
Low-profile visualized intraluminal support-within-Enterprise overlapping-stent technique versus flow diversion in the treatment of intracranial vertebrobasilar trunk dissecting aneurysms
Introduction
An intracranial vertebrobasilar trunk dissecting aneurysm (VBTDA) is defined as an aneurysm originating from the arterial segment from the intradural segment of the vertebral artery (VA) up to the origin of the superior cerebellar artery (1). A VBTDA is a rare condition that has high risks of mortality and disability compared to an anterior circulation aneurysm due to its specific location, pathological features, and relationship to the perforating branches (1-5). Furthermore, due to the limited surgical accessibility and the relationship to the perforating branches, open surgical options are not recommended and are often associated with high morbidity and mortality rates (6,7).
Endovascular treatment has been a major tool for treating VBTDAs, but the safety and efficacy of different endovascular techniques require further exploration. Single-stent or multilayer-stent techniques with coil embolization have been described previously (5,8). Recently, the optional method of flow diversion (FD) has been used to treat intracranial aneurysms, but there are also potential risks of stroke when FD is used to treat posterior circulation nonsaccular aneurysms (9). Moreover, under normal circumstances, the procedural cost of FD treatment is higher than that of traditional endovascular therapy, and FD is currently not covered by social medical insurance in some areas in China. As VBTDAs are rare, there are limited reports on the treatment outcomes in relation to the angiographic results and the benefits and risks of different treatment techniques.
The potential flow-diverting effect of a low-profile visualized intraluminal support (LVIS) device may be beneficial in aneurysm occlusion. However, the transition zone of braided stents, including LVIS and flow diverters, creates a lower metal coverage rate area, which may affect the aneurysm healing process (10,11). In this study, we used LVIS-within-Enterprise overlapping-stent technique with coil embolization to treat VBTDAs. The Enterprise stent is used as an outer framework that constrains the expansion of the LVIS stent to increase metal coverage at the aneurysm neck. This study sought to assess the safety and long-term results of FD and LVIS-within-Enterprise overlapping-stent technique in the treatment of intracranial VBTDAs. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-970/rc).
Methods
Study design and patients
This was a retrospective, observational, cohort study. A total of 9,147 patients with intracranial aneurysms were screened between January 2014 and March 2022, and 91 patients with 95 VBTDAs who underwent LVIS-within-Enterprise overlapping-stent assisted–coiling or FD were included in this retrospective study. To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria: (I) have a classic dissecting aneurysm and segmental ectasia (according to the classification guidelines for intracranial dissecting aneurysms) (12); (II) have an unruptured VBTDA as confirmed by digital subtraction angiography (DSA), computer tomography (CT) angiography, or magnetic resonance (MR) angiography; and (III) have an aneurysm that could be treated with both FD and LVIS-within-Enterprise overlapping-stent technique. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had an aneurysm involving the extracranial segment of the VA or an aneurysm originating from the side branches; (II) had a basilar tip, vertebrobasilar dolichoectasia, or superior cerebellar artery aneurysm; (III) had previously undergone treatment for an intracranial aneurysm; and/or (IV) had a modified Rankin Scale (mRS) score >2 before the procedure. The study flowchart is shown in Figure 1.
The doctors informed each patient of all the treatment methods, including the price, insurance coverage, and the differences in the medications for each method. After individual discussions with their doctors, each patient chose either the FD treatment or LVIS-within-Enterprise overlapping-stent technique based on the their preferences and their family’s financial situation.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of The First Affiliated Hospital of Harbin Medical University approved this retrospective study, and written informed consent was obtained from all the patients before the procedure.
Antiplatelet protocol
Daily dual antiplatelet therapy (DAT) (75 mg of clopidogrel plus 100 mg of aspirin) was prescribed for all the patients at least 3–5 days before the procedure. Thromboelastography was used to study the platelet function. For the clopidogrel hyporesponders, the clopidogrel was changed to ticagrelor (90 mg, twice daily). After the procedure, the DAT was maintained for at least 3 months (6 months for patients treated with FD), and the single-antiplatelet therapy (usually aspirin, 100 mg daily) was maintained indefinitely.
