
Vulnerable carotid plaque characteristics on magnetic resonance vessel wall imaging: potential predictors for hemodynamic instability during carotid artery stenting
Introduction
Stroke has become the leading cause of death in Chinese population (1). Carotid artery stenotic disease is one of the major etiologies of ischemic stroke (2), and carotid artery stenting (CAS) is a common treatment strategy for this condition (3). However, several studies have shown that about 30–70% of the patients who undergo CAS treatment experience perioperative transient hypotension and bradycardia, defined as hemodynamic instability (HI) (4). This phenomenon may lead to cerebral infarction and cause a decline in cognitive function and subsequent cerebrovascular dementia (5). Although several investigators have identified many factors associated with HI (4), predicting perioperative HI before CAS surgery is still challenging. In previous studies, only computed tomography angiography (CTA) or ultrasound was used for the qualitative analysis of plaque characteristics, however, both are less accurate than is magnetic resonance (MR) vessel wall imaging, particularly for evaluating intraplaque hemorrhage (IPH).
The HI might be a manifestation of sequela of the sinus reflex due to the stenting procedures, and the interaction between the stent and atherosclerotic plaques might be related to the extent of the sinus reflex (6). Previous studies have demonstrated that carotid plaque morphology and compositions were associated with perioperative HI. The HI-associated plaque morphological and compositional features include extensive or eccentric lesions, a distance between the carotid bifurcation and maximum stenotic lesion <10 mm, and calcified or fibrous lesions (7-9). The stiffness of atherosclerotic plaque is associated with vulnerability (10). Histological studies indicate that vulnerable plaque is characterized by specific compositional features, such as a larger lipid-rich necrotic core (LRNC) and IPH (11,12). When a stent adds pressure to the atherosclerotic plaque, different plaque compositions transmit the pressure in different ways. However, it is unclear which composition plays the key role in occurrence of HI. Among noninvasive imaging modalities, MR vessel wall imaging has been shown to be an ideal approach to characterize carotid vulnerable plaque compositional features (13,14). However, few studies have used MR imaging to assess the relationship between carotid vulnerable plaque features and perioperative HI.
This study aimed to investigate the association between characteristics of carotid atherosclerotic plaques determined by MR vessel wall imaging and perioperative HI in patients with moderate-to-severe carotid artery atherosclerotic stenosis who underwent CAS surgery. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-865/rc).
Methods
Study sample
In this retrospective study, patients with carotid stenotic disease in the outpatient clinic who were referred for CAS treatment and admitted to the Department of Vascular Surgery of Beijing Tsinghua Changgung Hospital from January 2017 to December 2019 and subsequently underwent multicontrast MR vessel wall imaging for carotid arteries before CAS surgery were included. The inclusion criteria were symptomatic carotid stenosis (stenosis ≥50%, with ipsilateral black-out syndrome, transient ischemic attacks, or stroke) or asymptomatic carotid stenosis (stenosis ≥70%). The diagnosis of luminal stenosis was determined by ultrasound, according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria. The diagnostic criterion for ipsilateral black-out syndrome is transient ipsilateral blurred vision lasting for several minutes. The diagnostic criterion for transient ischemic attacks is an acute loss of contralateral focal cerebral or monocular function with symptoms lasting less than 24 hours. The diagnostic criterion for stroke is ipsilateral focal cerebral lesions confirmed by brain MR before CAS surgery (15). Participants with the following conditions were excluded: (I) conditions affecting perioperative blood pressure, such as renal artery stent implantation at the same time of CAS surgery or pacemaker placement in advance; (II) severe heart, liver, or kidney dysfunction; (III) intracranial hemorrhage occurring within 3 months prior to surgery or extensive cerebral infarction occurring within 4 weeks prior to surgery; (IV) allergies to iodine contrast agent or other angiographic contraindications; and (V) contraindications to MR examination. Clinical data, including age, gender, body mass index (BMI), history of hypertension, blood pressure before surgery, history of smoking, hyperlipidemia, diabetes mellitus, stroke, coronary heart disease, left ventricular ejection fraction (LVEF) as measured by echocardiography, and antihypertensive drug usage, were collected from the patients’ medical records. All data were collected from January 2020 to April 2020, and postprocessing work was completed from May 2020 to October 2020. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the ethics board of Beijing Tsinghua Changgung Hospital. Individual consent for this retrospective analysis was waived.
