
Accuracy and applicability of the novel pneumoperitoneum three-dimensional volume rendering technique in adhesive small bowel obstruction
Introduction
Small bowel obstruction (SBO) refers to the partial or complete blockage of the small intestine; it is a common surgical condition, and 20% of all emergency surgical procedures are SBO (1-3). Abdominal adhesions are the most common reason for SBO, accounting for approximately 60–75% of all cases (1,3). Approximately 80–91% of all patients with adhesive small bowel obstruction (ASBO) have previously undergone abdominal surgery (4,5).
Approximately 70% of patients with ASBO can be relieved by nonsurgical treatment (1,3,4). The Bologna guidelines recommend nonsurgical treatment in the absence of strangulation signs (6). However, the disadvantage of nonsurgical treatment is that pathogenic adhesions are not removed, and recurrence often increases the suffering of patients. Surgical treatment to release adhesions is one way to relieve the cause of the obstruction, but common complications include postoperative readhesion obstruction and iatrogenic bowel lesions, which may aggravate the problem.
ASBO cases are classified into two types according to the site of the obstruction adhesion: parietal adhesions (between the abdominal wall and bowel) and interintestinal adhesions (between bowel or mesentery structure and bowel) (7,8). The number of adhesion sites is typically divided into single adhesions and multiple adhesions (6-8). The type of ASBO is still closely related to the postoperative course of disease and the long-term efficacy and success of laparoscopic surgery. Surgical treatment has a better effect in treating single adhesions (1,7,9). Therefore, improving the diagnostic accuracy of adhesions that cause SBO, and screening suitable patients for surgery will reduce the risk of surgical uncertainty and optimize the effect of surgical treatment.
At present, the diagnosis of ASBO is mainly based on abdominal X-ray and computerized tomography (CT) (9-12). When evaluating patients suspected of having ASBO, radiologists play a crucial role and should address the issues that will arise during emergency diagnostic imaging. However, adhesive bands are not typically visible on CT imaging, and it is still difficult to diagnose the degree and amount of adhesions with medical imaging (6,9-13). Surgeons typically use personal experience and imaging to obtain a presumptive diagnosis. A definite diagnosis must be made using laparoscopy or laparotomy. Obtaining a diagnosis of adhesion in the clinical setting with high precision is currently difficult.
Three-dimensional volume rendering (3DVR) is a very mature imaging technology and is widely used in vascular and skeletal reconstruction (14-16). However, the density of gastrointestinal tissue is similar to that of abdominal soft tissue. These tissues lack a contrast interface and thus 3D reconstruction cannot be achieved. Pneumoperitoneum is established by injecting gas through abdominal puncture, and the density difference between the gas and soft-tissue interface is then used to reconstruct 3D CT (17). However, 3DVR is very challenging and has only been used in a few studies for parietal adhesions.
Therefore, this study aimed to demonstrate the use of pneumoperitoneum 3DVR to obtain an accurate diagnosis and to determine the applicability 3DVR in diagnosing interintestinal and parietal adhesions in ASBO. We present the following article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1096/rc).
Methods
Study design and population
This retrospective study enrolled patients with ASBO in the Department of Emergency Surgery of Fujian Medical University Union Hospital between October 2021 and May 2022. All patients provided written informed consent prior to the pneumoperitoneum procedure. The study protocol was approved by the Ethics Committee of Fujian Medical University Union Hospital (No. 2022KY154) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
The diagnosis of ASBO was based on clinical and radiological findings. The patients underwent CT examination before admission. Enrolled patients were subjected to the following inclusion criteria: (I) visualization with pneumoperitoneum 3DVR and (II) treatment with surgery. Patients who underwent nonsurgical treatment (n=2) and had poor 3DVR image quality (n=1) were excluded. Ultimately, a total of 22 patients were included in the analysis (Figure 1).
Pneumoperitoneum procedure
The surgeon selected a point away from the abdominal incision for pneumoperitoneum puncture and then administered local anesthesia. The surgeon and his assistant lifted the abdominal wall and punctured point with an 18G needle (Becton Dickinson Infusion Therapy Systems, Inc., Sandy, UT, USA). When the needle entered the peritoneal cavity, the metal needle core was withdrawn, the trocar was retained in the peritoneal cavity, and the trocar was connected to a 5-mL syringe of saline. Entrance to the peritoneal cavity was considered successful when the saline level decreased, after which sterile carbon dioxide was injected into the peritoneal cavity. The initial gas injection was slow and was accelerated after a successful pneumoperitoneum was confirmed. With a gradually enlarging abdomen, the patients exhibited different degrees of abdominal distension. The intra-abdominal pressure was usually 7–8 mmHg, depending on the patient’s tolerance, and was measured with a micromanometer. Generally, approximately 2,000–3,000 mL of gas was injected over 30 minutes. The gas was removed after the CT scan was completed.
