
Staging liver fibrosis in patients with chronic hepatitis B using two-dimensional shear wave elastography based on histopathological findings: a prospective multicenter study
Introduction
Chronic hepatitis B (CHB) virus infection is the main cause of liver fibrosis in China (1). Liver fibrosis can progress to liver cirrhosis, liver cancer, and liver failure. Clinical treatment strategies differ depending on the stage of liver fibrosis (2). Mild to moderate liver fibrosis can be reversed with prompt and specific treatment (3). Therefore, for patients with CHB, accurate evaluation and dynamic monitoring of the degree of liver fibrosis are of great significance in guiding clinicians to implement timely treatment and control the course of the disease (2).
Liver biopsy is recognized as the gold standard for the staging of liver fibrosis. However, liver biopsy is an invasive examination and thus has poor repeatability and low acceptance by patients (4). Therefore, noninvasive examination is preferred and has become a much debated topic in clinical research (5). Although serum markers can predict liver fibrosis to a certain extent, their predictive accuracy is mediocre, because they are not liver specific (6). Some studies in the field of medical imaging have reported that magnetic resonance elastography has a higher diagnostic performance than transient elastography (TE) in the staging of liver fibrosis; however, its high cost and low accessibility have limited its clinical utility and patient acceptance (7-9). Ultrasound elastography has gradually become the primary method for the assessment of liver stiffness due to its convenience, high speed, real-time capability, and low cost (10).
Ultrasound elastography predicts the degree of liver fibrosis by quantifying liver tissue stiffness. Two-dimensional shear wave elastography (2D SWE) is an ultrasound elastography technique which can predict the degree of liver fibrosis. Compared with TE, which has been widely reported, 2D SWE has a higher success rate and a wider area of fibrosis assessment (11-13). ElastQ, available on the Philips EPIQ system (Philips, Bothell, WA, USA), is based on the same principles as other 2D SWE imaging systems (14). Stiffness values or shear wave speed values involving the same subject vary among manufacturers due to system and engineering-dependent factors such as differences in shear wave excitation settings and reconstruction algorithms (15). Therefore, any new shear wave elastography system needs to be validated in clinical practice to establish reference thresholds.
The aim of this prospective multicenter study was to evaluate the diagnostic performance of the ElastQ in a large sample of patients with CHB compared with serum biomarkers, with histopathological findings as the reference standard, and to obtain the optimal cut-off values for fibrosis staging. We present the following article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-831/rc).
Methods
Patients and study design
Between August 2020 and December 2021, consecutive patients diagnosed with CHB virus infection were considered for enrollment in a prospective multicenter study involving 14 hospitals. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).The study was approved by West China Hospital of Sichuan University Biomedical Research Ethics Committee (approval No. 2020638), Tianjin Third Central Hospital Institutional Review Board, the Affiliated Hospital of Southwest Medical University Institutional Review Board, Beijing Youan Hospital of Capital Medical University Ethics Committee, Panzhihua Central Hospital Institutional Review Board, the First Affiliated Hospital of Xiamen University Ethics Committee, the First Affiliated Hospital of Guangxi Medical University Ethics Committee, the First People's Hospital of Yinchuan Ethics Committee, Sichuan Cancer Hospital Ethics Committee, Guizhou Provincial People's Hospital Ethics Committee, the Second People’s Hospital Ethics Committee, Qinghai University Affiliated Hospital Ethics Committee, the Fourth People’s Hospital of Qinghai Province Ethics Committee, and Shijiazhuang Fifth Hospital Ethics Committee. Written informed consent was obtained from all patients.
The inclusion criteria for patients were as follows: (I) aged 18 years or older; (II) had been hepatitis B surface antigen (HBsAg)-positive for more than 6 months; and (III) underwent liver biopsy after sonography. The following patients were excluded: (I) patients who had previously undergone liver transplantation; (II) patients who were pregnant or lactating; (III) patients with a body mass index (BMI) ≥30 kg/m²; (IV) patients with other hepatic diseases such as hepatic vein tumor thrombus, intrahepatic bile duct dilatation, or biliary obstruction; (V) patients who had liver biopsy specimen of inadequate quality; and (VI) patients with an unreliable liver stiffness measurement (LSM).
The sample size was determined based on the desired sensitivity and specificity. The maximum expected sensitivity and specificity were taken from previous publications (6,16-18). The power of test was set as 0.9 and the standard error as 0.05. The calculated required sample size to achieve 90% power was 562, but as a precautionary measure (5%), the sample size was set at 590.