Procedure
All the procedures were performed by neurointerventionists with >10 years of experience at Neurosurgery Department of the First Affiliated Hospital of Harbin Medical University. For patients treated with LVIS-within-Enterprise overlapping-stent technique, the aneurysm was first coiled with the assistance of the Enterprise stent (Codman Neuro, Raynham, MA, USA) using the jailing or semijailing technique until satisfactory aneurysm occlusion was achieved and/or additional packing was not possible. After embolization, the LVIS (MicroVention-Terumo, Aliso Viejo, CA, USA) was deployed within the Enterprise stent. For patients who underwent the FD treatment, a Pipeline embolization device (PED) (Covidien, Irvine, CA, USA) or Tubridge FD (MicroPort NeuroTech, Shanghai, China) was deployed using either a Marksman microcatheter (Covidien) or a Fastrack microcatheter (MicroPort NeuroTech).
Data collection
An angiographic follow-up examination with DSA was performed to determine the aneurysm occlusion status of the patients, which was scheduled at 6 months and 1–2 years postsurgery. The endo-saccular occlusion status of the target aneurysms was recorded according to the Raymond-Roy grading scale (13). The clinical outcome data, which were collected via neurologic examination or telephone call at 30 days, 6 months, and the annual follow-up, were categorized as having a favorable outcome (an mRS score ≤2) or an unfavorable outcome (an mRS score >2) (14). The primary outcome was the complete occlusion rate at the last angiographic follow-up. The secondary outcomes included adequate aneurysm occlusion (grade 1–2 of the Raymond-Roy grading scale); the in-stent stenosis/thrombosis rate; general neurological complications in the corresponding vascular territories, including hemorrhagic events, thromboembolic events, or new cranial nerve deficits, diagnosed clinically or on MR/CT, assessed within 30 days and during the follow-up period; complication-related mortality; and an unfavorable outcome at the last clinical follow-up.
Statistical analysis
The continuous variables are presented as the mean ± standard deviation (SD), or the median and interquartile range (IQR). The categorical variables are presented as the number and percentage. The continuous variables were compared using an independent-sample t-test or Mann-Whitney test according to the data distribution. The categorical variables were compared using Fisher exact test and the chi-squared test. The association between treatment techniques and outcomes was adjusted using a multivariate logistic regression model. Variables of clinical interest or those with P values <0.05 on the unilateral logistic regression analysis were included in the model. Given the number of events available, the variables that were included in the model were selected with caution. Cases with missing follow-up data were omitted. The statistical analyses were conducted using SPSS 22.0 software (IBM Corp., Armonk, NY, USA), and a P value <0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 91 patients (63 male, 69.2%) with 95 VBTDAs were included in this study, of whom 55 (with 57 aneurysms) were treated with LVIS-within-Enterprise overlapping-stent technique (the LE group) and 36 patients (with 38 aneurysms) were treated with FDs (the FD group) (Figure 1). The patients had a mean age of 57.0±9.4 years. Among the 95 VBTDAs, 70 (73.7%) were located in the VA, and 25 (26.3%) were located in the basilar artery (BA) or in both the VA and BA. The median maximal diameter of aneurysms was 10.1 mm (IQR: 6.1–14.4 mm). In total, 15 (15.8%) aneurysms involved the posterior inferior cerebellar artery or anterior inferior cerebellar artery, and 5 (5.3%) were complicated by proximal or distal parent artery stenosis. The pretreatment mRS scores were 0 in 46 (50.5%) patients, 1 in 40 (44.0%) patients, and 2 in 5 (5.5%) patients. A comparison of the baseline characteristics between the 2 groups showed comparable distribution. For further details, see Table 1.
