
Two cases of Birt-Hogg-Dubé syndrome combined with congenital contractural arachnodactyly
Introduction
Birt-Hogg-Dubé syndrome (BHD) is a rare disease caused by a mutation in the folliculin (FLCN) gene (1). The clinical manifestations of BHD mainly include skin fibrofolliculomas, pulmonary cysts, pneumothorax, and renal cancer (1). The syndrome can be easily misdiagnosed as primary spontaneous pneumothorax. In China, the average delay from first onset to final diagnosis is 10 years because of the high rate of misdiagnosis of the disease (2). Computed tomography (CT) is an important method for diagnosing BHD, and familiarity with the chest CT features of the syndrome can shorten the time to diagnosis (3). However, the mechanism of pulmonary cyst formation in BHD has yet to be fully elucidated.
Reduced elastin expression due to FLCN mutations may contribute to the formation of lung cysts in BHD (4). Congenital contractural arachnodactyly (CCA) is an autosomal dominant connective tissue disease caused by a mutation in the fibrillin-2 (FBN2) gene (5). Fibrillins are essential for elastin production and functional stability, and elastin is necessary for normal alveolar development (6). When FLCN and FBN2 are mutated simultaneously, patients may exhibit more prominent lung manifestations.
Here, we report two cases of BHD combined with CCA from the same family. Both patients had heterozygous variants in the FLCN gene, c.1015C > T (p. Gln339Ter), and the FBN2 gene, c.3485G > A (p. Cys1162Tyr) (7). These specific mutation loci have previously been reported by Qiu et al. (7). We focused on the lung CT manifestations of the two patients to further analyze whether their pulmonary cysts differed from those previously reported in patients with BHD.
Case report
Case A was a 58-year-old man. On October 27, 2020, the patient came to the First Affiliated Hospital of Henan University of Science and Technology for the first time for repeated spontaneous pneumothorax, which he had had four times in total. He had no history of smoking or occupational dust exposure. Multislice CT of the chest showed predominantly pulmonary cysts in the lower lungs and subpleural distribution (Figure 1A,1B). This patient has a total of 241 pulmonary cysts in both lungs. Pulmonary cysts in the lower lungs accounted for 63% (153/241), and subpleural cysts accounted for 68% (164/241). The largest cyst, measuring 52 mm ×48 mm ×112 mm in diameter, was located in the left lower lung. The large cysts near the spine of the bilateral lower lobes were fusiform (Figure 1C). Pulmonary vessels could be observed inside some cysts (Figure 1D). In the hand examination, the patient showed bilateral finger flexion contracture and could not extend the fingers fully. Digital radiography showed finger deformity and narrowing of the finger joint space (Figure 2). No substantial lesions were found in the heart and kidney ultrasound.
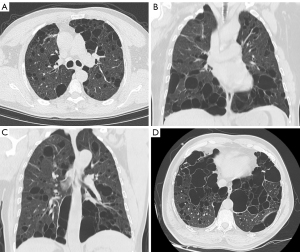
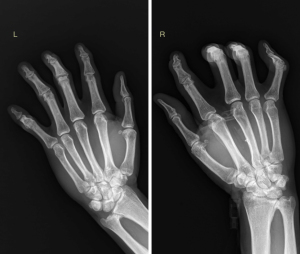
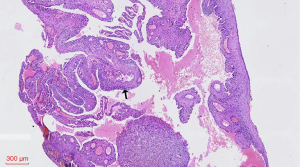
Case B was the 29-year-old son of case A. He first came to our hospital on May 19, 2014 with spontaneous pneumothorax, which he had had three times in total. The patient had no history of smoking or occupational dust exposure. Chest CT showed pulmonary cysts, which were mainly distributed in the lower lung and bilateral subpleural lungs (Figure 3A). This patient has a total of pulmonary cysts in both lungs. Pulmonary cysts in the lower lungs accounted for 52% (43/82) and subpleural accounted for 71% (58/82). The largest cyst, measuring 35 mm ×21 mm ×44 mm in diameter, was in the left upper lobe (Figure 3B). In the hand examination, the patient showed double finger deformities similar to those of his father. The patient underwent bullae resection, and pathological examination of the resected specimens confirmed lung bullae (Figure 4).