Patients with each grade of fibrosis were randomly divided into two groups at a ratio of 7:3. Of the patients, 70% were assigned to the training cohort to determine the optimal cut-off values, and the remaining 30% became the validation cohort to validate the diagnostic performance of the cut-off values. To investigate the influence of inflammatory activity grade and alanine transaminase (ALT) levels in identifying the fibrosis stage, subgroup analysis was performed by dividing the patients into groups according to inflammatory activity grade (G1, G2, G3, and G4). However, since there were only a small number of patients with G4 (n=10), the G4 and G3 groups were combined for analysis. Additionally, patients were divided into two groups based on ALT levels: ALT < 2 × upper limit of normal (ULN) (n=352) and ALT ≥ 2 × ULN (n=68).
Liver stiffness measurement by 2D SWE
All the patients underwent regular B-mode imaging and 2D SWE examination within 14 days before liver biopsy. The B-mode and 2D SWE examinations were performed with the EPIQ 7 ultrasound system (Philips, Bothell, WA, USA) with a C5-1 convex probe. All the ultrasound operators had more than 2 years of extensive clinical experience and were proficient in using ultrasound equipment. In addition, all had familiarized themselves with the research protocol and followed a detailed operating procedure manual. All operators were blinded to the clinical information and biopsy results of the patients.
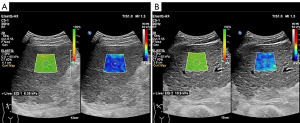
During each ElastQ imaging examination, homogeneous liver parenchyma, without large vessels or gallbladder interference, was selected as the region of interest. The ElastQ sampling box was placed 1–2 cm below the liver capsule. During a 6-second breath hold, the operator froze the image when the color inside the ElastQ sampling box was stabilized with the side-by-side confidence map filled mostly with yellow-green color (red = confidence score 0, green = confidence score 100). The operator then employed a circular measurement caliper with a diameter of 1 cm inside the high-confidence region for stiffness quantification. The ElastQ imaging and measurement interface is shown for a fibrotic liver at S1 and a fibrotic liver at S4 in Figure 1A,1B, respectively. The operator repeated the measurement five times. Between the consecutive acquisitions, the patient could maintain normal breathing. When less than two-thirds of the ElastQ confidence map was green, or when the big vessels were not avoided, the measurement was considered failed or unreliable. The quantified LSM was expressed in kilopascals (kPa). The measurement reliability for each patient was defined by the ratio of the interquartile range to the median (IQR/M). When the IQR/M was 30% or less, the patient’s 2D SWE values were recorded. Measurements were considered unreliable if the IQR/M exceeded 30% (14). The mean 2D SWE value of the five measurements was calculated for further analysis.
Liver biopsy and histologic staging
Percutaneous liver biopsies were performed under ultrasound guidance. Samples of more than 15 mm in length and with more than six portal tracts were considered eligible. Needle biopsy specimens were fixed with 10% formalin. Liver biopsies were subjected to hematoxylin and eosin and histochemical trichrome staining. For pathological quality control, all pathological specimens were gathered from the 14 hospitals for central reading by 2 pathologists (with 9 and 12 years of experience). The pathologists were blinded to the patients’ 2D SWE results. The fibrosis stage and inflammatory activity grade were evaluated using the Scheuer score. The liver fibrosis stages were: S0 (no fibrosis), S1 (portal fibrosis without septa), S2 (portal fibrosis and few septa), S3 (numerous septa without cirrhosis), and S4 (cirrhosis). The inflammation activity grades were: G0 (none), G1 (mild), G2 (moderate), G3 (obvious), and G4 (severe) (19).
Laboratory tests
Laboratory tests, such as liver function tests, blood counts, and serum markers for the hepatitis B virus, were performed 14 days before the liver biopsy. Each patient’s platelet count (PLT), γ-glutamyl transferase (GGT), alkaline phosphatase (ALP), ALT, aspartate aminotransferase (AST), total bilirubin, and direct bilirubin were recorded. Two noninvasive fibrosis scores based on patients’ laboratory tests and demographic information were calculated as follows: aspartate aminotransferase-to-platelet ratio index (APRI) = [AST (upper limit of normal U/L)/platelet count (×109/L) × 100 and fibrosis-4 index (FIB-4) = [age (years) × AST (U/L)]/[platelet count (×109/L) × ALT (U/L)1/2] (20,21).
