
Insertion of a laryngeal mask airway guided with a transesophageal fiber bronchoscope for a patient with an irregular airway: a case description
Introduction
Bronchoscopic interventional procedures are novel means of treating airway lesions, but intra-airway manipulation creates challenges for airway management (1). As a common supraglottic device, laryngeal mask airways (LMAs) do not compete with the airway, do not interfere with the progress of bronchoscopic interventional surgery, and do not interrupt the continuous supply of oxygen, which facilitates assisted bronchoscopic interventional procedures (1). However, correct placement is a prerequisite for effective ventilation. Previous research suggests that 40% to 60% of blindly placed LMAs do not reach an ideal or nearly ideal position (2). In the case of irregular airways, the success rate of LMA blind insertion greatly decreases. Recurrent insertion not only interrupts the supply of oxygen but may result in edema, bleeding, or spasm of the larynx. Malpositioning can considerably increase the risk of gastric air insufflation, regurgitation, and aspiration (3). This case report discusses a new method to improve the success rate and accuracy of laryngeal mask placement for irregular airways. In the case of an irregular anatomy of the larynx and/or presence of a nasal feeding tube, blind placement of the LMA recurrently failed. We inserted a fiber bronchoscope into the esophageal passage through the drainage tube of the LMA and gradually pushed the laryngeal mask into the hypopharynx with the guidance of a fiber bronchoscope which achieved an ideal position and effective ventilation.
Case presentation
A 50-year-old male, with a weight of 61 kg and a height of 165 cm, was admitted into the emergency department with respiratory distress. On review of symptoms, the patient was initially diagnosed with posterior hypopharyngeal wall carcinoma with concomitant invasion of the cervical esophagus (T4N0M0 IV). In January 2020, the patient underwent hypopharyngeal wall carcinoma resection, cervical esophagus resection, esophageal reconstruction using the pectoralis major myocutaneous, and tracheostomy. In October 2021, the patient was diagnosed with a tracheal tumor and underwent fiberoptic bronchoscopic interventional surgery and bare airway stent (18–60 mm) implantation. In August 2022, the patient again complained of coughing, wheezing, and dyspnea. Chest computed tomography (CT) revealed patchy shadows of the left upper lung field, tracheal stent implantation status, soft tissue density shadow in the stent, and an unclear boundary between the local trachea and the esophagus. A tracheal CT scan revealed discontinuous bronchial walls, a narrow tracheal cavity, and a local tracheal fistula (Figure 1). Given the endotracheal tumor recurrence, further surgical treatment was needed. Diagnosis on admission was indicated as endotracheal tumors, radiation pneumonia of the left upper lung, posthypopharyngeal carcinoma resection status, tracheal stenosis, posttracheostomy status, and tracheal stent implantation status. After anti-inflammatory treatment with improved respiratory distress, the patient was scheduled to receive a fiberoptic bronchoscopic interventional procedure for removal of the lesions.
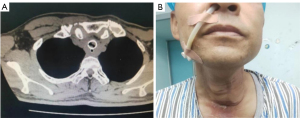
A previous tracheal stent was located about 3 cm below the glottis, this tracheal intubation could have limited the activity of a fiber bronchoscope and the interventional procedure in the tracheal cavity. Therefore, an LMA was chosen to facilitate assisted bronchoscopic interventional procedures. Endotracheal intubation was considered as a last resort to ensure the safety of the patient in case the LMA insertion failed. The patient had significant displacement of the larynx after hypopharyngeal carcinoma resection. The laryngeal node was positioned flush with the right clavicle midline rather than with the midsternal line. Moreover, the previous tracheostomy incision did not heal well owing to long-term radiotherapy, with an ensuing 0.25-cm fistula along the cervical surface with bubbling and airway secretions from the fistula on coughing. It was considered that high airway pressure may lead to failed ventilation with gas leakage from the fistula. Another point was that an unclear demarcation line between the local trachea and esophagus was suspected given the presence of a tracheoesophageal fistula. Malpositioning could considerably increase the pressure in the esophagus and result in gastric air insufflation, regurgitation, aspiration, or even severe intrapulmonary infection. Therefore, it was particularly important to ensure accurate positioning of the LMA and appropriate airway pressure during mechanical ventilation. Owing to severe cough and dysphagia, the patient was only fed through a feeding tube. Considering the possible difficulty of reimplantation of a feeding tube and the benefit of drainage of the gastric air and a reduction of reflux aspiration, we decided not to pull out the feeding tube (Figure 1). The insertion of the LMA undoubtedly became more difficult given the irregular anatomy of the larynx and the presence of a feeding tube.