MR imaging protocol
All patients underwent carotid artery plaque imaging on a 3.0T MR scanner (Discovery 750, GE Healthcare, Chicago, IL, USA) with a carotid artery 8-channel phased array coil. The imaging sequences and parameters were as follows: 3-dimensional time-of-flight (3D TOF) with spoiled gradient echo, repetition time (TR)/echo time (TE) 21/3.3 ms, and flip angle 30°; 2-dimensional black-blood T1-weighted imaging with fast spin-echo, double inversion recovery, and TR/TE 800/10 ms; and 2-dimensional black-blood T2-weighted imaging with fast spin-echo, double inversion recovery, and TR/TE 4,412/103 ms. The carotid MR imaging was centered on the index carotid artery, which is defined as artery with the most severe stenosis and referred for CAS treatment. All sequences were acquired with an identical field of view of 14 cm × 14 cm, a slice thickness of 2 mm, and a longitudinal coverage of 32 mm.
MR imaging analysis
The MR vessel wall images of carotid arteries were interpreted by 2 radiologists with more than 3 years of experience in carotid artery MR imaging who were blinded to all clinical information. A custom-designed software (CASCADE; University of Washington, Seattle, WA, USA) was used for image analysis (16). The lumen and wall boundaries of the carotid arteries were outlined manually to measure lumen area, wall area, total vessel area, and wall thickness at each axial location. The normalized wall index (NWI) was calculated using the following equation: wall area/total vessel area ×100%. Calcification, LRNC, IPH, and fibrous cap rupture were identified and quantified using the published criteria (17,18). Vulnerable plaque was defined as plaque with a large LRNC (percent of the area of LRNC occupying vessel wall >40%), IPH, or fibrous cap rupture. A previous study found good to excellent intrareader and interreader reproducibility in measuring wall thickness, calcification area, LRNC area, and IPH area (19).
Endovascular procedure
Two experienced surgeons followed the same surgical procedure. Antiplatelet treatment (aspirin 100 mg and clopidogrel 75 mg per day) was administered for a minimum of 7 days prior to the surgical procedure. The CAS was performed under local anesthesia via the percutaneous transfemoral route. The surgical procedures were performed by an experienced vascular surgeon team. An embolism protective device (Spider FX, EV3; Medtronic, Minneapolis, MN, USA) was released at the distal end of the internal carotid artery. Patients with hypotension and/or bradycardia received 0.5 mg of atropine before balloon angioplasty. A predilatation balloon (4–20 mm, Sterling; Boston Scientific, Marlborough, MA, USA) was placed into the carotid artery to slowly expand the stenosis area at the speed of 1 atm/s to the normal balloon pressure. After removal of the balloon, 1 or 2 self-expandable nitinol stents (8–6 mm ×40 mm or 9–7 mm ×40 mm as determined by artery diameter; Protégé, EV3; Medtronic) was placed at the most stenotic area and completely covered the plaque region. A final angiogram, was performed to confirm the stent coverage, residual stenosis (less than 30%, otherwise, postdilatation was performed), or distal embolization (20). If there was poor expansion of the stenting, a poststent dilatation was performed.
Perioperative HI and treatment
Monitoring the upper arm cuff blood pressure was performed routinely. Blood pressure was recorded before predilatation and every minute after predilatation until 10 minutes after the stent implantation. The blood pressure drop (BPD) was defined as the difference of the systolic blood pressure (SBP) before predilatation minus the lowest SBP after stent implantation. The BPD was considered to be 0 mmHg when BPD <0 mmHg. Patients were divided into 2 groups: the HI group (BPD ≥30 mmHg or the lowest SBP <90 mmHg) and the non-HI group (BPD <30 mmHg and the SBP ≥90 mmHg) (4). Patients in the HI group were immediately injected with 0.5 mg of atropine. If SBP was still lower than 90 mmHg, an additional 0.5 mg of atropine was injected. In case of severe and prolonged HI (SBP <90 mmHg and/or heart rate <50 bpm lasting over 10 min), an infusion of dopamine 5–15 µg/kg/min was administered.