Statistical analysis
Statistical analyses were performed using SPSS (version 23.0, IBM, Armonk, NY, USA) and MedCalc software (version 11.2, MedCalc Software, Ostend, Belgium). Quantitative variables were expressed as medians and interquartile ranges, and categorical variables as absolute and relative frequencies. Quantitative data were compared between two groups using Student’s t-test or the Mann-Whitney test, and categorical data using the χ2 test or Fisher’s test. A P value of less than 0.05 was considered statistically significant. Spearman’s correlation coefficient were used to evaluate the correlations between the two groups of variables. Intraobserver variability in LSM was assessed using the intraclass correlation coefficient (ICC). An ICC of more than 0.80 was regarded as excellent agreement. The area under the receiver operating characteristic curve (AUROC) and its 95% confidence interval (CI) were used to evaluate the diagnostic performance of 2D SWE, and the Delong test was used to compare the AUROCs. The optimal cut-off values of ElastQ in ROC curve analysis were determined by maximizing the Youden index.
Results
Between August 2020 and December 2021, a total of 667 patients with CHB were recruited for 2D SWE examination. After a detailed clinical data review and 2D SWE image quality assessment, 65 patients were excluded. Of the excluded patients, 8 had an age or BMI outside the eligible ranges, and another 8 were coinfected with the hepatitis C virus. The other 49 patients were excluded due to having poor measurement quality (n=37) or an insufficient number of 2D SWE measurements (n=12); this translated to a 94.3% success rate for 2D SWE (Figure 2). Finally, 602 patients, with an average age of 51 years (range, 18 to 83 years), were enrolled. The patients were divided on a 70:30 basis into a training cohort (n=420) and a validation cohort (n=182). The demographic and baseline characteristics of the patients are summarized in Table 1. There were no significant differences in patient clinical characteristics between the training and validation cohorts (P>0.05).
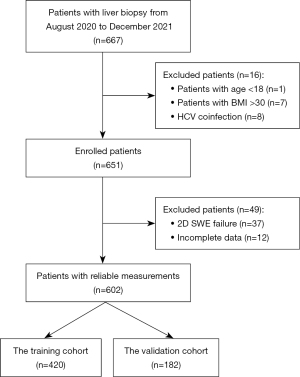
Table 1
| Characteristics | Reference value | Training cohort | Validation cohort | P value |
|---|---|---|---|---|
| No. of patients | NA | 420 (69.8) | 182 (30.2) | |
| Age (years) | NA | 53 [40.3–61] | 51 [43–58] | 0.501 |
| Sex | ||||
| Male | NA | 316 (75.2) | 138 (75.8) | |
| Female | NA | 104 (24.8) | 44 (24.2) | |
| BMI (kg/m²) | NA | 23.6 [21.6–26] | 22.9 [20.8–25] | 0.324 |
| PLT (×109/L) | 100–300 | 152 [105–210] | 145 [99–182.3] | 0.138 |
| AST (U/L) | <40 | 31.5 [23–54.8] | 36 [23–76.3] | 0.090 |
| ALT (U/L) | <50 | 36 [22–70] | 44 [25–125.5] | 0.147 |
| GGT (U/L) | <60 | 35 [20–66] | 41 [24–74] | 0.649 |
| ALP (U/L) | 51–160 | 82 [66.3–102] | 80 [63–104] | 0.936 |
| Total bilirubin (µmol/L) | 5.0–28.0 | 15.1 [11.3–19.7] | 14.8 [11.1–21.3] | 0.952 |
| Direct bilirubin (µmol/L) | <8.8 | 3.7 [2.4–5.7] | 4.3 [2.8–7.3] | 0.753 |
| Scheuer fibrosis stage | ||||
| S0 | NA | 30 (7.1) | 12 (6.6) | |
| S1 | NA | 84 (20.0) | 35 (19.2) | |
| S2 | NA | 79 (18.8) | 33 (18.1) | |
| S3 | NA | 93 (22.1) | 45 (24.7) | |
| S4 | NA | 134 (31.9) | 57 (31.3) | |
| Inflammation grade | ||||
| G1 | NA | 120 (28.6) | 44 (24.2) | |
| G2 | NA | 180 (42.9) | 91 (50.0) | |
| G3 | NA | 110 (26.2) | 41 (22.5) | |
| G4 | NA | 10 (2.4) | 6 (3.3) |
Qualitative variables are in n (%), and quantitative variables are median [interquartile range]. NA, not available; BMI, body mass index; PLT, platelet count; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ-glutamyl transferase; ALP, alkaline phosphatase.
The ICC for LSM by 2D SWE, based on five measurement from each patient, was 0.923 (95% CI: 0.911–0.933), indicating high reproducibility and stability of 2D SWE.
