
Qualitative and quantitative superb vascular imaging in the diagnosis of thyroid nodules ≤10 mm based on the Chinese Thyroid Imaging Reporting and Data System 4 (C-TIRADS 4)
Introduction
As a common clinical problem, thyroid nodules (TNs) can be detected in more than half of healthy people, most of which are asymptomatic and discovered incidentally (1). According to the International Agency for Research on Cancer, there were about 586,200 new cases of thyroid cancer worldwide in 2020, ranking ninth among all malignancies in terms of incidence (2). Ultrasonography (US) is considered one of the most important methods of managing TNs. TNs with several ultrasonographic features, such as hypo- or marked hypo-echogenicity, microcalcification, a taller-than-wide shape (TTW), and a spiculated or microlobulated margin, are suspicious for malignancy. However, some benign TNs also present these features (3). Hence, it can sometimes be difficult to identify malignant nodules by using gray-scale US alone, and additional information is needed for accurate diagnosis.
According to the Chinese Thyroid Imaging Reporting and Data System (C-TIRADS) published by the Superficial Organ and Vascular Ultrasound Group of the Society of Ultrasound in Medicine of the Chinese Medical Association, solid nodules classified as C-TIRADS 4 (C-TR4), especially 4B and 4C, indicate moderate and high risks of malignancy, respectively (4). Accurate diagnosis of C-TR4–5 TNs with a maximum diameter ≤10 mm is critical for better clinical management and prognosis (5). Angiogenesis is a characteristic of malignant tumors, which is essential for tumor growth and development. Tumor-associated vasculature is irregular and overly branched (6). Superb microvascular imaging (SMI), a recently developed US imaging modality, can better display microvascular information by eliminating clutter and preserving low-flow signals. Several researchers have applied SMI to grade disease activities and monitor treatment responses (7,8). SMI technology has also been reported to show good agreement with contrast-enhanced ultrasound (CEUS) in detecting vessels (9,10). Moreover, several studies (11-18) have used SMI to record the number of blood vessels in TNs, and the performance in differentiating benign from malignant nodules was shown to be significantly improved (11-16,18). However, these researches did not study whether different SMI sections could make a difference when diagnosing malignant TNs.
At present, there are no studies comparing qualitative with quantitative SMI in the diagnosis of benign and malignant TNs. Therefore, our study aimed to explore whether there is a difference between qualitative and quantitative SMI in the diagnosis of thyroid cancer. A study found that the combination of quantitative and qualitative vascularity grading can improve diagnostic performance by optimizing both sensitivity and specificity (19). Hence, we also explored the performance of quantitative and qualitative SMI in differentiating TNs. We present the following article in accordance with the Standards for Reporting Diagnostic accuracy studies (STARD) reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1193/rc).
Methods
Patients
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was reviewed and approved by the Ethics Committee of Peking Union Medical College Hospital (No. JS-2881) and individual consent for this retrospective analysis was waived. From October 2020 to June 2022, 202 consecutive patients (142 women and 60 men) with 220 suspicious malignant TNs who underwent US examination at the Peking Union Medical College Hospital were initially selected in our study. The inclusion criteria were as follows: (I) one or more suspected malignant TNs (C-TR4); (II) definite fine needle aspiration (FNA) diagnosis or surgical pathological results in our hospital; (III) maximum diameter of the nodules ≤10 mm; (IV) nodules with SMI both in longitudinal and transverse sections. The exclusion criteria were as follows: (I) incomplete or missing SMI data; (II) nodules without definite diagnosis; (III) nodules confirmed as malignant in another hospital. Finally, 109 TNs (81 malignant, 28 benign) met our criteria and were enrolled in this research (Figure 1). A total sample size of 109 achieves 91% power to detect a change in sensitivity of 0.8 using a one-sided binomial test. The target significance level was 0.023, and the actual significance level achieved by the sensitivity test was 0.0178. The prevalence of the disease was 0.5.