Statistical analysis
The continuous variables are described as the mean ± standard deviation (SD) or median and interquartile range (IQR) depending on the results of the normal distribution test. The lumen area, wall area, total vessel area, maximum wall thickness, NWI, volume of calcification, volume of LRNC, and volume of IPH were compared between the HI group and the non-HI group and between the prolonged HI group and the non-prolonged HI group using an independent t-test or the Mann-Whitney U test as appropriate. The prevalence of calcification, LRNC and IPH, fibrous cap rupture, and any vulnerable plaque features present between the 2 groups was compared using the Chi-squared test. Logistic regression was used to determine the odds ratio (OR) and corresponding 95% CI of carotid plaque features in predicting HI. A P value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS 24.0 (IBM Corp., Armonk, NY, USA).
Results
A total of 69 patients with carotid artery stenosis were scheduled to undergo CAS from January 2017 to December 2019. Of these patients, 8 were excluded due to the exclusion criteria, 5 were excluded due to poor image quality of the MR imaging (n=3) and lack of blood pressure data in the operation record (n=2). Ultimately, 56 patients were included in the statistical analysis. Among these 56 patients, the mean age was 68.7±8.3 years old, 44 (79%) were males, 8 (14%) had a history of ipsilateral stroke, 46 (82%) had hypertension, 17 (30%) had diabetes, and 20 (36%) had cardiovascular disease. The patient recruitment process is detailed in Figure 1. The detailed clinical information is listed in Table 1.
Table 1
| Clinical characteristics | All patients (n=56) | HI group (n=26) | Non-HI group (n=30) | P value |
|---|---|---|---|---|
| Age, years old | 68.7±8.3 | 70.6±8.4 | 67.1±7.9 | 0.12 |
| Sex, male | 44 (79.0) | 23 (88.0) | 21 (70.0) | 0.09 |
| BMI, kg/m2 | 25.5±3.3 | 25.2±3.2 | 25.7±3.4 | 0.57 |
| Hypertension | 46 (82.0) | 23 (88.0) | 23 (76.0) | 0.25 |
| SBP, mmHg | 132±14 | 134±14 | 131±15 | 0.39 |
| DBP, mmHg | 73±8 | 73±10 | 72±8 | 0.52 |
| Smoke | 34 (61.0) | 17 (65.0) | 17 (57.0) | 0.51 |
| Hyperlipidemia | 33 (59.0) | 17 (65.0) | 16 (53.0) | 0.36 |
| HDL, mmol/L | 0.94±0.28 | 0.91±0.22 | 0.97±0.33 | 0.50 |
| LDL, mmol/L | 2.31±0.92 | 2.38±0.93 | 2.24±0.93 | 0.61 |
| TC, mmol/L | 3.87±1.08 | 3.96±1.05 | 3.80±1.11 | 0.57 |
| TG, mmol/L | 1.55±1.04 | 1.57±1.15 | 1.53±0.96 | 0.89 |
| Diabetes | 17 (30.0) | 10 (43.0) | 7 (23.0) | 0.22 |
| History of stroke | 8 (14.0) | 4 (15.0) | 4 (13.0) | 0.83 |
| History of CHD | 20 (36.0) | 9 (35.0) | 11 (37.0) | 0.87 |
| LVEF, % | 64.3±3.2 | 64.7±3.6 | 63.9±2.9 | 0.32 |
| ACEI | 16 (29.0) | 7 (27.0) | 9 (30.0) | 0.90 |
| β-blocker | 18 (32.0) | 9 (34.0) | 9 (30.0) | 0.71 |
| CCB | 32 (57.0) | 16 (62.0) | 16 (53.0) | 0.54 |
| Statin | 33 (59.0) | 13 (50.0) | 20 (67.0) | 0.21 |
| Side, left | 31 (55.0) | 13 (50.0) | 18 (60.0) | 0.45 |
| Symptomatic | 29 (52.0) | 15 (58.0) | 14 (47.0) | 0.68 |
| Stenosis rate, % | 78±11 | 79±10 | 76±12 | 0.48 |
| Poststent dilatation | 10 (18.0) | 5 (19.0) | 5 (17.0) | 0.80 |
Data are shown as mean ± SD or n (%). HI, hemodynamic instability; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TC, total cholesterol; TG, triglyceride; CHD, coronary heart disease; LVEF, left ventricular ejection fraction; ACEI angiotensin-converting enzyme inhibitors; CCB, calcium channel blocker; SD, standard deviation.