All 2D SWE values ranged from 3.08 kPa to 21.00 kPa. According to patients’ histopathological results, the distribution of liver fibrosis stages in the training cohort was as follows: S0 (n=30, 7.1%), S1 (n=84, 20%), S2 (n=79, 18.8%), S3 (n=93, 22.1%), and S4 (n=134, 31.9%) (Table 1 and Figure 3). Overall, 72.8% of patients had significant fibrosis (≥ S2) and 31.9% of patients had cirrhosis (S4). The 2D SWE values showed a strong correlation with fibrosis stage (r=0.71, P<0.001). In the training cohort, the AUROCs of ElastQ for diagnosing fibrosis stages ≥S1, ≥S2, ≥S3, and S4 were 0.817 (95% CI: 0.777–0.853), 0.887 (95% CI: 0.852–0.915), 0.912 (95% CI: 0.881–0.937), and 0.832 (95% CI: 0.793–0.866), respectively. The optimal liver stiffness cut-off values for predicting fibrosis stages ≥S1, ≥S2, ≥S3, and S4 were 5.72 kPa (sensitivity: 78%, specificity: 70%), 6.85 kPa (sensitivity: 77%, specificity: 86%), 7.43 kPa (sensitivity: 80%, specificity: 86%), and 8.03 kPa (sensitivity, 81%; specificity: 73%), respectively (Table 2).

Table 2
| Fibrosis stage | AUROC | P value | Cut-off value | Sensitivity | Specificity | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| ≥S1 | |||||||
| 2D SWE | 0.817 | <0.001 | >5.69 kPa | 0.78 | 0.7 | 97 | 20 |
| APRI | 0.725 | <0.001 | >0.43 | 0.64 | 0.77 | 97 | 14 |
| FIB-4 | 0.75 | <0.001 | >1.53 | 0.66 | 0.83 | 98 | 16 |
| ≥S2 | |||||||
| 2D SWE | 0.887 | <0.001 | >6.85 kPa | 0.77 | 0.86 | 94 | 58 |
| APRI | 0.782 | <0.001 | >0.37 | 0.82 | 0.64 | 86 | 57 |
| FIB-4 | 0.824 | <0.001 | >1.57 | 0.76 | 0.78 | 90 | 54 |
| ≥S3 | |||||||
| 2D SWE | 0.912 | <0.001 | >7.43 kPa | 0.8 | 0.86 | 87 | 79 |
| APRI | 0.746 | <0.001 | >0.42 | 0.82 | 0.59 | 70 | 74 |
| FIB-4 | 0.78 | <0.001 | >1.6 | 0.79 | 0.65 | 73 | 72 |
| S4 | |||||||
| 2D SWE | 0.832 | <0.001 | >8.02 kPa | 0.81 | 0.73 | 59 | 89 |
| APRI | 0.674 | <0.001 | >0.42 | 0.85 | 0.52 | 43 | 87 |
| FIB-4 | 0.725 | <0.001 | >1.57 | 0.89 | 0.52 | 47 | 91 |
AUROC, area under the receiver operating characteristic curve; PPV, positive predictive value; NPV, negative predictive value; 2D SWE, two-dimensional shear wave elastography; APRI, aspartate aminotransferase-to-platelet ratio index; FIB-4, fibrosis-4 index.
Next, the diagnostic performance of the cut-off values was assessed in the validation cohort. The AUROCs of ElastQ for diagnosing fibrosis stages ≥S1, ≥S2, ≥S3, and S4 were 0.807 (95% CI: 0.742–0.861), 0.868 (95% CI: 0.810–0.914), 0.855 (95% CI: 0.796–0.903), and 0.851 (95% CI: 0.791–0.900), respectively. The diagnostic performance of the cut-off values in the training cohort and validation cohort is shown in Table 3.
Table 3
| Fibrosis stage | AUROC | P value | Cut-off value (kPa) | Sensitivity | Specificity | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| ≥S1 | |||||||
| Training cohort | 0.817 | >5.69 | 0.78 | 0.7 | 97 | 20 | |
| Validation cohort | 0.807 | >0.05 | >5.69 | 0.72 | 0.75 | 98 | 16 |
| ≥S2 | |||||||
| Training cohort | 0.887 | >6.85 | 0.77 | 0.86 | 94 | 58 | |
| Validation cohort | 0.868 | >0.05 | >6.85 | 0.68 | 0.94 | 97 | 51 |
| ≥S3 | |||||||
| Training cohort | 0.912 | >7.43 | 0.8 | 0.86 | 87 | 79 | |
| Validation cohort | 0.855 | >0.05 | >7.43 | 0.73 | 0.84 | 85 | 71 |
| S4 | |||||||
| Training cohort | 0.832 | >8.02 | 0.81 | 0.73 | 59 | 89 | |
| Validation cohort | 0.851 | >0.05 | >8.02 | 0.82 | 0.74 | 60 | 90 |
AUROC, area under the receiver operating characteristic curve; PPV, positive predictive value; NPV, negative predictive value.