Table 1
| Characteristics | LE group (n=55) | FD group (n=36) | P value |
|---|---|---|---|
| Male, n (%) | 37 (67.3) | 27 (75.0) | 0.43 |
| Age (years) (mean ± SD) | 58.3±9.6 | 55.1±8.9 | 0.12 |
| Risk factors, n (%) | |||
| Hypertension | 36 (65.5) | 20 (55.6) | 0.34 |
| Diabetes | 6 (10.9) | 6 (16.7) | 0.63 |
| Current/previous smoking | 17 (30.9) | 15 (41.7) | 0.29 |
| Alcohol abuse | 17 (30.9) | 10 (27.8) | 0.74 |
| Presented with ischemic symptoms, n (%) | 21 (38.2) | 13 (36.1) | 0.84 |
| Number of aneurysms | 57 | 38 | – |
| Aneurysm location, n (%) | 0.05 | ||
| BA/VBJ | 11 (19.3) | 14 (36.8) | |
| VA | 46 (80.7) | 24 (63.2) | |
| Aneurysm size, n (%) | 0.07 | ||
| <15 mm | 49 (86.0) | 27 (71.1) | |
| ≥15 mm | 8 (14.0) | 11 (28.9) | |
| Parent artery stenosis, n (%) | 4 (7.0) | 1 (2.6) | 0.63 |
| Side branch involved, n (%) | 7 (12.3) | 8 (21.1) | 0.25 |
LE, low-profile visualized intraluminal support-within-Enterprise overlapping-stent technique; FD, flow diversion; SD, standard deviation; BA, basilar artery; VBJ, vertebrobasilar junction; VA, vertebral artery.
Procedure-related data
Of the 57 aneurysms in the LE group, 3 (5.3%) were treated with 3 overlapping stents (1 Enterprise plus 2 LVIS or 2 Enterprise plus 1 LVIS), 1 (1.7%) with 4 overlapping stents (2 Enterprise plus 2 LVIS), and 53 (93.0%) were treated with 2 overlapping stents. Coil embolization was performed in all patients in the LE group. Of the 38 aneurysms in the FD group, 35 (92.1%) aneurysms were treated with PEDs and 3 (7.9%) with Tubridge FDs. Overlapping FDs were used in 6 (15.8%) aneurysms. All the patients in the FD group were treated with FD alone without adjunctive coiling. Additionally, 2 (3.6%) patients in the LE group and 2 (5.6%) patients in the FD group underwent unilateral VA sacrifice.
Primary outcomes
The median follow-up time was 9 months (IQR, 6–14 months) for the LE group and 7 months (IQR, 6–10 months) for the FD group. Based on the last follow-up, the aneurysms treated with LVIS-within-Enterprise overlapping-stent technique were associated with a higher rate of complete occlusion (Figures 2,3). The complete occlusion rate was 90.0% for the LE group and 60.9% for the FD group, with an adjusted odds ratio (OR) of 5.79 (95% CI: 1.35–24.85; P=0.01) (Table 2).
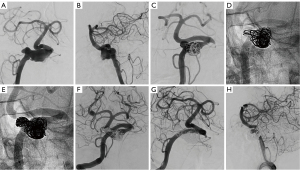
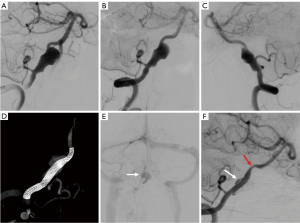
Table 2
| Outcomes | LE group, n (%) | FD group, n (%) | OR | 95% CI | Adjusted P value |
|---|---|---|---|---|---|
| Complete occlusion† | 27/30 (90.0) | 14/23 (60.9) | 5.79 | 1.35–24.85 | 0.01* |
| Adequate occlusion† | 28/30 (93.3) | 20/23 (87.0) | 0.98 | 0.09–10.83 | 0.98 |
| In-stent stenosis/thrombosis‡ | 2/30 (6.7) | 3/23 (13.0) | 0.43 | 0.04–4.16 | 0.46 |
| General neurological complications in the corresponding vascular territories§ | 8/55 (14.5) | 5/36 (13.9) | 0.32 | 0.05–2.05 | 0.22 |
| Neurological complications in the corresponding vascular territories within 30 days after the procedure§ | 5/55 (9.1) | 4/36 (11.1) | 0.61 | 0.08–4.64 | 0.63 |
| Unfavorable outcome§ | 6/55 (10.9) | 4/36 (11.1) | 0.07 | 0.01–1.26 | 0.07 |
| Complication-related mortality§ | 4/55 (7.3) | 3/36 (8.3) | 0.24 | 0.02–3.79 | 0.31 |
*, P<0.05; LE, low-profile visualized intraluminal support-within-Enterprise overlapping-stent technique; FD, flow diversion; OR, odds ratio; CI, confidence interval. †, adjusted for aneurysm maximal diameter, age, and aneurysm involving the basilar artery; ‡, adjusted for patient presenting with ischemic symptoms, age, and hypertension; §, adjusted for patient presenting with ischemic symptoms, unilateral vertebral artery sacrifice, aneurysm involving the basilar artery, age, and aneurysm maximal diameter.