The two patients’ genetic sequencing maps are described in detail in the supplementary file (Figure S1). All procedures performed in this study were conducted in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for the publication of the case reports and accompanying images was obtained from the 2 patients. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
In East Asian patients, BHD mainly presents as pulmonary cysts and spontaneous pneumothorax, while typical skin and kidney involvement are less common than in other groups (8). Pulmonary cysts are the most common radiological manifestation of BHD (8), and chest CT is an important method used for their evaluation. In BHD, pulmonary cysts are mainly located in the lower lobes of both lungs and the subpleural area (9,10). The cysts can include or be adjacent to blood vessels (11). Most large cysts (>20 mm) are irregular, while the cysts near the spine can show plastic changes (9). In the present case report, the two patients presented with pulmonary cysts and pneumothorax without typical skin and renal lesions. Their pulmonary cysts were mainly distributed in the lower lobe of both lungs and the subpleural regions.
Among diffuse cystic lung lesions, BHD mainly needs to be distinguished from lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis, and lymphocytic interstitial pneumonia. Sporadic lymphangioleiomyomatosis almost exclusively occurs in women of childbearing age and also shows as multiple cysts in both lungs on CT. However, lymphangioleiomyomatosis cysts are smaller, rounder, and more uniformly distributed than those in in BHD (10). Pulmonary Langerhans cell histiocytosis is closely related to smoking. On chest CT, it shows as cysts with multiple nodules distributed mainly in both upper lungs, whereas pulmonary cysts in BHD are mainly distributed in the lower lungs (12). Lymphocytic interstitial pneumonia, which is mostly secondary to desiccation syndrome, has more diverse manifestations than BHD on chest CT. In addition to pulmonary cysts, lymphocytic interstitial pneumonia can be accompanied by ground-glass shadows and centrilobular nodules (13).
The mechanism of the formation of BHD pulmonary cysts has yet to be fully elucidated. Previous studies have suggested that FLCN mutations lead to reduced mTORC1 and Wnt activity, which may reduce alveolar growth (14,15). Chu et al. (4) found that, after FLCN gene knockout in mouse pulmonary mesenchymal cells, the expression of elastin in the lung decreased, leading to alveolar destruction, which may be one reason for the formation of pulmonary cysts in BHD. FBN2 is involved in the regulation of elastin synthesis (16). In this case, two patients had mutations in both FLCN and FBN2. Compared with data previously reported in China, we found that the average number of pneumothoraxes in these two patients was more remarkable than previously reported in China (3.5 vs. 1.8) and the diameter of the largest cyst was larger (112 vs. 85 mm) (2). Xu et al. (10) suggested that most patients with BHD had only a small number of pulmonary cysts (<50), while our two patients had a relatively large number of cysts (241 and 82). Both FLCN mutations and FBN2 mutations lead to elastin dysfunction; therefore, we speculate that this provides the conditions for creating more and larger pulmonary cysts in the patient’s lungs. Toro et al. (17) showed that pneumothorax was significantly associated with the number and size of cysts, which explains pneumothorax occurring a more significant average number of pneumothoraxes in the present cases.
The main clinical manifestations of CCA are joint contracture, arachnodactyly, crumpled ears, and kyphoscoliosis (18). Usually, CCA is not associated with lung abnormalities. In contrast, Marfan syndrome caused by FBN1 mutations is associated with pulmonary maculopathy and pneumothorax, although at a low incidence (19). The generation of pulmonary cysts in BHD may involve a cooperative formation of mechanisms (20). FBN2 mutations cause elastin dysfunction, which may not be a sufficient mechanism to cause pulmonary cysts alone but may contribute to the formation of pulmonary cysts at a later stage.