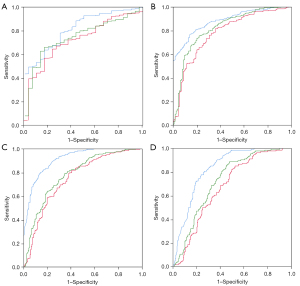
The comparison of the diagnostic performance of 2D SWE, the APRI, and the FIB-4 is shown in Figure 4 and reported in Table 2. In the correlation analysis with liver fibrosis stage, 2D SWE showed a significantly stronger correlation than APRI or FIB-4 (r=0.719 vs. r=0.44 vs. r=0.521, respectively, P<0.001). Additionally, 2D SWE had higher AUROCs than both APRI and FIB-4 for all liver fibrosis stages (≥S1: 0.82 vs. 0.73 vs. 0.75, respectively, ≥S2: 0.89 vs. 0.78 vs. 0.82, respectively, ≥S3: 0.91 vs. 0.75 vs. 0.78, respectively, and S4: 0.83 vs. 0.67 vs. 0.73, respectively, P<0.05 for all).
The distribution of inflammatory activity grades in the study cohort was G1 (n=120, 28.6%), G2 (n=180, 42.9%), G3 (n=110, 26.2%), and G4 (n=10, 2.4%). A moderate correlation of LSM with inflammation grade was observed (r=0.528, P<0.001). In the diagnosis of fibrosis stage S4, the AUROC in the low-activity group (G1) was higher than those in the moderate-activity group (G2) and the high-activity group (G3–4) (0.921 vs. 0.786 vs. 0.749, respectively; P<0.05). The correlation between ALT and LSM was weak (r=0.14, P<0.05). In the diagnosis of fibrosis stages ≥S3 and S4, the AUROC in the normal ALT level group (ALT < 2 × ULN, n=352) was higher than that in the elevated ALT level group (ALT ≥ 2 × ULN, n=68) (Table 4).
Table 4
| Variable | ≥S1 | ≥S2 | ≥S3 | S4 |
|---|---|---|---|---|
| Inflammation stage | ||||
| G1 (n=120) | 0.689 (0.598, 0.771) | 0.863 (0.788, 0.919) | 0.908 (0.842, 0.953) | 0.921 (0.857, 0.962) |
| G2 (n=180) | 0.673 (0.600, 0.741) | 0.84 (0.778, 0.890) | 0.907 (0.855, 0.945) | 0.786 (0.719, 0.843) |
| G3–4 (n=120) | 0.764 (0.678, 0.837) | 0.879 (0.807, 0.932) | 0.866 (0.792, 0.921) | 0.749 (0.662, 0.824) |
| P value | >0.05 | >0.05 | >0.05 | <0.05 |
| ALT level | ||||
| ALT ≤ 2 × ULN (n=352) | 0.81 (0.74, 0.88) | 0.891 (0.86, 0.92) | 0.924 (0.90, 0.95) | 0.87 (0.83, 0.91) |
| ALT > 2 × ULN (n=68) | 0.803 (0.56, 1.00) | 0.781 (0.65, 0.91) | 0.816 (0.71, 0.92) | 0.63 (0.49, 0.76) |
| P value | 0.968 | 0.111 | 0.049 | 0.001 |
Data in parentheses are 95% confidence intervals. ALT, alanine aminotransferase; ULN, upper limit of normal.
Discussion
This large prospective multicenter study primarily evaluated the diagnostic performance of a novel 2D SWE elastography system (ElastQ) for liver fibrosis staging and determined the optimal diagnostic cut-off values in patients with CHB. Using histopathological results as the reference standard, our study showed that ElastQ was convenient to operate and demonstrated stable results. Furthermore, its diagnostic performance was significantly superior to that of serum markers (FIB-4 and APRI).