CT scans and reconstruction
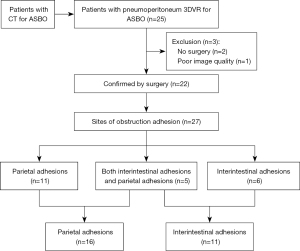
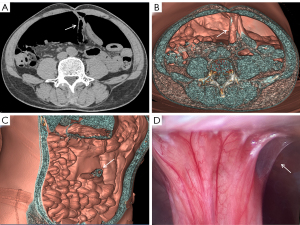
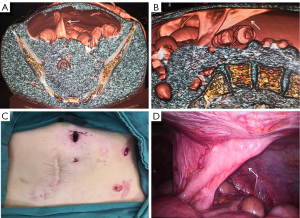
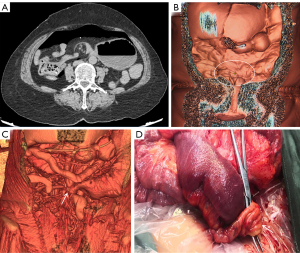
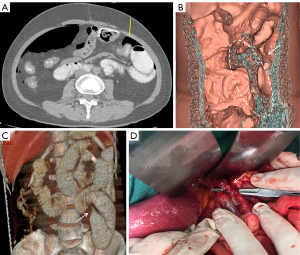
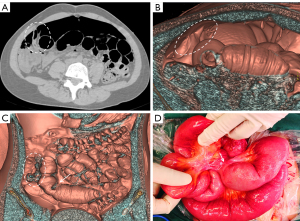
The patient underwent a CT examination in the radiology department after successful pneumoperitoneum establishment. The abdomen was scanned using a 256-slice spiral revolution CT machine (GE Healthcare, Chicago, IL, USA) with a slice thickness of 1.25 mm, a pitch of 1.375, a voltage of 120 kV, a current of 35 mA, and a speed of 0.28 s/r. The scanning range was from the top of the diaphragm to the upper edge of the pubic symphysis. The imaging data gathered from the abdominal CT scan were used to reconstruct the 3DVR images of the peritoneal cavity using the volume rendering technique with the use of computer-assisted design software (GE Advantage Workstation AW 4.7, GE Healthcare). All pneumoperitoneum CT scans were reconstructed and evaluated by 2 senior radiologists (radiologist Zhiyong Chen with 12 years of experience in abdominal radiology and radiologist Enshuang Zheng with 8 years of experience in general radiology). A 3DVR image was used to show the best angle to view the adhesions (Figures 2-6).
ASBO treatment
For patients with ASBO and signs of radiological evidence of strangulation or peritonitis, emergency surgery was mandatory. Conversely, nonsurgical treatment was performed in the absence of strangulation signs. If abdominal dilatation or nausea was observed at the time of admission, a nasogastric tube was inserted or a nasointestinal tube decompression was performed. After the bowel obstruction was relieved, the surgeon determined the need for semi-emergency surgery based on the type of the patient’s adhesions and the patient’s consent. The surgeon chose the appropriate surgical approach according to the patient’s 3DVR results and performed the operation with the patient under general anesthesia.
Data collection
Patient data including sex, age, history of previous surgery, nasogastric or nasointestinal tube decompression, pneumoperitoneum 3DVR results, surgical findings, pneumoperitoneum complications, and recurrent bowel obstruction were collected. The surgical findings were taken as the gold standard and compared with the diagnostic results of pneumoperitoneum 3DVR.
Statistical analysis
The normality of the data distribution was evaluated using the Shapiro-Wilk test. Normally distributed data are presented as mean ± SD. Data with a skewed distribution are described using medians and quartiles [M (P25, P75)]. Categorical data are presented as numbers and percentages, and the consistency of the pneumoperitoneum 3DVR diagnostic results and surgical findings was evaluated with a kappa test. Kappa 0.8–1.0 indicated almost perfect consistency, 0.6–0.8 substantial consistency, and 0.4–0.6 moderate consistency. Statistical significance was defined as a P value <0.05. We performed all analyses using SPSS 25.0 software (IBM Corp., Armonk, NY, USA).