Ultrasonographic examinations
Gray-scale US examinations of the thyroid gland were performed using a 5–12 MHz linear transducer (iU22; Philips Medical Systems, Bothell, WA, USA) by an expert with more than 20 years of experience in thyroid US. Sonographic features such as location, maximum diameter, shape composition, echogenicity, calcification, and other characteristics were recorded. Based on these features, C-TIRADS classification was also performed by the expert mentioned above. Next, 2 radiologists with 2 and 5 years of experience in thyroid US used SMI to evaluate the vascular distribution and morphology of each nodule. SMI was obtained using a high-frequency (14 MHz) linear array probe (Canon Medical Systems, Tokyo, Japan). The settings were as follows: frame rate 25–45 fps; velocity less than 2.5 cm/s; and color gain 40–50%. Qualitative SMI was defined by the modality of blood vessels of TNs, which was classified into 3 types (20): type I, no vascularity; type II, peripheral vascularity; type III, intra-nodular vascularity (Figure 2). Then, 2 reviewers independently analyzed the vascular pattern of each nodule. Before analysis, the reviewers were blinded to the clinical information of patients and the results of FNA or surgery. If the 2 reviewers yielded the same results, they would be accepted, otherwise, a third reviewer would read the image and determine the final result. The intraclass correlation coefficient (ICC) was calculated to estimate inter-observer agreement.
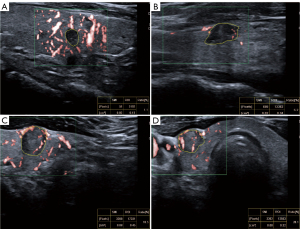
Quantitative SMI can be obtained using the vascular index (VI) of the nodule, which represents the ratio between the pixels of the Doppler signal and those of the whole nodule. Previous studies have utilized the color mode when conducting quantitative analysis (21,22). Hence, both qualitative and quantitative SMI were obtained in color mode in our study. VI can be automatically calculated by delineating the region of interest (ROI) in freestyle, which is the color SMI image with the most abundant blood flow (Figure 2). We collected qualitative and quantitative SMI in both longitudinal and transverse sections within 1 month before FNA. Intra-nodular vascularity was reported to be a feature of malignant nodules (14,23). VIsum represents the sum of VI in the longitudinal and transverse sections of each nodule. If a C-TR4B nodule had intra-nodular vascularity or a VIsum > optimal cut-off value, the original C-TIRADS was upgraded to C-TR4C. If a C-TR4C or C-TR4B nodule manifested a VIsum ≤ optimal cut-off value and no intra-nodular vascularity, the original C-TIRADS was downgraded to C-TR4A.
Ultrasound-guided FNA was conducted by an expert in US intervention. The pathologic results of US-FNA or surgery were regarded as the reference standard. The FNA results were based on the Bethesda System for Reporting Thyroid Cytopathology (24).
Statistical analysis
The chi-squared (χ2) test or Fisher’s exact test was applied to categorical variables, and an independent t-test was used to compare continuous variables. The maximum diameter of the nodules was compared between the benign and malignant TNs using a Wilcoxon rank sum test. Diagnostic performance was evaluated by sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy. A receiver operating characteristic (ROC) curve with a 95% confidence interval (CI) was formulated to determine the diagnostic value of qualitative and quantitative SMI in different sections, and the area under the curve (AUC) was compared using a Z test. The interclass correlation coefficient (ICC) was used to test the interobserver reliability. All statistical analyses were conducted using SPSS Statistical Software version 26 (IBM Corp., Armonk, NY, USA) and MedCalc Statistical Software version 20.022 (MedCalc Software, Ltd., Ostend, Belgium). Differences were considered statistically significant at P<0.05. The sample size was calculated by PASS V.15 (NCSS, Kaysville, UT, USA).
