al. Nonvascularized Bone Grafts for Reconstruction of Segmental Mandibular Defects: Is Length of Graft a Factor of Success? J Oral Maxillofac Surg 2019;77:2557-66. [Crossref] [PubMed]
- Tepper OM, Rudy HL, Lefkowitz A, et al. Mixed Reality with HoloLens: Where Virtual Reality Meets Augmented Reality in the Operating Room. Plast Reconstr Surg 2017;140:1066-70. [Crossref] [PubMed]
- Zhu M, Liu F, Zhou C, et al. Does intraoperative navigation improve the accuracy of mandibular angle osteotomy: Comparison between augmented reality navigation, individualised templates and free-hand techniques. J Plast Reconstr Aesthet Surg 2018;71:1188-95. [Crossref] [PubMed]
- Meng FH, Zhu ZH, Lei ZH, et al. Feasibility of the application of mixed reality in mandible reconstruction with fibula flap: A cadaveric specimen study. J Stomatol Oral Maxillofac Surg 2021;122:e45-9. [Crossref] [PubMed]
- Andrews CM, Henry AB, Soriano IM, et al. Registration Techniques for Clinical Applications of Three-Dimensional Augmented Reality Devices. IEEE J Transl Eng Health Med 2020;9:4900214. [Crossref] [PubMed]
- Schulz KL, Kesting MR, Nobis CP, Matta R, Lutz R. Three-dimensional evaluation of condylar position after mandibular reconstruction with a fibula free flap-comparison of different surgical techniques. Int J Oral Maxillofac Surg 2022:S0901-5027(22)00415-5.
- Winnand P, Ayoub N, Redick T, Gesenhues J, Heitzer M, Peters F, Raith S, Abel D, Hölzle F, Modabber A. Navigation of iliac crest graft harvest using markerless augmented reality and cutting guide technology: A pilot study. Int J Med Robot 2022;18:e2318. [Crossref] [PubMed]
- Koyachi M, Sugahara K, Odaka K, et al. Accuracy of Le Fort I osteotomy with combined computer-aided design/computer-aided manufacturing technology and mixed reality. Int J Oral Maxillofac Surg 2021;50:782-90. [Crossref] [PubMed]
- Pose-Díez-de-la-Lastra A, Moreta-Martinez R, García-Sevilla M, et al. HoloLens 1 vs. HoloLens 2: Improvements in the New Model for Orthopedic Oncological Interventions. Sensors (Basel) 2022;22:4915. [Crossref] [PubMed]
- Ito T, Kawashima Y, Yamazaki A, et al. Application of a virtual and mixed reality-navigation system using commercially available devices to the lateral temporal bone resection. Ann Med Surg (Lond) 2021;72:103063. [Crossref] [PubMed]
- Yamazaki A, Ito T, Sugimoto M, et al. Patient-specific virtual and mixed reality for immersive, experiential anatomy education and for surgical planning in temporal bone surgery. Auris Nasus Larynx 2021;48:1081-91. [Crossref] [PubMed]