Results
Clinical characteristics
A total of 22 patients were included in this study (Figure 1). The patients had a mean age of 52.82±11.63 years, and 12 (54.5%) were female. All patients had a history of previous surgery, 18 patients underwent nasogastric tube decompression, and 1 patient underwent nasointestinal tube decompression. The patient clinical features are summarized in Table 1.
Table 1
| Variable | Patients (n=22) |
|---|---|
| Sex | |
| Male | 10 (45.5%) |
| Female | 12 (54.5%) |
| Age (years), mean ± SD | 52.82±11.63 |
| History of previous surgery | 22 (100%) |
| Previous surgical approach | |
| Open | 18 (81.8%) |
| Laparoscopic | 4 (18.2%) |
| Nasogastric tube decompression | 18 (81.8%) |
| Nasointestinal tube decompression | 1 (4.5%) |
SD, standard deviation.
Comparison of 3DVR results and surgical findings
Pneumoperitoneum 3DVR results and surgical findings of the patients are shown in Table2. In total, 27 sites of obstruction adhesions were found during surgery, and 5 of the patients had both parietal adhesions and interintestinal adhesions. Sixteen parietal adhesions (16/16) were found using pneumoperitoneum 3DVR (κ=1.00; P<0.001), and the diagnosis of parietal adhesions on pneumoperitoneum 3DVR was perfectly consistent with the surgical findings. Eight (8/11) interintestinal adhesions were found using pneumoperitoneum 3DVR (κ=0.727; P<0.001), and the diagnosis of interintestinal adhesions on pneumoperitoneum 3DVR was substantially consistent with the surgical findings. Therefore, pneumoperitoneum 3DVR could objectively display adhesion morphology and provide high-quality objective clinical evidence (Figures 2-6).
Outcomes of pneumoperitoneum
The pneumoperitoneum procedures were technically successful in all 22 patients. No intestinal injuries or hematomas occurred. The incidence of subcutaneous emphysema was 4.5% (1/22), and none of the patients who developed subcutaneous emphysema required intervention (Table 2).
Table 2
| Variable | Patients (n=22) |
|---|---|
| Site of obstruction adhesion | |
| Parietal adhesions (3DVR/surgical findings) | 16/16 (100.0) |
| Interintestinal adhesions (3DVR/surgical findings) | 8/11 (72.7) |
| Surgical approach | |
| Open | 6 (27.3) |
| Laparoscopic | 16 (72.7) |
| 3DVR imaging to operation time (days) | 2 [1–3] |
| Subcutaneous emphysema | 1 (4.5) |
| Recurrent bowel obstruction | 1 (4.5) |
Data are shown as n (%) or medians and quartiles. 3DVR, 3-dimensionalvolume rendering.
Outcomes of surgery
Sixteen patients underwent laparoscopic surgery, and six patients underwent open surgery. All patients showed significant relief of their bowel obstruction after surgery. One patient (4.5%) was readmitted for recurrences within the study period among the 22 patients undergoing ASBO surgery (Table 2).
Discussion
In this study, 3DVR based on pneumoperitoneum was technically successful in all 22 patients, and parietal adhesions and interintestinal adhesions were clearly displayed using 3DVR. 3DVR can provide adequate preoperative evaluation and allow the development of an efficient surgical plan. All patients showed significant relief of their bowel obstruction after surgery. Therefore, this study demonstrated that the accuracy and applicability of novel 3DVR are feasible for ASBO.
The diagnosis of ASBO is based on clinical manifestations, previous patient history, and CT features (1,5,6). The radiologist is able to determine the site, level, and cause of bowel obstruction from CT images (9-12). However, the adhesion is generally not identifiable on CT scans. In previous studies, several CT features predictive of adhesive bands in the transition zone have been described, such as a closed loop, the beak sign, the fat notch sign, the whirl sign, and the feces sign (9,12,18). In addition, coronal and sagittal multiplanar CT images have proven helpful in detecting signs of features in the transition zone (11,12,18). However, in the majority of emergency patients, typical CT features in the presumptive diagnosis of adhesions as the cause of SBO are lacking. Therefore, it is very difficult for radiologists to diagnose the degree and amount of adhesions with medical imaging.