Secondary outcomes
The adequate aneurysm occlusion rates at the last angiography follow-up were similar between the LE group and the FD group (93.3% versus 87.0%, adjusted OR =0.98, 95% CI: 0.09–10.83; P=0.98). In the LE group, 2 (6.7%) aneurysms were recanalized, and 1 (3.3%) underwent retreatment. The incidence of in-stent stenosis/thrombosis did not differ significantly between the LE group and the FD group, which had rates of 6.7% and 13.0%, respectively (adjusted OR =0.43, 95% CI: 0.04–4.16; P=0.46).
All the patients’ clinical outcome data were available for analysis. The patients had a median follow-up time of 27 (IQR, 12–45) months. A total of 13 (14.3%) patients experienced postprocedural neurological complications in the corresponding vascular territories. Among these 13 patients, 9 (9.9%) experienced complications within 30 days of the surgery, and 4 (4.4%) experienced complications after 30 days postsurgery. As Table 2 shows, the general rates of postprocedural neurological complications in the corresponding vascular territories were comparable between the 2 groups (14.5% for the LE group and 13.9% for the FD group; adjusted OR =0.32, 95% CI: 0.05–2.05; P=0.22). The complication rates within 30 days after procedure were also comparable (9.1% for the LE group and 11.1% for the FD group; adjusted OR =0.61, 95% CI: 0.08–4.64; P=0.63). Ischemic events occurred in 13 (14.4%) patients, 8 (14.5%) of whom were in the LE group and 5 (13.9%) of whom were in the FD group. Hemorrhagic events occurred in 2 (2.2%) patients, all of whom were in the FD group (5.6%). The unfavorable clinical outcome (an mRS score >2) rates at the last follow-up were also similar between the 2 groups (10.9% for the LE group and 11.1% for the FD group; adjusted OR =0.07, 95% CI: 0.01–1.26; P=0.07). The complication-related mortality was 7.7% (7/91). Of the 7 deaths, 4 (7.3%) occurred in the LE group and 3 (8.3%) in occurred the FD group, but the difference between the 2 groups was not statistically significant (adjusted OR =0.24, 95% CI: 0.02–3.79; P=0.31).
Complication details
Among the 8 patients with neurological complications in the LE group, 1 patient experienced bilateral limb weakness and aphasia 1 day after the procedure. Brain magnetic resonance imaging (MRI) showed acute brainstem infarctions (the patient’s mRS score at discharge was 4). The patient died due to acute cerebral infarction in the corresponding vascular territories during the follow-up period. One patient experienced new onset dizziness perioperatively, and MRI showed acute cerebellar infarctions. The patient died due to an acute cerebral infarction in the corresponding vascular territories during the follow-up period. One patient experienced dysphagia after the procedure (the patient’s mRS score at discharge was 1). Unfortunately, the patient died due to acute cerebral infarctions in the corresponding vascular territories during the follow-up period. One patient experienced weakness in the bilateral limb and dysphagia perioperatively (the patient’s mRS score at discharge was 4). One patient experienced unilateral limb weakness 1 day after surgery (the patient’s mRS score at discharge was 4). The patient died due to cerebral infarctions in the corresponding vascular territories. Moreover, 3 patients experienced weakness in the unilateral limb during the follow-up, including 1 with aphasia, and these 3 patients had final mRS scores of 4, 2, and 2, respectively.
Among the 5 patients with neurological complications in the FD group, there were 3 deaths and 1 patient with disability during the follow-up period. Acute in-stent thrombosis directly after the procedure was observed in 1 patient, and intra-arterial thrombolysis was performed. The patient was discharged without a neurologic deficit. However, the patient experienced acute cerebral infarctions in the corresponding vascular territories during the follow-up period and consequently died. In addition, 2 patients experienced bilateral limb weakness and aphasia during the periprocedural period, and head CT showed intracerebral hemorrhage and cerebral infarctions. The mRS scores of these 2 patients at discharge were 4 and 5, respectively, and 1 of these patients died due to the target aneurysm rupturing during the follow-up period. Moreover, 1 patient experienced unilateral limb weakness during the periprocedural period, but the symptom resolved after medical therapy. Additionally, 1 patient died due to in-stent thrombosis during the follow-up period.