Our study presents several unique advantages. First, the proportion of patients was uniform across liver fibrosis stages, which reduced the effects of potential bias on the statistical results. Second, we strictly excluded patients with medical conditions or diseases that might affect liver stiffness and collected pathological specimens for central reading to ensure the reliability of our results. Third, to the best of our knowledge, this is the first multicenter prospective report on ElastQ with a large sample size.
Many studies have reported that TE has great potential for diagnosing fibrosis stage and is recommended as a noninvasive technique in guidelines (22). However, an increasing number of studies suggest that 2D SWE has many advantages over TE (23-26). Poynard et al. (24) reported that 2D SWE had a higher success rate than TE in patients with obesity and ascites (86% vs. 55%, P=0.04) and that it produced more reliable results. The ICC in our study was higher than those reported by Joo et al. (25) for point shear wave elastography and Ronot et al. (26) for TE (0.923 vs. 0.848 vs. 0.85), showing 2D SWE to have good repeatability. The ElastQ imaging system used in this study provides side-by-side and concurrent confidence and stiffness maps. The quality index presented in the confidence map can guide the user to dynamically monitor the acquisition performance and make reliable measurements.
In this study, the optimal cut-off values for identifying fibrosis stages ≥S1, ≥S2, ≥S3, and S4 were 5.72 kPa, 6.85 kPa, 7.43 kPa, and 8.03 kPa, respectively. Our previous study reported that the optimal cut-off values of elastography point quantification (ElastPQ) in identifying fibrosis stages ≥S1, ≥S2, ≥S3, and S4 were ≥5.8 kPa, ≥6.8 kPa, ≥9.1 kPa, and ≥10.3 kPa, respectively (6). The cut-off values obtained in this study are closer to each other. In this analysis, the maximum 2D SWE value of ElastQ was 21 kPa, while the maximum value of ElastPQ was 38 kPa (6). A likely explanation for this difference is that the 2D SWE value measured by ElastQ is more stable than that measured by ElastPQ. The closeness of cut-off values impacts on clinical practice. Therefore, we recommend that the number of measurements obtained must be five. Also, when measuring liver stiffness, it is important to ensure that the confidence score is in the range of 90 to 100. Credible and stable results can only be achieved through strict compliance with operational norms. Moreover, our study included more patients with significant fibrosis and cirrhosis. That is because cirrhosis (S4) accounts for a larger proportion of patients in larger hospitals, while smaller hospitals have a higher proportion of no fibrosis or mild fibrosis (S0–2). This difference is related to the psychology of the patient’s choice of institution and the local treatment mechanism. Therefore, the results of our study are more generalizable to groups of patients with a high prevalence of fibrosis, such as those in the infection department and those undergoing liver surgery. Furthermore, the characteristics of different ultrasound machines should also be taken into consideration for system-dependent SWE measurements (27), and it should be re-emphasized that the cut-off values recommended for different elastography systems are not interchangeable (28).
Our study also found that the diagnostic performance of APRI and FIB-4 was inferior to that of 2D SWE, which is consistent with previous research findings (6,29-31). Serum markers have a poor real-time performance; if the disease changes, the changes in serum marker values will be delayed (32). However, 2D SWE can directly measure the stiffness of the liver (33), and it can therefore predict the stage of liver fibrosis with high accuracy.
Consistent with the elastography guidelines (2), our study showed good correlations between different inflammation grades and LSM (r=0.528, P<0.001). In the diagnosis of fibrosis stage S4, the diagnostic performance of LSM in the low-activity group (G1) was better than that in the moderate-activity (G2) and high-activity (G3–4) groups. In our study, the correlation between ALT and LSM was weak (r=0.14, P<0.05). Wu et al. (34) have reported that they used 1 × ULN of ALT as the grouping cut-off point, and the difference in diagnostic performance between the two groups was moderate (P=0.45). In the guidelines for hepatitis diagnosis and treatment, liver function damage or hepatitis virus activity is considered for patients with an ALT level of 2 × ULN or above (35). Therefore, we used 2 × ULN as the grouping cut-off point. Our study showed that when the ALT level was below 2 × ULN, the diagnostic performance of LSM was higher (P<0.001) and the 2D SWE values were more reliable. These results confirmed the inflammation activity grade and ALT level to be important confounding factors in the diagnosis of fibrosis stage (33,36).