Pneumoperitoneum puncture is a critical first step in this procedure. Previous studies have reported the feasibility of pneumoperitoneum puncture for patients, and clinical experience shows that pneumoperitoneum puncture is a low-risk procedure (17,19,20). According to Wang et al. (17), 6.6% of the 331 patients assessed developed complications of subcutaneous emphysema, and 24 patients in the study by Palmer et al. (20) experienced no immediate major adverse events. Compared with the procedure of establishing pneumoperitoneum by laparoscopy, we used a slim-diameter needle to inject gas, which significantly reduced the probability of developing a puncture injury to internal organs (21). Pneumoperitoneum puncture is suitable for patients with remission or partial remission of bowel obstruction, when the patient’s abdominal wall is loose and can expand easily and when the patient’s cardiopulmonary function is basically normal. For obese patients or patients with multiple operations for diffuse adhesions, the dynamic observation of free gas under the diaphragm can be used to determine if pneumoperitoneum has been successfully obtained, which can improve the success rate of puncture (17,20). For patients with extensive intraperitoneal intestinal dilatation or a strangulated intestinal obstruction, it is not easy to establish a satisfactory pneumoperitoneal space. Moreover, the probability of an intestinal puncture injury is increased, and, therefore, this technique is not suitable for pneumoperitoneum puncture. Before this technology is applied, the patient’s indications should be carefully considered, and the surgeon must have good surgical skills.
Pneumoperitoneum 3DVR can accurately display the site, scope, structural composition, and shape of adhesions causing ASBO. Since the abdominal wall is separated from the abdominal organs during the state of pneumoperitoneum, the parietal adhesions can be accurately displayed (Figures 2,3). In our study, the diagnostic accuracy of parietal adhesions was 100%, which is consistent with the reported accuracy of pneumoperitoneum CT diagnosis, which was 95.46% in a previous study (17). However, no previous study has reported the diagnosis of interintestinal adhesions with the assistance of pneumoperitoneum 3DVR. Interintestinal adhesions are sometimes not clearly displayed, and different filter values need to be used to better visualize interintestinal adhesions in three dimensions (Figure 4). In this study, 3 cases of interintestinal adhesions were not clearly displayed with 3DVR, and after the intraoperative confirmation of interintestinal matted adhesions, it was noted that these adhesions were too thin and difficult to capture using 3DVR (Figure 6). Future application of cinematic rendering technology can generate photorealistic images with the potential to depict the anatomic details more accurately, which can further improve the diagnostic accuracy of interintestinal adhesions (22,23).
Pneumoperitoneum 3DVR enables the surgeon to accurately assess the patient’s intra-abdominal adhesions before the operation and can be used for the analysis and judgment of the obstruction mechanism to make a reasonable surgical plan (24,25). The surgeon can correctly select the position of the first trocar for the placement of the laparoscope, which is one of the key steps in laparoscopic surgery (Figure 3C). Pneumoperitoneum 3DVR can predict the difficulty of laparoscopic surgery, which can be conducive to timely conversion to laparotomy during surgery to avoid serious complications. If pneumoperitoneum 3DVR indicates extensive and dense parietal adhesions or clumps of interintestinal adhesions that can easily cause iatrogenic intestinal rupture, laparoscopic surgery is not suitable (Figure 5). For the patient, 3DVR allows the surgeon to easily obtain an understanding of any complex anatomy, the expected outlook of the surgical effect, and the specific and objective risk measurement (25). For medical students, 3DVR provides a visualized educational tool for ASBO.
At present, 3D printing is another means of performing good preoperative surgical planning (24,26). This method allows surgeons to understand individual anatomical features and customize the 3D printing of different materials for the patients (27). However, most of the prototypes could not achieve a good simulation due to limitations of the materials (26). Compared with 3D printing, 3DVR is readily available, less expensive, and less time-consuming. In addition, 3DVR can be integrated in holographic navigation surgery, in which the patient’s anatomy appears on the screen as a holographic 3D image, allowing the surgeon to then visualize the patient’s anatomy from multiple angles (28,29). 3DVR intraoperative navigation is a safe and effective adjunct to surgical procedures. The future of pneumoperitoneum 3DVR intraoperative navigation in ASBO surgery is worth exploring further.
In this study, local anesthesia was administered, gas was injected through a slim-diameter needle, and the abdominal wall and intestinal loops were separated. Consequently, this provided sufficient space and density contrast for 3DVR and therefore increased the accuracy for adhesions and reduced the number of surgical complications. In the current study, 3DVR allowed for an accurate diagnosis, and no major complications occurred during the pneumoperitoneum procedure. In addition, the 3D reconstruction algorithm is relatively easy to establish, has the potential to reduce the overreliance on personal experience, and can promote the popularity of pneumoperitoneum 3DVR.