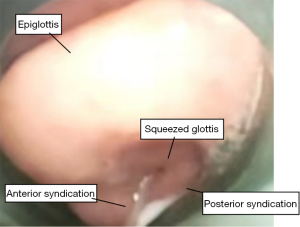
After arriving at the operating theatre, the patient was monitored with a multifunctional monitor electrocardiogram. The baseline vital signs were as follows: heart rate (HR), 76 bpm; respiratory rate (RR), 22 bpm; blood pressure, 20/70 mmHg; and oxygen saturation level (SpO2), 93%. After the tracheal fistula was covered with an oil veil, 5-minute preoxygenation was commenced using mask ventilation with oxygen flow at 6 L/min to increase SpO2 from 93% to 100%. Considering the risk of failure of LMA insertion and difficult ventilation, a spontaneous breath preserved anesthesia induction scheme without muscle relaxant was adopted. Subsequently, 100 mg of propofol and 5 µg of sufentanyl were administered. Topical anesthesia of the airway was reinforced by spraying 1% tetracaine into the trachea. A size 4 double-lumen LMA (LMA Supreme, Teleflex, Wayne, PA, USA) was then inserted blindly with the cuff deflated 20 mL and fully lubricated. The cuff was filled to 20 mL after the insertion. A significant audible leak and obscure chest expansion were observed after manual control of ventilation was initiated. A triangle capnograph and peak airway pressure exceeding 30 cmHg were observed after mechanical ventilation with a tidal volume of 300 mL and a ventilatory frequency of 14 bpm. The SpO2 then dropped from 100% to 85%. Fiberoptic bronchoscopy was performed to observe the glottic situation in a timely manner. Using a broncho fiberscope guided through the LMA tube to a position just proximal to the mask aperture, we observed the anterior epiglottis, deformation of the glottis, displacement of the right vocal fold, and oppression of the left vocal fold (Figure 2). The feeding tube was located in the esophagus, which resulted in the distal end of the LMA not aligning with the upper esophageal sphincter, and, thus, the mask was mismatched with the glottis. After multiple adjustments using visual guidance of a fiber bronchoscope, the mask was still not located at an ideal position. LMA Supreme is a new double-lumen tube, which is composed of a drainage tube for inserting a gastric tube and a classic ventilation cuff. The distal end of the drainage tube should align with the upper esophageal sphincter to relieve pressure of the stomach and facilitate the escape of gas from the stomach. Hence, a new method was attempted to guide the laryngeal mask placement. Under direct vision, the fiber bronchoscope was inserted into the esophageal passage through the drainage tube of the LMA (Figure 3). The laryngeal mask was then gradually advanced into the hypopharynx along the fiber bronchoscope until resistance was felt. After inflation of the cuff with air, fiberoptic bronchoscopy was performed again to observe the position of the LMA. We found that the laryngeal mask was 80% aligned, and the distal end of the laryngeal mask was aligned with the esophagus. There was adequate chest expansion and the absence of an audible leak after manual control of ventilation. A square wave capnograph and peak airway pressure less than 20 cmHg were observed after mechanical ventilation under the same parameters, and the SpO2 was up to 98%. Anesthesia was maintained through continuous administration of 6–10 mg/kg/h of propofol and 0.3 mg/kg of rocuronium as well as intermittent administration of 5 µg of sufentanyl (total 40 µg). The whole airway insertion was completed within 10 min. The lesion was removed, and a new covered stent was implanted. The bronchoscopist was satisfied with the procedures. SpO2 was over 95% at all times. The new stent was implanted 2 cm below the glottis. The fistula was successfully covered from the inside with no air leakage. After extubation, the patient was transferred to the ward without any LMA insertion–related complications, including sore throat and hoarseness.

All procedures performed in this study were conducted in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Bronchoscopic interventional procedures are novel means of treating airway lesions, but intra-airway manipulation creates challenges for airway management (4). In cases with subglottic and upper tracheal stenotic lesions, an airway device placed inside the lumen can interfere with the procedure (4). Therefore, a supra-glottic device is required for effective oxygenation. Many studies have confirmed that an LMA can facilitate assisted bronchoscopic interventional procedures, maintain better oxygenation and hemodynamic stability, and lead to fewer complications (1,3,4). Fadaizadeh et al. (4) reported that an LMA could be introduced as a reliable alternative for ventilation during interventional airway procedures. Schieren et al. (5) also suggested that supraglottic airway devices have the advantages of being safe and convenient for tracheal resection and reconstruction in cases of severe and proximally located subglottic stenosis. The case described here had proximally located subglottic lesions and airway stenosis with a suspicious tracheoesophageal fistula and open airway fistula; as a consequence, a flexible bronchoscope was used for the operation, and LMA was preferred for airway management. Moreover, the LMA was attached via a swivel 3-way connector to the anesthesia breathing circuit. A flexible fiber bronchoscope was passed through the free port of the connector. The arrangement allowed for the passage of an endoscope through the lumen of the LMA and into the trachea without interrupting the continuous supply of oxygen (Figure 4). One percent tetracaine sprayed onto the vocal folds prior to passing the bronchoscope through the glottic airway reduced airway irritation and inhibited the laryngeal reflex during fiberoptic bronchoscopy, which was the key to successfully achieving the spontaneous breath preserved anesthetic induction scheme.