Discussion
In this current study, the complete occlusion rate of intracranial VBTDAs was significantly higher in the patients treated with LVIS-within-Enterprise overlapping-stent technique than in those treated with FD. However, the overall morbidity, mortality, and unfavorable clinical outcome rates were comparable between the 2 groups.
Reconstructive treatment of VBTDAs with single stent–assisted coiling or conventional stent-only therapy is associated with risks of delayed aneurysm occlusion or recurrence (15,16). As previously reported, overlapping stents with coiling embolization may have advantages in preventing recurrence and may be a better treatment option for VBTDAs (15). In addition, LVIS stent-assisted coil embolization has shown promising results in treating VBTDAs (17-19). The LVIS device is a self-expanding, single-wire braided, compliant, retrievable intracranial stent designed to treat wide-necked intracranial aneurysms with a metal coverage of approximately 23% (19). In a hemodynamic analysis, the overlapping deployment of 2 LVIS stents increased the metal coverage to 36.3%, which in turn increased the FD effect to match that of a PED (20). However, due to the braid design, in fusiform aneurysms, the LVIS stent expands outwardly along the neck, and the metal coverage along the neck is unevenly distributed. After the stent deployment, there is a transition zone on the stent, which may affect the aneurysm healing process. The transition zone is located at the proximal and distal ends of the stent at the aneurysm neck, and represents the stent’s transition from the restrained parent artery to the unrestrained aneurysm neck. In a bench-top study, the transition zone of a LVIS stent showed higher porosity and lower neck coverage than did a proper-sized stent, especially when the stent was compressed in the high-density zone (11). The Enterprise stent is a laser-cut, closed-cell stent with greater radial force but less metal coverage (approximately 8%) than the LVIS stent. At the aneurysm neck, which contains the unconstrained segment, the outward expansion of the Enterprise stent is relatively small. Thus, the Enterprise stent could be used as an outer framework that constrains the outward expansion of the LVIS stent, reduces the transition zone, and thus increases the metal coverage across the aneurysm neck. The LVIS-within-Enterprise overlapping stent-assisted coiling embolizes the aneurysm using coils and provides a flow-diverting effect to the parent artery, which may contribute to the complete occlusion of the VBTDAs.
Previous studies have reported the overall angiography outcomes of VBTDAs following conventional stent-assisted coiling versus those following FD treatment; however, the results of different studies have varied (5,21,22). In some case series, for patients with VBTDAs, the FD treatment was associated with a higher possibility of follow-up complete aneurysm occlusion than was stent-assisted coiling (21,22). A meta-analysis by Domingo et al. demonstrated that complete/near complete occlusion was comparable between the 2 treatment modalities (5). In another study, Jeon et al. (23) found that the complete occlusion rate of VBTDAs treated with stent-assisted coiling was 78.7% at the mean follow-up time of 20.2 months, while Wang et al. (18) reported a complete occlusion rate of 76.7% in patients with vertebral aneurysms treated with LVIS stent-assisted coiling or LVIS stenting alone at a mean follow-up time of 8.3 months. In the present study, we attempted to use the LVIS-within-Enterprise overlapping-stent technique with coil embolization in the treatment of VBTDAs and found a relatively higher complete occlusion rate and a lower recurrence rate compared to the results reported in previous studies (8,18,23,24).
The complete occlusion rate of aneurysms treated with FD in our study was 60.9%, which was similar to the results of posterior circulation aneurysms with FD reported in previous studies (5,25,26) but was significantly lower than the rates reported for aneurysms treated with LVIS-within-Enterprise overlapping-stent technique. In the current study, no patients in the FD group received coil embolization, which might explain the lower complete occlusion rate. Previous studies have demonstrated that FD plus coil embolization affects complete aneurysm occlusion (27,28). However, the coil increases the cost of the procedure and increases the risk of procedure-related complications. In addition, the relatively high coil packing density may also contribute to the persistence of the mass effect of the aneurysm. The adequate aneurysm occlusion rate of aneurysms treated with FD in our study was 87.0%, which was comparable to the rates reported previously in the literature (5,26). The relatively high rate of adequate occlusion demonstrates one of the advantages of FD in the treatment of VBTDAs.