The study has some limitations. First, there was no control group for comparison. Second, there was a lack of a standardized grading system for adhesions. Third, the number of patients included in this study was not sufficiently high, and thus more patients need to be included in future studies.
Conclusions
The novel pneumoperitoneum 3DVR helped visualize the type of adhesions and is accurate and applicable in ASBO. This can help personalize the treatment of patients and can be useful in planning a more effective surgical path.
Acknowledgments
Funding: The project was supported by the Natural Science Foundation of Fujian Province (grant No. 2022J01247); the Education Department of Fujian Province Young and Middle-Aged Teacher Education Research Project (grant No. JAT210109); the Joint Funds for the Innovation of Science and Technology, Fujian Province (grant No. 2018Y9054); and the Medical “Creating Double High” Construction Funds, Fujian Province (grant No. [2021]76).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-1096/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1096/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Fujian Medical University Union Hospital (No. 2022KY154), and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tong JWV, Lingam P, Shelat VG. Adhesive small bowel obstruction - an update. Acute Med Surg 2020;7:e587. [Crossref] [PubMed]
- Millet I, Ruyer A, Alili C, Curros Doyon F, Molinari N, Pages E, Zins M, Taourel P. Adhesive small-bowel obstruction: value of CT in identifying findings associated with the effectiveness of nonsurgical treatment. Radiology 2014;273:425-32. [Crossref] [PubMed]
- Behman R, Nathens AB, Mason S, Byrne JP, Hong NL, Pechlivanoglou P, Karanicolas P. Association of Surgical Intervention for Adhesive Small-Bowel Obstruction With the Risk of Recurrence. JAMA Surg 2019;154:413-20. [Crossref] [PubMed]
- Ng YY, Ngu JC, Wong AS. Small bowel obstruction in the virgin abdomen: time to challenge surgical dogma with evidence. ANZ J Surg 2018;88:91-4. [Crossref] [PubMed]
- Mullan CP, Siewert B, Eisenberg RL. Small bowel obstruction. AJR Am J Roentgenol 2012;198:W105-17. [Crossref] [PubMed]
- Ten Broek RPG, Krielen P, Di Saverio S, Coccolini F, Biffl WL, Ansaloni L, et al. Bologna guidelines for diagnosis and management of adhesive small bowel obstruction (ASBO): 2017 update of the evidence-based guidelines from the world society of emergency surgery ASBO working group. World J Emerg Surg 2018;13:24. [Crossref] [PubMed]
- Duron JJ, Silva NJ, du Montcel ST, Berger A, Muscari F, Hennet H, Veyrieres M, Hay JM. Adhesive postoperative small bowel obstruction: incidence and risk factors of recurrence after surgical treatment: a multicenter prospective study. Ann Surg 2006;244:750-7. [Crossref] [PubMed]
- Ariake K, Yokoyama S, Doi T, Takemura S, Kajiwara T, Kuroda F. Effect of omentum removal on the risk for postoperative adhesive small bowel obstruction recurrence: a case-control study. Int J Surg 2015;13:27-32. [Crossref] [PubMed]
- Guerrini J, Zugna D, Poretti D, Samà L, Costa G, Mei S, Ceolin M, Biloslavo A, Zago M, Viganò L, Kurihara H. Adhesive small bowel obstruction: Single band or matted adhesions? A predictive model based on computed tomography scan. J Trauma Acute Care Surg 2021;90:917-23. [Crossref] [PubMed]
- Santillan CS. Computed tomography of small bowel obstruction. Radiol Clin North Am 2013;51:17-27. [Crossref] [PubMed]
- Paulson EK, Thompson WM. Review of small-bowel obstruction: the diagnosis and when to worry. Radiology 2015;275:332-42. [Crossref] [PubMed]
- Zins M, Millet I, Taourel P. Adhesive Small Bowel Obstruction: Predictive Radiology to Improve Patient Management. Radiology 2020;296:480-92. [Crossref] [PubMed]