The laryngeal mask requires high air tightness, and correct positioning is the prerequisite for effective ventilation. The ideal position for the laryngeal mask is the glottis covered by the cuff, with the proximal end opposite to the root of the tongue and with the distal end aligned with the upper sphincter in the esophagus, thus forming a sealed space with the larynx. Ideal or nearly ideal positioning of the LMA is required to minimize the risk of adverse airway events and maximize its intended functionality. Previous studies have suggested that 40% to 60% of blindly placed LMAs do not reach the ideal or nearly ideal position (4,6,7). Successful insertion is usually assessed clinically with a square wave on the capnograph, appropriate chest excursion, or the absence of an audible leak at a peak, which are all indirect assessment methods. While these methods are not visual (2), Fadaizadeh et al. (4) and Zhao et al. (2) suggested that fiberoptic visualization of the LMA position at the laryngeal entrance may be the gold standard of LMA positioning. Fiberoptic bronchoscopic grade can be classified as follows: grade 1 (A), the vocal cords are not visible, but function is adequate; grade 2 (B), the vocal cords plus the anterior epiglottis are visible; grade 3 (C), the vocal cords plus the posterior epiglottis are visible; and grade 4 (D), only the vocal cords are visible, and the position is considered optimal (6). However, fiberoptic bronchoscopy has only been considered a positioning method to be performed after LMA insertion. In our opinion, a fiber bronchoscope passing through the bypass port to align it with the upper esophagus for guidance allows for direct observation of the whole LMA insertion procedure, with adjustments of the angle being possible until the final ideal or nearly ideal position is achieved. This method makes the process of implantation visible and the position of LMA more accurate while avoiding the complications caused by blind repositioning, such as hypoxia and laryngospasm, sore throat, hoarseness, and bleeding. The difficulty of this case was the postoperative anatomic variation, with the whole larynx being shifted to the left, which posed a potential risk for airway management. The presence of a feeding tube and deformation of the glottis both resulted in malpositioning, such that the distal end was not aligned with the esophageal opening. Although it is not possible to change the shape of a curved rigid structure, the direction of insertion can be adjusted. When the fiber bronchoscope was inserted into the esophageal passage through the drainage tube of the LMA, the laryngeal mask was gradually advanced into the hypopharynx along the support and guidance of the fiber bronchoscope. With the distal end of the laryngeal mask aligned with the esophagus, the airbag mask could be aligned with the glottis, which was the key to successful insertion.
In summary, the fiber bronchoscope is the gold standard of LMA positioning. In face of failure of blind LMA insertion, or suboptimal or variant anatomy of the laryngeal and glottis, an LMA guided by transesophageal fiberoptic bronchoscopy could be an effective method.
Conclusions
Guidance with a fiber bronchoscope allows for the direct observation of the whole LMA insertion procedure and for the timely adjustment of the laryngeal mask to the ideal position. Insertion of the LMA guided by transesophageal fiberoptic bronchoscopy is a novel approach that can be used in case blind LMA insertion fails or when there is a suboptimal or variant anatomy of the laryngeal and glottis.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1180/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review from the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fadaizadeh L, Hosseini MS, Dabir S. Role of laryngeal mask airway in interventional bronchoscopy procedures for upper tracheal stenosis: case series. Middle East J Anaesthesiol 2013;22:223-7. [PubMed]
- Zhao L, Zhang J, Zhou Q, Jiang W. Comparison of a new visual stylet (Discopo)-guided laryngeal mask airway placement vs conventional blind technique: a prospective randomized study. J Clin Anesth 2016;35:85-9. [Crossref] [PubMed]
- Gunasekaran A, Govindaraj K, Gupta SL, Vinayagam S, Mishra SK. Comparison of Gastric Insufflation Volume Between Ambu AuraGain and ProSeal Laryngeal Mask Airway Using Ultrasonography in Patients Undergoing General Anesthesia: A Randomized Controlled Trial. Cureus 2022;14:e27888. [Crossref] [PubMed]
- Fadaizadeh L, Hoseini MS, Bagheri M. Anaesthesia Management During Interventional Bronchoscopic Procedures: Laryngeal Mask Airway or Rigid Bronchoscope. Turk J Anaesthesiol Reanim 2014;42:302-7. [Crossref] [PubMed]
- Schieren M, Egyed E, Hartmann B, Aleksanyan A, Stoelben E, Wappler F, Defosse JM. Airway Management by Laryngeal Mask Airways for Cervical Tracheal Resection and Reconstruction: A Single-Center Retrospective Analysis. Anesth Analg 2018;126:1257-61. [Crossref] [PubMed]
- Park JY, Yu J, Hong JH, Hwang JH, Kim YK. Head elevation and laryngeal mask airway Supreme insertion: A randomized controlled trial. Acta Anaesthesiol Scand 2021;65:343-50. [Crossref] [PubMed]
- Simsek T, Saracoglu A, Sezen O, Cakmak G, Saracoglu KT. Blind vs. video-laryngoscope-guided laryngeal mask insertion: A prospective randomized comparison of oropharyngeal leak pressure and fiberoptic grading. J Clin Monit Comput 2022;36:1249-55. [Crossref] [PubMed]