There were several limitations in our study. First, the proportions of patients with different fibrosis grades were not uniform, and patients with cirrhosis (S4, 31.9%) accounted for a relatively large proportion of the study population. Therefore, that may affect the generalization of truncation value. Second, our study included few patients with severe inflammation and, as such, patients with obvious inflammation (G3) and severe inflammation (G4) were not analyzed separately. Third, with the development of standardized treatment for CHB, an increasing number of patients are treated with long-term antiviral therapy. However, antiviral therapy was not found to be an independent risk factor for predicting the grade of liver fibrosis in univariate logistic regression (P>0.05). Therefore, we did not discuss the impact of antiviral therapy in depth in this study. Further studies are needed to explore the dynamics of LSM in patients on antiviral therapy. Finally, to avoid the influence of abdominal wall thickness on LSM, we excluded patients with a BMI higher than 30. This exclusion criterion was intended to prevent the influence of fatty liver, but it also limited the generalizability of the results of this study (37).
Conclusions
In conclusion, 2D SWE performed well in the diagnosis and staging of liver fibrosis, with convenient operation and stable results. It is expected to become an alternative method for the noninvasive assessment of the degree of liver fibrosis in the future.
Acknowledgments
We would like to thank Jiali Yang and Jie Zhou for assisting with the research, and Hua Xie and Pingping Ji for their invaluable contributions to this work.
Funding: This work was supported by the China International Medical Foundation (grant No. Z-2017-24-1989).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-831/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-831/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383:1749-61. [Crossref] [PubMed]
- Barr RG, Wilson SR, Rubens D, Garcia-Tsao G, Ferraioli G. Update to the Society of Radiologists in Ultrasound Liver Elastography Consensus Statement. Radiology 2020;296:263-74. [Crossref] [PubMed]
- Tan-Garcia A, Lai F, Sheng Yeong JP, Irac SE, Ng PY, Msallam R, et al. Liver fibrosis and CD206(+) macrophage accumulation are suppressed by anti-GM-CSF therapy. JHEP Rep 2019;2:100062. [Crossref] [PubMed]
- Tapper EB, Lok AS. Use of Liver Imaging and Biopsy in Clinical Practice. N Engl J Med 2017;377:756-68. [Crossref] [PubMed]
- Martínez SM, Crespo G, Navasa M, Forns X. Noninvasive assessment of liver fibrosis. Hepatology 2011;53:325-35. [Crossref] [PubMed]
- Lu Q, Lu C, Li J, Ling W, Qi X, He D, Liu J, Wen T, Wu H, Zhu H, Luo Y. Stiffness Value and Serum Biomarkers in Liver Fibrosis Staging: Study in Large Surgical Specimens in Patients with Chronic Hepatitis B. Radiology 2016;280:290-9. [Crossref] [PubMed]
- Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, Fujita K, Yoneda M, Taguri M, Hyogo H, Sumida Y, Ono M, Eguchi Y, Inoue T, Yamanaka T, Wada K, Saito S, Nakajima A. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology 2016;150:626-37.e7. [Crossref] [PubMed]
- Fu F, Li X, Chen C, Bai Y, Liu Q, Shi D, Sang J, Wang K, Wang M. Non-invasive assessment of hepatic fibrosis: comparison of MR elastography to transient elastography and intravoxel incoherent motion diffusion-weighted MRI. Abdom Radiol (NY) 2020;45:73-82. [Crossref] [PubMed]
- Lefebvre T, Wartelle-Bladou C, Wong P, Sebastiani G, Giard JM, Castel H, Murphy-Lavallée J, Olivié D, Ilinca A, Sylvestre MP, Gilbert G, Gao ZH, Nguyen BN, Cloutier G, Tang A. Prospective comparison of transient, point shear wave, and magnetic resonance elastography for staging liver fibrosis. Eur Radiol 2019;29:6477-88. [Crossref] [PubMed]
- Wang J, Wu M, Linghu R, Chang J, Wu M, Feng C, et al. Usefulness of New Shear Wave Elastography Technique for Noninvasive Assessment of Liver Fibrosis in Patients with Chronic Hepatitis B: A Prospective Multicenter Study. Ultraschall Med 2022;43:e1-10. [Crossref] [PubMed]
- Sande JA, Verjee S, Vinayak S, Amersi F, Ghesani M. Ultrasound shear wave elastography and liver fibrosis: A Prospective Multicenter Study. World J Hepatol 2017;9:38-47. [Crossref] [PubMed]