- Catena F, Di Saverio S, Coccolini F, Ansaloni L, De Simone B, Sartelli M, Van Goor H. Adhesive small bowel adhesions obstruction: Evolutions in diagnosis, management and prevention. World J Gastrointest Surg 2016;8:222-31. [Crossref] [PubMed]
- Duran AH, Duran MN, Masood I, Maciolek LM, Hussain H. The Additional Diagnostic Value of the Three-dimensional Volume Rendering Imaging in Routine Radiology Practice. Cureus 2019;11:e5579. [Crossref] [PubMed]
- Jiang Z, Nimura Y, Hayashi Y, Kitasaka T, Misawa K, Fujiwara M, Kajita Y, Wakabayashi T, Mori K. Anatomical annotation on vascular structure in volume rendered images. Comput Med Imaging Graph 2013;37:131-41. [Crossref] [PubMed]
- Stull KE, Tise ML, Ali Z, Fowler DR. Accuracy and reliability of measurements obtained from computed tomography 3D volume rendered images. Forensic Sci Int 2014;238:133-40. [Crossref] [PubMed]
- Wang GS, Zhang ZY, Qi XT, Liu J, Liu T, Zhao JW, Chen XX, Chen Y. The technology of artificial pneumoperitoneum CT and its application in diagnosis of abdominal adhesion. Sci Rep 2021;11:20785. [Crossref] [PubMed]
- Petrovic B, Nikolaidis P, Hammond NA, Grant TH, Miller FH. Identification of adhesions on CT in small-bowel obstruction. Emerg Radiol 2006;12:88-93; discussion 94-5. [Crossref] [PubMed]
- Martínez-Hoed J, Bonafe-Diana S, Bueno-Lledó J. A systematic review of the use of progressive preoperative pneumoperitoneum since its inception. Hernia 2021;25:1443-58. [Crossref] [PubMed]
- Palmer SL, Abdoli S, Crookes PF. Preoperative Pneumoperitoneum: Low-Risk Surgical Adjunct to the Surgical Management of Dense Abdominal Adhesions. J Vasc Interv Radiol 2019;30:761-4. [Crossref] [PubMed]
- Cornette B, Berrevoet F. Trocar Injuries in Laparoscopy: Techniques, Tools, and Means for Prevention. A Systematic Review of the Literature. World J Surg 2016;40:2331-41. [Crossref] [PubMed]
- Elshafei M, Binder J, Baecker J, Brunner M, Uder M, Weber GF, Grützmann R, Krautz C. Comparison of Cinematic Rendering and Computed Tomography for Speed and Comprehension of Surgical Anatomy. JAMA Surg 2019;154:738-44. [Crossref] [PubMed]
- Gehrsitz P, Rompel O, Schöber M, Cesnjevar R, Purbojo A, Uder M, Dittrich S, Alkassar M. Cinematic Rendering in Mixed-Reality Holograms: A New 3D Preoperative Planning Tool in Pediatric Heart Surgery. Front Cardiovasc Med 2021;8:633611. [Crossref] [PubMed]
- Etherton D, Tee L, Tillett C, Wong YH, Yeong CH, Sun Z. 3D visualization and 3D printing in abnormal gastrointestinal system manifestations of situs ambiguus. Quant Imaging Med Surg 2020;10:1877-83. [Crossref] [PubMed]
- Rodriguez-Acevedo O, Elstner K, Read JW, Jacombs A, Ibrahim N. Functional 3DVR imaging of abdominal wall hernias. J Med Imaging Radiat Oncol 2020;64:663-7. [Crossref] [PubMed]
- Tejo-Otero A, Buj-Corral I, Fenollosa-Artés F. 3D Printing in Medicine for Preoperative Surgical Planning: A Review. Ann Biomed Eng 2020;48:536-55. [Crossref] [PubMed]
- Shan Q, Huang W, Shang M, Wang Z, Xia N, Xue Q, Wu Z, Ding X, Mao A, Wang Z. Customization of stent design for treating malignant airway stenosis with the aid of three-dimensional printing. Quant Imaging Med Surg 2021;11:1437-46. [Crossref] [PubMed]
- Du C, Li J, Zhang B, Feng W, Zhang T, Li D. Intraoperative navigation system with a multi-modality fusion of 3D virtual model and laparoscopic real-time images in laparoscopic pancreatic surgery: a preclinical study. BMC Surg 2022;22:139. [Crossref] [PubMed]
- Takiguchi S, Fujiwara Y, Yamasaki M, Miyata H, Nakajima K, Nishida T, Sekimoto M, Hori M, Nakamura H, Mori M, Doki Y. Laparoscopic intraoperative navigation surgery for gastric cancer using real-time rendered 3D CT images. Surg Today 2015;45:618-24. [Crossref] [PubMed]