Results
Baseline characteristics
Finally, 106 patients (77 women, 29 men; mean age ± SD =43.41±11.69 years), and 109 TNs that met our criteria were enrolled in this study. Among the suspected 109 TNs, 81 nodules (74.3%) were malignant and 28 nodules (25.7%) were benign. Some 51 TNs underwent surgery, and pathological results were obtained (48 cases of papillary carcinoma, 1 medullary carcinoma, and 2 cases of nodular goiter). The mean age difference between patients with malignant and benign nodules was not statistically significant (42.41±11.41 vs. 46.32±12.22, P=0.13). The maximum diameters of nodules ranged from 3 to 10 mm (mean: 7.8 mm). Compared with the maximum diameter [median, inter-quartile range (IQR)] of the benign nodules (7.0, 4.0 mm), the size of malignant nodules (8.0, 2.0 mm) exhibited no statistical difference (P=0.18). Table 1 shows the demographic characteristics and US features according to the final diagnosis. Gray-scale US features of TNs, such as location, microcalcification, were also different between the benign and malignant groups, but this difference was not significantly significant (all P>0.05). The ICC for the vascular pattern was 0.816 in the longitudinal sections and 0.817 in the transverse sections.
Table 1
| Parameter | Thyroid nodules | P-value | |
|---|---|---|---|
| Benign | Malignant | ||
| Gender | 0.99 | ||
| Male | 8 | 23 | |
| Female | 20 | 58 | |
| Ages (mean ± SD), years | 46.32±12.22 | 42.41±11.41 | 0.13 |
| Nodule location | 0.23 | ||
| Isthmus | 2 | 5 | |
| Left side | 9 | 41 | |
| Right side | 17 | 35 | |
| Maximum diameter (median, IQR), mm | 7.0, 4.0 | 8.0, 2.0 | 0.18 |
| Solid composition | >0.99 | ||
| Yes | 28 | 79 | |
| No | 0 | 2 | >0.99 |
| Marked hypoechoic | |||
| Yes | 1 | 2 | |
| No | 27 | 79 | |
| Taller-than-wide shape | 0.64 | ||
| Yes | 22 | 60 | |
| No | 6 | 21 | |
| Ill-defined/irregular margin or extrathyroidal extension | 0.80 | ||
| Yes | 26 | 74 | |
| No | 2 | 7 | |
| Microcalcifications | 0.19 | ||
| Yes | 19 | 40 | |
| No | 9 | 41 | |
| C-TIRADS | 0.30 | ||
| 4A | 0 | 0 | |
| 4B | 2 | 12 | |
| 4C | 26 | 69 | |
| SMI + C-TIRADS | <0.001 | ||
| 4A | 11 | 5 | |
| 4B | 0 | 0 | |
| 4C | 17 | 76 | |
SD, standard deviation; IQR, interquartile range; C-TIRADS, Chinese Thyroid Imaging Reporting and Data System; SMI, superb microvascular imaging.
Comparison between qualitative and quantitative SMI
The results of qualitative and quantitative SMI between malignant and benign nodules in the longitudinal and transverse sections are demonstrated in Table 2. A significant difference was observed in qualitative SMI in the longitudinal and transverse sections (P=0.01 and 0.002, respectively), indicating that the vascular modality of malignant nodules is prone to be type III. As for quantitative SMI, the VI was significantly higher in malignant nodules than that in benign nodules both in the longitudinal (19.9±11.4 vs. 13.8±10.6, P=0.01) and transverse (20.2±12.1 vs. 11.3±8.7, P=0.001) sections. However, we found that there was no significant difference between the longitudinal and transverse sections in benign (13.8±10.6 vs. 11.3±8.7, P=0.35) and malignant (19.9±11.4 vs. 20.2±12.1, P=0.91) nodules. To further explore whether there were differences between qualitative and quantitative SMI, we compared the diagnostic performance of SMI in the same section. As shown in Table 3, the AUC values of qualitative and quantitative SMI were not statistically different in the longitudinal [0.657 (95% CI: 0.560–0.745) vs. 0.646 (95% CI: 0.549–0.735), P=0.79] and transverse [0.696 (95% CI: 0.600–0.780) vs. 0.725 (95% CI: 0.632–0.806), P=0.51] sections. Next, we found no significant difference between qualitative SMI [AUC: 0.657 (95% CI: 0.560–0.745) vs. 0.696 (95% CI: 0.600–0.780), P=0.42] and quantitative SMI [AUC: 0.646 (95% CI: 0.549–0.735) vs. 0.725 (95% CI: 0.632–0.806), P=0.06] in the longitudinal and transverse sections.