In this study, we reported comparable postprocedural and periprocedural complication rates between patients who received the LVIS-within-Enterprise overlapping stent-assisted coil embolization and those who received the FD treatment. Ischemic events occurred in all 13 (14.3%) patients with complications. In a meta-analysis of patients treated with FD for posterior circulation nonsaccular aneurysms, Kiyofuji et al. (9) reported that the overall mortality and morbidity rates were 21% and 26%, respectively, and another meta-analysis reported that the periprocedural complication rates of patients with nonsaccular posterior circulation were 6% after stent-assisted coiling, and 18% after FD treatment (5). It has also been reported that ischemic events account for most complications after the endovascular treatment of VBTDAs (29,30). Taken together, our findings show that during the endovascular treatment of VBTDAs, it is necessary to pay attention to the occurrence of postoperative complications, especially ischemic events. Inadequate stent expansion, antiplatelet drug hyporesponsiveness, acute or delayed perforating vessel occlusion, a delayed cobalt allergic reaction, and the detachment of intraluminal thrombus during the stenting or coiling might be potential causes of ischemic events after the endovascular treatment (30,31).
To the best of our knowledge, this was the first study to compare the safety and efficacy of LVIS-within-Enterprise overlapping-stent technique to FD in the treatment of intracranial VBTDAs. However, future studies need to be conducted to further explore the hemodynamic changes between LVIS-within-Enterprise overlapping-stent technique, FD treatment, and other conventional stenting techniques.
The present study had a few limitations. The CIs were relatively wide when analyzing the patients’ primary or secondary outcomes, which might have decreased the power in the multivariate analysis. Moreover, the relationship between aneurysm size, evolution, and treatment techniques was not additionally analyzed in this study. In addition, 41.8% of the patients were lost to angiography follow-up, and the last follow-up time was short and varied greatly, which might have biased the efficacy analysis. Finally, due to the retrospective, nonrandomized design, the potential selection bias inherent to all retrospective studies is unavoidable. Thus, randomized controlled trials need to be conducted in the future.
Conclusions
This study showed that VBTDAs treated with LVIS-within-Enterprise overlapping-stent technique had a significantly higher complete occlusion rate than those treated with FD. The 2 treatment modalities had comparable adequate occlusion rates and safety profiles. Thus, the LVIS-within-Enterprise overlapping-stent technique might be a good option for treating VBTDAs. Future randomized controlled trials on ruptured aneurysms need to be conducted.
Acknowledgments
Funding: This work was supported by the Key Research and Development Projects in Heilongjiang Province (No. 2022ZX06C03), the Foundation of The First Affiliated Hospital of Harbin Medical University (No. 2019L02), and the Natural Science Foundation of Heilongjiang Province of China (No. YQ2019H05).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-970/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-970/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work including ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the Institutional Review Board of The First Affiliated Hospital of Harbin Medical University. Informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Saliou G, Sacho RH, Power S, Kostynskyy A, Willinsky RA, Tymianski M, terBrugge KG, Rawal S, Krings T. Natural history and management of basilar trunk artery aneurysms. Stroke 2015;46:948-53. [Crossref] [PubMed]