Baseline characteristics of carotid plaques on MR imaging
Of the 56 patients, 26 (46%) were in the HI group and 30 (54%) were in the non-HI group. Patients in the HI group showed a significantly larger wall area [43.2 (34.9–50.5) vs. 35.9 (32.3–39.4); P=0.008] and total vessel area (79.7±17.2 vs. 69.9±17.3 mm2; P=0.03) of the carotid artery compared to those in the non-HI group. Significant differences were also found in the prevalence of IPH (62% vs. 30%; P=0.02) and vulnerable plaque (77% vs. 43%; P=0.01) and in the volume of LRNC [344.7 (IQR, 155.1–665.7) vs. 103.1 (53.9–62.9) mm3; P=0.001] between the HI group and the non-HI group. No significant differences were found in other carotid plaque characteristics between the HI group and the non-HI group (all P values >0.05). Of the 56 patients, 23 (41.1%) developed prolonged HI. Patients with prolonged HI showed greater plaque length [21.4 (16.5–26.6) vs. 16.5 (13.5–21.3) mm; P=0.02] compared to those non-prolonged HI (Table S1). Detailed information is listed in Table 2.
Table 2
| Plaque characteristics | Patients with HI (n=26) | Patients without HI (n=30) | P value |
|---|---|---|---|
| Plaque morphology | |||
| Lumen area, mm2 | 33.6 (29.4, 39.5) | 29.2 (20.5, 40.4) | 0.19 |
| Wall area, mm2 | 43.2 (34.9, 50.5) | 35.9 (32.3, 39.4) | 0.008* |
| Total vessel area, mm2 | 79.7±17.2 | 69.9±17.3 | 0.04* |
| Maximum wall thickness, mm | 4.86 (4.01, 6.25) | 4.21 (3.84, 4.87) | 0.08 |
| Plaque length, mm | 18.7 (15.7, 25.0) | 16.8 (13.3, 22.9) | 0.20 |
| Distance from carotid bifurcation to maximum stenotic lesion*, mm | 3.75 (0, 6.82) | 3.75 (0, 9.37) | 0.77 |
| NWI, % | 56.7±6.2 | 55.6±6.7 | 0.53 |
| Plaque compositions | |||
| Presence of calcification | 17 (65.0) | 18 (60.0) | 0.8 |
| Presence of LRNC | 25 (96.0) | 29 (97.0) | 0.72 |
| Presence of IPH | 16 (62.0) | 9 (30.0) | 0.02* |
| Volume of calcification, mm3 | 13.8 (4.8, 56.7) | 26.6 (8.7, 65.7) | 0.39 |
| Volume of LRNC, mm3 | 344.7 (155.1, 665.7) | 103.1 (53.9, 162.9) | 0.001* |
| Volume of IPH, mm3 | 124.0 (40.7, 377.5) | 54.1 (14.9, 127.0) | 0.15 |
| Fibrous cap rupture | 13 (50.0) | 9 (30.0) | 0.13 |
| Vulnerable plaque | 20 (77.0) | 13 (43.0) | 0.01* |
| Death | 0 | 0 | – |
| Stroke | 1 (4.0) | 0 | 0.46 |
Data are shown as mean ± SD, or n (%), or median (IQR). *, distance was calculated by the absolute value. SD, standard deviation; IQR, interquartile range; HI, hemodynamic instability; NWI, normalized wall index; LRNC, lipid-rich necrotic core; IPH, intraplaque hemorrhage.