Table 2
| Parameter | Thyroid nodules | P value | |
|---|---|---|---|
| Benign | Malignant | ||
| Longitudinal section | |||
| Qualitative SMI | 0.01 | ||
| Type I | 5 | 4 | |
| Type II | 12 | 22 | |
| Type III | 11 | 55 | |
| Quantitative SMI-VI (mean ± SD), % | 13.8±10.6 | 19.9±11.4 | 0.01 |
| Transverse section | |||
| Qualitative SMI | 0.002 | ||
| Type I | 6 | 3 | |
| Type II | 12 | 24 | |
| Type III | 10 | 54 | |
| Quantitative SMI-VI (mean ± SD), % | 11.3±8.7 | 20.2±12.1 | 0.001 |
SMI-VI, superb microvascular imaging-based vascular index; SD, standard deviation.
Table 3
| Parameter | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | AUC (95% CI) | P value |
|---|---|---|---|---|---|---|---|
| Longitudinal section | |||||||
| Qualitative SMI | 67.9 | 60.7 | 83.3 | 60.7 | 66.1 | 0.657 (0.560–0.745) | 0.005 |
| Quantitative SMI | 81.5 | 50.0 | 82.5 | 48.3 | 73.4 | 0.646 (0.549–0.735) | 0.02 |
| Transverse section | |||||||
| Qualitative SMI | 66.7 | 67.9 | 85.7 | 41.3 | 76.1 | 0.696 (0.600–0.780) | <0.001 |
| Quantitative SMI | 53.1 | 82.1 | 89.6 | 36.7 | 60.6 | 0.725 (0.632–0.806) | <0.001 |
SMI, superb microvascular imaging; PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve; CI, confidence interval.
Combination of SMI and C-TIRADS to diagnose thyroid carcinoma
We then combined SMI and C-TIRADS to diagnose thyroid carcinoma. The optimal cut-off value for the SMI pixel count for predicting malignant TNs was 12.2. Hence, if a C-TR4B nodule had a VIsum >12.2 or intra-nodular vascularity, the original C-TIRADS was upgraded to C-TR4C. If a C-TR4C or C-TR4B nodule manifested a VIsum ≤12.2 and no intra-nodular vascularity, the original C-TIRADS was downgraded to C-TR4A. As a result, 18 C-TR4C nodules were downgraded to C-TR4A and 14 C-TR4B nodules were upgraded to C-TR4C. The diagnostic performance of the SMI + C-TIRADS for TNs was as follows: sensitivity 93.8%, specificity 39.3%, PPV 81.7%, NPV 68.8%, and accuracy 79.8%. The AUC value for the new TIRADS was 0.666 (95% CI: 0.569–0.753, P<0.001). According to C-TIRADS, we found that 26 benign nodules were defined as C-TR4C. Based on our new TIRADS, 11 benign nodules were downgraded to C-TR4A; patients with these nodules might avoid FNA and instead undergo active surveillance. We also found that 5 malignant nodules were downgraded to C-TR4A.
Discussion
Like other previous studies (5,16,18,25), our study did not cover all types of TNs. The present study focused on nodules classified as C-TIRADS 4, which are more important and meaningful in clinical practice. Conventional US features showed no statistical difference between benign and malignant TNs in our study (all P>0.05), highlighting the difficulty in distinguishing malignant from benign TNs by conventional US features.