- Wang GX, Liu LL, Yang Y, Wen L, Duan CM, Yin JB, Zhang D. Risk factors for the progression of unruptured intracranial aneurysms in patients followed by CT/MR angiography. Quant Imaging Med Surg 2021;11:4115-24. [Crossref] [PubMed]
- Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, Forbes GS, Thielen K, Nichols D, O'Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman GL, Torner JCInternational Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003;362:103-10. [Crossref] [PubMed]
- Tawk RG, Hasan TF, D'Souza CE, Peel JB, Freeman WD. Diagnosis and Treatment of Unruptured Intracranial Aneurysms and Aneurysmal Subarachnoid Hemorrhage. Mayo Clin Proc 2021;96:1970-2000. [Crossref] [PubMed]
- Domingo RA, Tripathi S, Perez-Vega C, Vivas-Buitrago T, Lu VM, Todnem ND, Quinones-Hinojosa A, Tawk RG. Treatment of posterior circulation non-saccular aneurysms with flow diversion versus stent-assisted coiling: a systematic review and meta-analysis. J Neurointerv Surg 2021;13:159-63. [Crossref] [PubMed]
- Tjahjadi M, Niemelä M, Kivelev J, Serrone J, Maekawa H, Jahromi BR, Kerro O, Hafez A, Lehto H, Kivisaari R, Hernesniemi J. Presigmoid Approach to Vertebrobasilar Artery Aneurysms: A Series of 31 Patients and Review of the Literature. World Neurosurg 2016;92:313-22. [Crossref] [PubMed]
- Frisoli FA, Srinivasan VM, Catapano JS, Rudy RF, Nguyen CL, Jonzzon S, Korson C, Karahalios K, Lawton MT. Vertebrobasilar dissecting aneurysms: microsurgical management in 42 patients. J Neurosurg 2021; Epub ahead of print. [Crossref] [PubMed]
- Zhao K, Zhao R, Yang X, Guan S, Liang G, Wang HL, et al. Predictors of unfavorable outcome in stent-assisted coiling for symptomatic unruptured intracranial spontaneous vertebral artery dissecting aneurysms (uis-VADAs): results from a multicenter study. J Neurointerv Surg 2022;14:1008-13. [Crossref] [PubMed]
- Kiyofuji S, Graffeo CS, Perry A, Murad MH, Flemming KD, Lanzino G, Rangel-Castilla L, Brinjikji W. Meta-analysis of treatment outcomes of posterior circulation non-saccular aneurysms by flow diverters. J Neurointerv Surg 2018;10:493-9. [Crossref] [PubMed]
- Shapiro M, Raz E, Becske T, Nelson PK. Building multidevice pipeline constructs of favorable metal coverage: a practical guide. AJNR Am J Neuroradiol 2014;35:1556-61. [Crossref] [PubMed]
- Matsuda Y, Chung J, Keigher K, Lopes D. A comparison between the new Low-profile Visualized Intraluminal Support (LVIS Blue) stent and the Flow Redirection Endoluminal Device (FRED) in bench-top and cadaver studies. J Neurointerv Surg 2018;10:274-8. [Crossref] [PubMed]
- Zhang Y, Tian Z, Sui B, Wang Y, Liu J, Li M, Li Y, Jiang C, Yang X. Endovascular Treatment of Spontaneous Intracranial Fusiform and Dissecting Aneurysms: Outcomes Related to Imaging Classification of 309 Cases. World Neurosurg 2017;98:444-55. [Crossref] [PubMed]
- Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke 2001;32:1998-2004. [Crossref] [PubMed]
- Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke 1999;30:1538-41. [Crossref] [PubMed]
- Park SI, Kim BM, Kim DI, Shin YS, Suh SH, Chung EC, Kim SY, Kim SH, Won YS. Clinical and angiographic follow-up of stent-only therapy for acute intracranial vertebrobasilar dissecting aneurysms. AJNR Am J Neuroradiol 2009;30:1351-6. [Crossref] [PubMed]
- Cho KC, Jeon P, Kim BM, Lim SM, Jung WS, Kim JJ, Suh SH. Saccular or dissecting aneurysms involving the basilar trunk: Endovascular treatment and clinical outcome. Neurol Res 2019;41:671-7. [Crossref] [PubMed]