Association between carotid plaque characteristics and HI
The results of the logistic regression analysis are summarized in Table 3. Univariate regression analysis showed that the carotid wall area (OR =1.098; 95% CI: 1.022–1.180; P=0.01) and total vessel area (OR =1.035; 95% CI: 1.001–1.070; P=0.05) were significantly associated with HI. Maximum wall thickness showed marginal association with HI (P=0.05). In addition, the presence of IPH (OR =3.733; 95% CI: 1.229–11.338; P=0.02), the volume of LRNC (OR =1.004; 95% CI: 1.001–1.007; P=0.005), and the presence of vulnerable plaque (OR =4.359; 95% CI: 1.362–13.954; P=0.01) were found to be significantly associated with HI. After adjustments were made for age, sex, and BMI (model 1), the wall area (P=0.04), volume of LRNC (P=0.01), and presence of vulnerable plaque (P=0.03) were still significantly associated with HI. After further adjustments were made for the plaque burden of NWI (model 2), only the associations of LRNC volume (P=0.01) and the presence of vulnerable plaque (P=0.06) with HI were statistically and marginally significant, respectively. The incidence of perioperative death and stroke was not significantly different between the 2 groups. Figure 2 shows a typical case of HI. Since the wall area had collinearity with NWI, it was not included in the model 2 analysis.
Table 3
| Plaque characteristics | HI | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate regression | Multivariate regression (model 1) | Multivariate regression (model 2) | ||||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |||
| Wall area | 1.098 (1.022–1.180) | 0.01 | 1.085 (1.002–1.174) | 0.04 | – | – | ||
| Total vessel area | 1.035 (1.001–1.070) | 0.05 | 1.024 (0.985–1.064) | 0.24 | – | – | ||
| Maximum wall thickness | 1.509 (0.994–2.293) | 0.05 | 1.420 (0.919–2.194) | 0.11 | – | – | ||
| Presence of IPH | 3.733 (1.229–11.338) | 0.02 | 2.934 (0.904–9.518) | 0.07 | 2.592 (0.712–9.440) | 0.15 | ||
| Volume of LRNC | 1.004 (1.001–1.007) | 0.005 | 1.004 (1.001–1.006) | 0.01 | 1.005 (1.001–1.009) | 0.01 | ||
| Vulnerable plaque | 4.359 (1.362–13.954) | 0.01 | 3.927 (1.171–13.165) | 0.03 | 4.038 (0.955–17.070) | 0.06 | ||
Model 1: adjusted for age, sex, and BMI; Model 2: adjusted for age, sex, BMI, and NWI. BMI, body mass index; NWI, normalized wall index; LRNC, lipid-rich necrotic core; IPH, intraplaque hemorrhage.
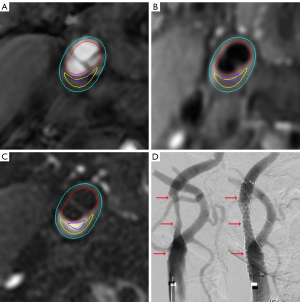
Discussion
This study investigated the association between carotid atherosclerotic plaque characteristics assessed by MR vessel wall imaging and HI in patients with carotid stenosis referred for CAS. We found that patients with a larger plaque burden and vulnerable plaque features, particularly a larger LRNC, had a higher risk of developing HI during the CAS procedure. Our results suggest that patients with a larger plaque burden and vulnerable plaque features in carotid arteries may need preventive treatment to reduce the risk of HI when undergoing CAS.
In the present study, the carotid plaque burden of the wall area was found to be the main risk factor for HI. The carotid plaque burden can be measured using wall thickness, wall area, luminal stenosis, plaque length, and plaque volume, which have been found to be associated with plaque vulnerability (21) and future vascular events (22). Previous studies have demonstrated that plaque burden is an independent risk factor for HI (8,23,24). A study by Popescu et al. (23) enrolled 120 patients who underwent CAS and reported that >90% of stenosis measured with digital subtraction angiography (DSA) was a risk factor for HI (OR =2.00; 95% CI: 0.95–4.18; P=0.07). Saleh et al. (8) investigated 728 patients who underwent CTA and CAS and found that patients with greater plaque length were more likely to develop HI (OR = 1.043, 95% CI: 1.012–1.076; P=0.007). Husmann et al. (24) reported that carotid plaque volume measured with 3D duplex sonography was significantly associated with a drop in heart rate (P=–0.57; P=0.01) during the CAS procedure. However, in these studies, the methodology of measuring carotid plaque burden was not uniform. Using angiographic approaches, such as CTA or DSA, will underestimate plaque length due to positive remodeling. In the present study, we used MR vessel wall imaging to measure plaque morphology, including plaque length, maximum wall thickness, and wall area, which is more accurate than angiographic approaches. We did not find a significant association between plaque length and HI in our study population. Our finding of an association between carotid wall area and HI indicates that carotid plaque size measured by wall area might be a more predictive risk factor for HI.