Doppler US has been reported to distinguish benign and malignant TNs. However, the feasibility of vascularity in differentiating malignant from benign nodules remains controversial (13). Up to now, some studies have researched the value of qualitative SMI in differentiating thyroid carcinoma by evaluating the vascularity of TNs, and vascularity was classified as 3–5 patterns in these studies according to different criteria (11-18,20). Some of them concluded that SMI may contribute to the identification of malignant nodules, but others (17,20) revealed that SMI might not improve the diagnostic performance of US in differentiating TNs. This may be because there is no consensus regarding the vascular pattern of TNs. Different classification criteria may lead to different results and more categories could provide more detailed vascularity information. Consequently, a widely accepted and effective vascular classification is needed in the future. We found that intra-nodular vascularity may be a feature of malignant nodules, which was consistent with previous studies (14,23). In our study, intra-nodular vascularity on SMI was insufficient to determine malignant and benign nodules, with unsatisfying specificity and sensitivity. Although several studies have been conducted on qualitative and quantitative nodular vascularity in nodule classification (19,20), there is no research comparing qualitative and quantitative SMI in the diagnosis of thyroid carcinoma. Therefore, we planned to compare quantitative and qualitative SMI in identifying malignant and benign nodules in different sections. Our results indicated that there was no significant difference between qualitative vascular patterns and quantitative VI in diagnosing malignant nodules both in the longitudinal (P=0.79) and transverse (P=0.51) sections. It has been suggested that SMI in longitudinal sections may be more effective than that in transverse sections in reducing the influence of carotid pulsation (13). However, we believe that not all nodules are affected, and only those that are close to the lateral thyroid capsule may be affected by carotid pulsation.
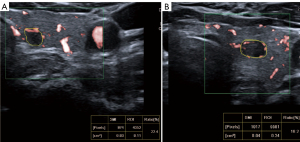
A study concluded that the combination of qualitative and quantitative CEUS can improve the diagnostic performance for malignant tumors (26). Meanwhile, we found some benign TNs with high VIs but peripheral vascularity on SMI (Figure 3). It is possible that the combination of qualitative and quantitative SMI could achieve better diagnostic performance. Hence, we combined qualitative and quantitative SMI to predict malignant TNs. A C-TR4B nodule was upgraded to C-TR4C if the sum of quantitative VIs was above 12.2 or had intra-nodular vascularity. However, if the sum of quantitative VIs was less than 12.2, a C-TR4C or C-TR4B nodule without intra-nodular vascularity would be downgraded to C-TR4A. After upgrading and downgrading, 16 nodules were downgraded into C-TR4A, and in these nodules, 11 nodules were benign. Although SMI + C-TIRADS had lower specificity, the diagnostic performance of combined SMI and C-TIRADS had high sensitivity (93.8%) and accuracy (79.8%). Moreover, 11 benign nodules could avoid FNA and instead undergo follow-up. Therefore, SMI may play an important role in improving the overdiagnosis of TNs.
It is known that the growth pattern of malignant nodules differs from that of benign nodules. Malignant nodules are prone to grow through the normal tissue plane in a centrifugal way (1), which may trigger angiogenesis in the same orientation. Our study did not find significantly different VIs between transverse sections and longitudinal sections in malignant nodules (P=0.91). Microcalcification is considered to contribute most highly to malignant nodules among several risk features (25). Malignant nodules may be difficult to distinguish from benign ones without microcalcification, and vascularity measurement may be affected by a twinkle artifact from calcification (23); hence, further studies can focus on identifying thyroid carcinoma from suspicious nodules without calcification by quantitative SMI.
This study had a few limitations. Firstly, there may have been a selection bias due to the small sample size. As a tertiary hospital in China, most of our patients were suspected of malignancy from local clinics or checkup centers, hence, we inevitably had a high malignant rate. This problem could be resolved by multicenter clinical studies including patients from different hospital levels. In addition, we only studied the effect of color SMI in the diagnosis of benign and malignant nodules, but found that the outlines of the nodules were clearer in color mode than that in monochrome mode. Thirdly, there was no consensus regarding the vascular pattern of malignant nodules. Our results may be different if various criteria were used. Lastly, the ROIs were drawn freestyle, which may involve problems in depicting the accurate margin and VIs of nodules. As a non-invasive method, more accurate ways of measuring the VI are needed to expand its application in the future.
Conclusions
In this study, we found there was no statistical difference between qualitative and quantitative SMI in the diagnosis of C-TIRADS 4 TNs ≤10 mm in the same sections. However, when combining qualitative and quantitative SMI to upgrade and downgrade C-TIRADS classification, the new model (SMI + C-TIRADS) had high sensitivity (93.8%) and accuracy (79.8%). Further studies should be conducted to explore the optimal classification of blood flow patterns and the combination of qualitative and quantitative SMI to predict malignant TNs.