- Hong Q, Li W, Ma J, Jiang P, Zhang Y. Endovascular treatment of vertebral and basilar artery aneurysms with low-profile visualized intraluminal support device. BMC Neurol 2021;21:198. [Crossref] [PubMed]
- Wang CC, Fang YB, Zhang P, Zhu X, Hong B, Xu Y, Liu JM, Huang QH. Reconstructive endovascular treatment of vertebral artery dissecting aneurysms with the Low-profile Visualized Intraluminal Support (LVIS) device. PLoS One 2017;12:e0180079. [Crossref] [PubMed]
- Fiorella D, Boulos A, Turk AS, Siddiqui AH, Arthur AS, Diaz O, Lopes DKLVIS investigators. The safety and effectiveness of the LVIS stent system for the treatment of wide-necked cerebral aneurysms: final results of the pivotal US LVIS trial. J Neurointerv Surg 2019;11:357-61. [Crossref] [PubMed]
- Kim S, Yang H, Hong I, Oh JH, Kim YB. Computational Study of Hemodynamic Changes Induced by Overlapping and Compacting of Stents and Flow Diverter in Cerebral Aneurysms. Front Neurol 2021;12:705841. [Crossref] [PubMed]
- Wang J, Jia L, Duan Z, Wang Z, Yang X, Zhang Y, Lv M. Endovascular Treatment of Large or Giant Non-saccular Vertebrobasilar Aneurysms: Pipeline Embolization Devices Versus Conventional Stents. Front Neurosci 2019;13:1253. [Crossref] [PubMed]
- Zhang Y, Liang F, Zhang Y, Yan P, Liang S, Ma C, Jiang C. Exploring the Feasibility of Pipeline Embolization Device Compared With Stent-Assisted Coiling to Treat Non-saccular, Unruptured, Intradural Vertebral Artery Aneurysms. Front Neurol 2019;10:275. [Crossref] [PubMed]
- Jeon JP, Cho YD, Rhim JK, Park JJ, Cho WS, Kang HS, Kim JE, Hwang G, Kwon OK, Han MH. Stent-Assisted Coil Embolization of Vertebrobasilar Dissecting Aneurysms: Procedural Outcomes and Factors for Recanalization. Korean J Radiol 2016;17:801-10. [Crossref] [PubMed]
- Kim JH, Ko YS, Kwon SM, Kim CH, Lee CY. Predictive Factors of Recurrence after Endovascular Treatment of Unruptured Vertebrobasilar Fusiform Aneurysms. Clin Neuroradiol 2023;33:73-86. [Crossref] [PubMed]
- Adeeb N, Ogilvy CS, Griessenauer CJ, Thomas AJ. Expanding the Indications for Flow Diversion: Treatment of Posterior Circulation Aneurysms. Neurosurgery 2020;86:S76-84. [Crossref] [PubMed]
- Wallace AN, Madaelil TP, Kamran M, Miller TR, Delgado Almandoz JE, Grossberg JA, Kansagra AP, Gandhi D, Kayan Y, Cawley CM, Moran CJ, Jindal G. CreveCoeur T, Howard BM, Cross DT, Kole MJ, Roy AK, Dion JE, Osbun JW. Pipeline Embolization of Vertebrobasilar Aneurysms-A Multicenter Case Series. World Neurosurg 2019;124:e460-9. [Crossref] [PubMed]
- Luo B, Kang H, Zhang H, Li T, Liu J, Song D, Zhao Y, Guan S, Maimaitili A, Wang Y, Feng W, Wang Y, Wan J, Mao G, Shi H, Yang X. Pipeline Embolization device for intracranial aneurysms in a large Chinese cohort: factors related to aneurysm occlusion. Ther Adv Neurol Disord 2020;13:1756286420967828. [Crossref] [PubMed]
- Zhang Q, Shao Q, Chang K, Zhang H, He Y, Andrade-Barazarte H, Sheng Z, Mo X, Zemmar A, Li L, Li T. Safety and Efficacy of Coils in Conjunction With the Pipeline Flex Embolization Device for the Treatment of Cerebral Aneurysms. Front Neurol 2021;12:651465. [Crossref] [PubMed]
- Wu Q, Xu S, Wang C, Ji Z, Li Y, Sun B, Meng Y, Shi H, Wu P. Endovascular Management of Vertebrobasilar Trunk Artery Large Aneurysms: Complications and Long-Term Results. Front Neurol 2022;13:839219. [Crossref] [PubMed]
- Wu Q, Shao Q, Li L, Liang X, Chang K, Li T, He Y. Prophylactic administration of tirofiban for preventing thromboembolic events in flow diversion treatment of intracranial aneurysms. J Neurointerv Surg 2021;13:835-40. [Crossref] [PubMed]
- Fujii S, Fujita K, Yamaoka H, Miki K, Hirai S, Nemoto S, Sumita K. Refractory in-stent stenosis after flow diverter stenting associated with delayed cobalt allergic reaction. J Neurointerv Surg 2022;14:e4. [Crossref] [PubMed]