In the present study, carotid vulnerable plaque features, including the volume of LRNC and the presence of IPH, were found to be significantly associated with HI. In related studies, the influence of carotid plaque vulnerable characteristics on HI was not examined. Rubio et al. (7) enrolled 90 patients and measured the degree of calcification with CTA; they found that the severity of calcium score was not a significant predictor for postoperative HI (P=0.68). On the contrary, Saleh et al. (8) found that hyperechoic/calcified plaques (OR =2.195; 95% CI: 1.458–3.304; P<0.001) were significant predictive factors for the occurrence of HI. Similarly, Nonaka et al. (9) investigated 33 patients and found that calcification at the carotid bifurcation was a risk factor for perioperative HI (P<0.001). MR vessel wall imaging is a better method for characterizing noncalcified vulnerable plaque features, particularly for LRNC and IPH. However, the mechanism underlying the association between vulnerable carotid features determined using MR vessel wall imaging and HI is unclear (25). Anatomically, baroreceptors are located medial or anterior to the surface of the internal carotid artery. Nerve terminals have been observed to be mainly in contact with elastin at the media-adventitial border and collagen fibers in the inner part of the adventitia (26). According to Pascal’s law, a fluid at rest creates pressure at a point that is the same in all directions. When a stent adds pressure on the atherosclerotic plaque with LRNC, it will transmit the pressure to a wide range of intima, which stimulates a large number of neuroreceptors and leads to a larger drop in blood pressure. Our findings indicate greater attention should be paid to patients with vulnerable plaque to prevent HI during CAS treatment.
In our study, HI was defined as BPD ≥30 mmHg or SBP ≤90 mmHg. The definition of HI varies in the related literature. Mylonas et al. (4) performed a meta-analysis of 27 studies and reported that most (n=13) used SBP ≤90 mmHg as the definition of hypotension, while the other 13 studies defined it as SBP ≤90 mmHg or BPD ≥30–50 mmHg, and only 1 study used SBP ≤80 mmHg as the definition of hypotension. In a study by Ito et al. (27), hypotension was defined as BPD ≥20 mmHg. Our definition of HI was relatively strict, which made our HI occurrence rate higher than that in other studies. However, although most studies tried to use both blood pressure and heart rate in the definition of HI, they only separately analyzed the relationship between HI and blood pressure or between HI and heart rate (7,9). Studies using these 2 different definitions usually showed incongruous results (23). In our study, we focused on clarifying the relationship between HI and blood pressure but did not mention heart rate change because blood pressure change was the key factor of cerebral perfusion pressure (28).
Our study has several limitations. First, the sample size was small, and future studies with larger sample sizes are warranted. Second, only blood pressure was analyzed, and investigation using more blood pressure and heart rate data before and after CAS should be conducted to validate the generalizability of our findings. Third, this study only focused on the imaging features of carotid plaques determined using MR vessel wall imaging. Other factors, such as carotid bifurcation angle and hemodynamics, need to be taken into account to clarify other influencing factors of HI. Fourth, 2 experienced surgeons performed the surgery, and although there was no significant difference in the prevalence of HI between these 2 surgeons (P=0.54), future studies may be improved by use of a single surgeon.
Conclusions
Carotid plaque burden and vulnerable plaque features, particularly a larger LRNC, are associated with HI during the CAS procedure, suggesting that patients with a larger plaque burden and vulnerable plaque may have a higher risk of developing HI when undergoing CAS treatment.