Acknowledgments
Funding: This research was funded by the National High Level Hospital Clinical Research Funding (Nos. 2022-PUMCH-B-066 and 2022-PUMCH-D-001).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-1193/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1193/coif). The authors report that this research was funded by the National High Level Hospital Clinical Research Funding (Nos. 2022-PUMCH-B-066 and 2022-PUMCH-D-001). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Peking Union Medical College Hospital (No. JS-2881) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rago T, Vitti P. Risk Stratification of Thyroid Nodules: From Ultrasound Features to TIRADS. Cancers (Basel) 2022;14:717. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Chung SR, Ahn HS, Choi YJ, Lee JY, Yoo RE, Lee YJ, Kim JY, Sung JY, Kim JH, Baek JH. Diagnostic Performance of the Modified Korean Thyroid Imaging Reporting and Data System for Thyroid Malignancy: A Multicenter Validation Study. Korean J Radiol 2021;22:1579-86. [Crossref] [PubMed]
- Zhou J, Yin L, Wei X, Zhang S, Song Y, Luo B, et al. 2020 Chinese guidelines for ultrasound malignancy risk stratification of thyroid nodules: the C-TIRADS. Endocrine 2020;70:256-79. [Crossref] [PubMed]
- Li X, Gao F, Li F, Han XX, Shao SH, Yao MH, Li CX, Zheng J, Wu R, Du LF. Qualitative analysis of contrast-enhanced ultrasound in the diagnosis of small, TR3-5 benign and malignant thyroid nodules measuring ≤1 cm. Br J Radiol 2020;93:20190923. [Crossref] [PubMed]
- Gordon MS, Mendelson DS, Kato G. Tumor angiogenesis and novel antiangiogenic strategies. Int J Cancer 2010;126:1777-87. [Crossref] [PubMed]
- Jiang ZZ, Huang YH, Shen HL, Liu XT. Clinical Applications of Superb Microvascular Imaging in the Liver, Breast, Thyroid, Skeletal Muscle, and Carotid Plaques. J Ultrasound Med 2019;38:2811-20. [Crossref] [PubMed]
- Zhao W, Lu R, Yin L, Guo R. The value of superb microvascular imaging (SMI) scoring assignment method in differentiating benign and malignant thyroid nodules by conventional ultrasound. Clin Hemorheol Microcirc 2021;78:355-63. [Crossref] [PubMed]
- Diao XH, Shen Y, Chen L, Zhan J, Fang L, Liu YC, Chen Y. Superb microvascular imaging is as sensitive as contrast-enhanced ultrasound for detecting synovial vascularity in rheumatoid arthritis. Quant Imaging Med Surg 2022;12:2866-76. [Crossref] [PubMed]
- Meng Q, Xie X, Li L, Jiang C, Zhao K, Bai Z, Zheng Z, Yang Y, Yu Y, Zhang H, Zhao X. Assessment of neovascularization of carotid artery atherosclerotic plaques using superb microvascular imaging: a comparison with contrast-enhanced ultrasound imaging and histology. Quant Imaging Med Surg 2021;11:1958-69. [Crossref] [PubMed]
- Ahn HS, Lee JB, Seo M, Park SH, Choi BI. Distinguishing benign from malignant thyroid nodules using thyroid ultrasonography: utility of adding superb microvascular imaging and elastography. Radiol Med 2018;123:260-70. [Crossref] [PubMed]
- Cappelli C, Pirola I, Gandossi E, Marini F, Cristiano A, Casella C, Lombardi D, Agosti B, Ferlin A, Castellano M. Ultrasound Microvascular Blood Flow Evaluation: A New Tool for the Management of Thyroid Nodule? Int J Endocrinol 2019;2019:7874890. [Crossref] [PubMed]