In conclusion, this case report describes the first reported cases of an FLCN mutation causing BHD with an FBN2 mutation causing CCA. Concurrent mutations in FLCN and FBN2 may lead to more and larger pulmonary cysts in patients, suggesting that elastin has an important role in the formation of BHD pulmonary cysts.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-806/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were conducted in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the 2 patients to publish this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gupta N, Sunwoo BY, Kotloff RM. Birt-Hogg-Dubé Syndrome. Clin Chest Med 2016;37:475-86. [Crossref] [PubMed]
- Hu X, Zhang G, Chen X, Xu KF. Birt-Hogg-Dubé syndrome in Chinese patients: a literature review of 120 families. Orphanet J Rare Dis 2021;16:223. [Crossref] [PubMed]
- Zhang G, Liu J, Wang Y, Wang Y, Jiang X, Peng Y, Xiao J, Wei W, Shen B, Yi L, Ryu JH, Hu X. Birt-Hogg-Dubé syndrome encountered at rare lung disease clinic in Anhui province, China. Orphanet J Rare Dis 2022;17:203. [Crossref] [PubMed]
- Chu L, Luo Y, Chen H, Miao Q, Wang L, Moats R, Wang T, Kennedy JC, Henske EP, Shi W. Mesenchymal folliculin is required for alveolar development: implications for cystic lung disease in Birt-Hogg-Dubé syndrome. Thorax 2020;75:486-93. [Crossref] [PubMed]
- Tunçbilek E, Alanay Y. Congenital contractural arachnodactyly (Beals syndrome). Orphanet J Rare Dis 2006;1:20. [Crossref] [PubMed]
- Mariani TJ, Sandefur S, Pierce RA. Elastin in lung development. Exp Lung Res 1997;23:131-45. [Crossref] [PubMed]
- Qiu J, Lou Y, Zhu Y, Wang M, Peng H, Hao Y, Jiang H, Mao Y. Clinical Characteristics and Genetic Analysis of a Family With Birt-Hogg-Dubé Syndrome and Congenital Contractural Arachnodactyly. Front Genet 2022;12:768342. [Crossref] [PubMed]
- Guo T, Shen Q, Ouyang R, Song M, Zong D, Shi Z, Long Y, Chen P, Peng H. The clinical characteristics of East Asian patients with Birt-Hogg-Dubé syndrome. Ann Transl Med 2020;8:1436. [Crossref] [PubMed]
- Yang J, Hu X, Li J, Zhang G, Ge Y, Wei W. Correlative analysis of lung CT findings in patients with Birt-Hogg-Dubé Syndrome and the occurrence of spontaneous pneumothorax: a preliminary study. BMC Med Imaging 2022;22:22. [Crossref] [PubMed]
- Xu W, Xu Z, Liu Y, Zhan Y, Sui X, Feng R, Peng M, Li X, Wang J, Meng S, Wang L, Tian X, Zhang X, Xu KF. Characterization of CT scans of patients with Birt-Hogg-Dubé syndrome compared with those of Chinese patients with non-BHD diffuse cyst lung diseases. Orphanet J Rare Dis 2020;15:176. [Crossref] [PubMed]
- Lee JE, Cha YK, Kim JS, Choi JH. Birt-Hogg-Dubé syndrome: characteristic CT findings differentiating it from other diffuse cystic lung diseases. Diagn Interv Radiol 2017;23:354-9. [Crossref] [PubMed]
- Abbott GF, Rosado-de-Christenson ML, Franks TJ, Frazier AA, Galvin JR. From the archives of the AFIP: pulmonary Langerhans cell histiocytosis. Radiographics 2004;24:821-41. [Crossref] [PubMed]
- Marchiori E, Damato S, Rodrigues R, Valiante PM, Bras MAAJR. Lymphocytic interstitial pneumonia: correlation of high-resolution computed tomography findings with anatomopathology. Radiol Bras 2002;35:199-203. [Crossref]
- Kennedy JC, Khabibullin D, Hougard T, Nijmeh J, Shi W, Henske EP. Loss of FLCN inhibits canonical WNT signaling via TFE3. Hum Mol Genet 2019;28:3270-81. [Crossref] [PubMed]
- Ren S, Luo Y, Chen H, Warburton D, Lam HC, Wang LL, Chen P, Henske EP, Shi W. Inactivation of Tsc2 in Mesoderm-Derived Cells Causes Polycystic Kidney Lesions and Impairs Lung Alveolarization. Am J Pathol 2016;186:3261-72. [Crossref] [PubMed]
- Jacob MP, Sauvage M, Osborne-Pellegrin M. Regulation of elastin synthesis. J Soc Biol 2001;195:131-41. [Crossref] [PubMed]
- Toro JR, Pautler SE, Stewart L, Glenn GM, Weinreich M, Toure O, Wei MH, Schmidt LS, Davis L, Zbar B, Choyke P, Steinberg SM, Nguyen DM, Linehan WM. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé syndrome. Am J Respir Crit Care Med 2007;175:1044-53. [Crossref] [PubMed]
- Meerschaut I, De Coninck S, Steyaert W, Barnicoat A, Bayat A, Benedicenti F, et al. A clinical scoring system for congenital contractural arachnodactyly. Genet Med 2020;22:124-31. [Crossref] [PubMed]
- Karpman C, Aughenbaugh GL, Ryu JH. Pneumothorax and bullae in Marfan syndrome. Respiration 2011;82:219-24. [Crossref] [PubMed]
- Hoshika Y, Takahashi F, Togo S, Hashimoto M, Nara T, Kobayashi T, Nurwidya F, Kataoka H, Kurihara M, Kobayashi E, Ebana H, Kikkawa M, Ando K, Nishino K, Hino O, Takahashi K, Seyama K. Haploinsufficiency of the folliculin gene leads to impaired functions of lung fibroblasts in patients with Birt-Hogg-Dubé syndrome. Physiol Rep 2016;4:e13025. [Crossref] [PubMed]