Acknowledgments
Funding: This study was supported by grants of the Beijing Tsinghua Changgung Hospital Fund (Nos. 12016C1005 and 12017C1015) and the National Natural Science Foundation of China (No. 81771825).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-865/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-865/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:439-58. [Crossref] [PubMed]
- Ntaios G, Hart RG. Embolic Stroke. Circulation 2017;136:2403-5. [Crossref] [PubMed]
- Sardar P, Chatterjee S, Aronow HD, Kundu A, Ramchand P, Mukherjee D, Nairooz R, Gray WA, White CJ, Jaff MR, Rosenfield K, Giri J. Carotid Artery Stenting Versus Endarterectomy for Stroke Prevention: A Meta-Analysis of Clinical Trials. J Am Coll Cardiol 2017;69:2266-75. [Crossref] [PubMed]
- Mylonas SN, Moulakakis KG, Antonopoulos CN, Kakisis JD, Liapis CD. Carotid artery stenting-induced hemodynamic instability. J Endovasc Ther 2013;20:48-60. [Crossref] [PubMed]
- Pancak J, Wagnerova H, Škultéty Szárazová A, Blaho A, Durovsky O, Durovska J. Multi-infarct dementia and Alzheimer disease, contribution of cerebral circulation ultrasonography to pathogenesis and differential diagnosis. Value of microembolisation. Neuro Endocrinol Lett 2016;37:137-40. [PubMed]
- Kikuta S, Iwanaga J, Kusukawa J, Tubbs RS. Carotid Sinus Nerve: A Comprehensive Review of Its Anatomy, Variations, Pathology, and Clinical Applications. World Neurosurg 2019;127:370-4. [Crossref] [PubMed]
- Rubio G, Karwowski JK, DeAmorim H, Goldstein LJ, Bornak A. Predicting Factors Associated with Postoperative Hypotension following Carotid Artery Stenting. Ann Vasc Surg 2019;54:193-9. [Crossref] [PubMed]
- Saleh M, Ali H, Atalla K, Shahat M, Cieri E. Predictors of Carotid Artery Stenting-Induced Hemodynamic Instability. Vasc Endovascular Surg 2021;55:475-81. [Crossref] [PubMed]
- Nonaka T, Oka S, Miyata K, Mikami T, Koyanagi I, Houkin K, Yoshifuji K, Imaizumi T. Prediction of prolonged postprocedural hypotension after carotid artery stenting. Neurosurgery 2005;57:472-7; discussion 472-7. [Crossref] [PubMed]
- Selwaness M, van den Bouwhuijsen Q, Mattace-Raso FU, Verwoert GC, Hofman A, Franco OH, Witteman JC, van der Lugt A, Vernooij MW, Wentzel JJ. Arterial stiffness is associated with carotid intraplaque hemorrhage in the general population: the Rotterdam study. Arterioscler Thromb Vasc Biol 2014;34:927-32. [Crossref] [PubMed]
- Takaya N, Yuan C, Chu B, Saam T, Underhill H, Cai J, Tran N, Polissar NL, Isaac C, Ferguson MS, Garden GA, Cramer SC, Maravilla KR, Hashimoto B, Hatsukami TS. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI--initial results. Stroke 2006;37:818-23. [Crossref] [PubMed]
- Meng Q, Xie X, Li L, Jiang C, Zhao K, Bai Z, Zheng Z, Yang Y, Yu Y, Zhang H, Zhao X. Assessment of neovascularization of carotid artery atherosclerotic plaques using superb microvascular imaging: a comparison with contrast-enhanced ultrasound imaging and histology. Quant Imaging Med Surg 2021;11:1958-69. [Crossref] [PubMed]
- Zhou Z, Li R, Zhao X, He L, Wang X, Wang J, Balu N, Yuan C. Evaluation of 3D multi-contrast joint intra- and extracranial vessel wall cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2015;17:41. [Crossref] [PubMed]
- Fan Z, Yu W, Xie Y, Dong L, Yang L, Wang Z, Conte AH, Bi X, An J, Zhang T, Laub G, Shah PK, Zhang Z, Li D. Multi-contrast atherosclerosis characterization (MATCH) of carotid plaque with a single 5-min scan: technical development and clinical feasibility. J Cardiovasc Magn Reson 2014;16:53. [Crossref] [PubMed]