- Chen L, Zhan J, Diao XH, Liu YC, Shi YX, Chen Y, Zhan WW. Additional Value of Superb Microvascular Imaging for Thyroid Nodule Classification with the Thyroid Imaging Reporting and Data System. Ultrasound Med Biol 2019;45:2040-8. [Crossref] [PubMed]
- Kong J, Li JC, Wang HY, Wang YH, Zhao RN, Zhang Y, Jin J. Role of Superb Micro-Vascular Imaging in the Preoperative Evaluation of Thyroid Nodules: Comparison With Power Doppler Flow Imaging. J Ultrasound Med 2017;36:1329-37. [Crossref] [PubMed]
- Lu R, Meng Y, Zhang Y, Zhao W, Wang X, Jin M, Guo R. Superb microvascular imaging (SMI) compared with conventional ultrasound for evaluating thyroid nodules. BMC Med Imaging 2017;17:65. [Crossref] [PubMed]
- Pei S, Cong S, Zhang B, Liang C, Zhang L, Liu J, Guo Y, Zhang S. Diagnostic value of multimodal ultrasound imaging in differentiating benign and malignant TI-RADS category 4 nodules. Int J Clin Oncol 2019;24:632-9. [Crossref] [PubMed]
- Yoon JH, Kim EK, Kwak JY, Park VY, Moon HJ. Application of Various Additional Imaging Techniques for Thyroid Ultrasound: Direct Comparison of Combined Various Elastography and Doppler Parameters to Gray-Scale Ultrasound in Differential Diagnosis of Thyroid Nodules. Ultrasound Med Biol 2018;44:1679-86. [Crossref] [PubMed]
- Zhang L, Gu J, Zhao Y, Zhu M, Wei J, Zhang B. The role of multimodal ultrasonic flow imaging in Thyroid Imaging Reporting and Data System (TI-RADS) 4 nodules. Gland Surg 2020;9:1469-77. [Crossref] [PubMed]
- Chambara N, Liu SYW, Lo X, Ying M. Diagnostic Value of AngioPLUS Microvascular Imaging in Thyroid Nodule Diagnosis Using Quantitative and Qualitative Vascularity Grading. Biomedicines 2022; [Crossref] [PubMed]
- Yoon JH, Shin HJ, Kim EK, Moon HJ, Roh YH, Kwak JY. Quantitative Evaluation of Vascularity Using 2-D Power Doppler Ultrasonography May Not Identify Malignancy of the Thyroid. Ultrasound Med Biol 2015;41:2873-83. [Crossref] [PubMed]
- Durmaz MS, Kara Gedik G, Batur A, Yılmaz F. Using 2-dimensional color superb microvascular imaging vascularization index technique in the assessment of thyroid surgical bed. Med Ultrason 2021;23:289-96. [Crossref] [PubMed]
- Kurt SA, Kayadibi Y, Saracoglu MS, Ozturk T, Korkmazer B, Cerit M, Velidedeoğlu M. Prediction of Molecular Subtypes Using Superb Microvascular Imaging and Shear Wave Elastography in Invasive Breast Carcinomas. Acad Radiol 2023;30:14-21. [Crossref] [PubMed]
- Sultan LR, Xiong H, Zafar HM, Schultz SM, Langer JE, Sehgal CM. Vascularity assessment of thyroid nodules by quantitative color Doppler ultrasound. Ultrasound Med Biol 2015;41:1287-93. [Crossref] [PubMed]
- Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2009;19:1159-65. [Crossref] [PubMed]
- Wang J, Jiang J, Zhang D, Zhang YZ, Guo L, Jiang Y, Du S, Zhou Q. An integrated AI model to improve diagnostic accuracy of ultrasound and output known risk features in suspicious thyroid nodules. Eur Radiol 2022;32:2120-9. [Crossref] [PubMed]
- Iwasa Y, Iwashita T, Ichikawa H, Mita N, Uemura S, Yoshida K, Iwata K, Mukai T, Yasuda I, Shimizu M. Efficacy of Contrast-Enhanced Harmonic Endoscopic Ultrasound for Pancreatic Solid Tumors with a Combination of Qualitative and Quantitative Analyses: A Prospective Pilot Study. Dig Dis Sci 2022;67:1054-64. [Crossref] [PubMed]