- Brott TG, Howard G, Roubin GS, Meschia JF, Mackey A, Brooks W, Moore WS, Hill MD, Mantese VA, Clark WM, Timaran CH, Heck D, Leimgruber PP, Sheffet AJ, Howard VJ, Chaturvedi S, Lal BK, Voeks JH, Hobson RW 2nd. CREST Investigators. Long-Term Results of Stenting versus Endarterectomy for Carotid-Artery Stenosis. N Engl J Med 2016;374:1021-31. [Crossref] [PubMed]
- Cai Y, He L, Yuan C, Chen H, Zhang Q, Li R, Li C, Zhao X. Atherosclerotic plaque features and distribution in bilateral carotid arteries of asymptomatic elderly population: A 3D multicontrast MR vessel wall imaging study. Eur J Radiol 2017;96:6-11. [Crossref] [PubMed]
- Li L, Chai JT, Biasiolli L, Robson MD, Choudhury RP, Handa AI, Near J, Jezzard P. Black-blood multicontrast imaging of carotid arteries with DANTE-prepared 2D and 3D MR imaging. Radiology 2014;273:560-9. [Crossref] [PubMed]
- Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002;106:1368-73. [Crossref] [PubMed]
- Xu Y, Yuan C, Zhou Z, He L, Mi D, Li R, Cui Y, Wang Y, Wang Y, Liu G, Zheng Z, Zhao X. Co-existing intracranial and extracranial carotid artery atherosclerotic plaques and recurrent stroke risk: a three-dimensional multicontrast cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2016;18:90. [Crossref] [PubMed]
- Powers CJ, Hirsch JA, Hussain MS, Patsalides AT, Blackham KA, Narayanan S, Lee SK, Fraser JF, Bulsara KR, Prestigiacomo CJ, Gandhi CD, Abruzzo T, Do HM, Meyers PM, Albuquerque FC, Frei D, Kelly ME, Pride GL, Jayaraman MV. Standards and Guidelines committee of the Society of NeuroInterventional Surgery. Standards of practice and reporting standards for carotid artery angioplasty and stenting. J Neurointerv Surg 2014;6:87-90. [Crossref] [PubMed]
- Porambo ME, DeMarco JK. MR imaging of vulnerable carotid plaque. Cardiovasc Diagn Ther 2020;10:1019-31. [Crossref] [PubMed]
- Selwaness M, Hameeteman R, Van 't Klooster R, Van den Bouwhuijsen Q, Hofman A, Franco OH, Niessen WJ, Klein S, Vernooij MW, Van der Lugt A, Wentzel JJ. Determinants of carotid atherosclerotic plaque burden in a stroke-free population. Atherosclerosis 2016;255:186-92. [Crossref] [PubMed]
- Popescu D, Mergeani A, Bajenaru OA, Antochi FA. Hemodynamic instability after elective carotid stenting: frequency and risk factors. Maedica (Bucur) 2011;6:258-61. [PubMed]
- Husmann M, Thalhammer C, Spring S, Meier T, Roffi M, Schwarz UR, Rousson V, Amann-Vesti BR. Influence of plaque volume on hemodynamic response and stress hormone release in patients undergoing carotid artery stenting. Int Angiol 2012;31:10-5. [PubMed]
- Mishani S, Belhoul-Fakir H, Lagat C, Jansen S, Evans B, Lawrence-Brown M. Stress distribution in the walls of major arteries: implications for atherogenesis. Quant Imaging Med Surg 2021;11:3494-505. [Crossref] [PubMed]
- Porzionato A, Macchi V, Stecco C, De Caro R. The Carotid Sinus Nerve-Structure, Function, and Clinical Implications. Anat Rec (Hoboken) 2019;302:575-87. [Crossref] [PubMed]
- Ito Y, Kato N, Matsumura A, Sonobe M. Hemodynamic instability increases new ischemic brain lesions on diffusion-weighted imaging after carotid artery stenting. Neurol Med Chir (Tokyo) 2013;53:375-80. [Crossref] [PubMed]
- Smith LA, Melbourne A, Owen D, Cardoso MJ, Sudre CH, Tillin T, Sokolska M, Atkinson D, Chaturvedi N, Ourselin S, Hughes AD, Barkhof F, Jäger HR. Cortical cerebral blood flow in ageing: effects of haematocrit, sex, ethnicity and diabetes. Eur Radiol 2019;29:5549-58. [Crossref] [PubMed]