- Mingkai L, Sizhe W, Xiaoying W, Ying L, Wu B. Diagnostic performance of elastography on liver fibrosis in antiviral treatment-naive chronic hepatitis B patients: a meta-analysis. Gastroenterol Rep (Oxf) 2022;10:goac005. [Crossref] [PubMed]
- Lee DH, Lee ES, Bae JS, Lee JY, Han JK, Yi NJ, Lee KW, Suh KS, Kim H, Lee KB, Choi BI. 2D shear wave elastography is better than transient elastography in predicting post-hepatectomy complication after resection. Eur Radiol 2021;31:5802-11. [Crossref] [PubMed]
- Kennedy P, Wagner M, Castéra L, Hong CW, Johnson CL, Sirlin CB, Taouli B. Quantitative Elastography Methods in Liver Disease: Current Evidence and Future Directions. Radiology 2018;286:738-63. [Crossref] [PubMed]
- Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol 2015;41:1126-47. [Crossref] [PubMed]
- Herrmann E, de Lédinghen V, Cassinotto C, Chu WC, Leung VY, Ferraioli G, et al. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology 2018;67:260-72. [Crossref] [PubMed]
- Gao Y, Zheng J, Liang P, Tong M, Wang J, Wu C, et al. Liver Fibrosis with Two-dimensional US Shear-Wave Elastography in Participants with Chronic Hepatitis B: A Prospective Multicenter Study. Radiology 2018;289:407-15. [Crossref] [PubMed]
- Zhuang Y, Ding H, Zhang Y, Sun H, Xu C, Wang W. Two-dimensional Shear-Wave Elastography Performance in the Noninvasive Evaluation of Liver Fibrosis in Patients with Chronic Hepatitis B: Comparison with Serum Fibrosis Indexes. Radiology 2017;283:873-82. [Crossref] [PubMed]
- Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol 2007;47:598-607. [Crossref] [PubMed]
- Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S, Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317-25. [Crossref] [PubMed]
- Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518-26. [Crossref] [PubMed]
- van Katwyk S, Coyle D, Cooper C, Pussegoda K, Cameron C, Skidmore B, Brener S, Moher D, Thavorn K. Transient elastography for the diagnosis of liver fibrosis: a systematic review of economic evaluations. Liver Int 2017;37:851-61. [Crossref] [PubMed]
- Paul SB, Das P, Mahanta M, Sreenivas V, Kedia S, Kalra N, Kaur H, Vijayvargiya M, Ghosh S, Gamanagatti SR. Shalimar, Gupta SD, Acharya SK. Assessment of liver fibrosis in chronic hepatitis: comparison of shear wave elastography and transient elastography. Abdom Radiol (NY) 2017;42:2864-73. [Crossref] [PubMed]
- Poynard T, Munteanu M, Luckina E, Perazzo H, Ngo Y, Royer L, Fedchuk L, Sattonnet F, Pais R, Lebray P, Rudler M, Thabut D, Ratziu V. Liver fibrosis evaluation using real-time shear wave elastography: applicability and diagnostic performance using methods without a gold standard. J Hepatol 2013;58:928-35. [Crossref] [PubMed]
- Joo I, Kim SY, Park HS, Lee ES, Kang HJ, Lee JM. Validation of a New Point Shear-Wave Elastography Method for Noninvasive Assessment of Liver Fibrosis: A Prospective Multicenter Study. Korean J Radiol 2019;20:1527-35. [Crossref] [PubMed]
- Ronot M, Ferraioli G, Müller HP, Friedrich-Rust M, Filice C, Vilgrain V, Cosgrove D, Lim AK. Comparison of liver stiffness measurements by a 2D-shear wave technique and transient elastography: results from a European prospective multi-centre study. Eur Radiol 2021;31:1578-87. [Crossref] [PubMed]
- Mulazzani L, Cantisani V, Piscaglia F. Different techniques for ultrasound liver elastography. J Hepatol 2019;70:545-7. [Crossref] [PubMed]
- Săftoiu A, Gilja OH, Sidhu PS, Dietrich CF, Cantisani V, Amy D, et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Elastography in Non-Hepatic Applications: Update 2018. Ultraschall Med 2019;40:425-53. [Crossref] [PubMed]
- Duan WJ, Wang XZ, Ma AL, Shang J, Nan YM, Gao ZL, et al. Multicenter prospective study to validate a new transient elastography device for staging liver fibrosis in patients with chronic hepatitis B. J Dig Dis 2020;21:519-25. [Crossref] [PubMed]
- Fouad R, Elbaz T, Abdel Alem S, Elsharkawy A, Negm M, Khairy M, Hassany M, Cordie A, El Akel W, Esmat G. Evaluation of accuracy of elastography point quantification versus other noninvasive modalities in staging of fibrosis in chronic hepatitis C virus patients. Eur J Gastroenterol Hepatol 2018;30:882-7. [Crossref] [PubMed]
- Mobarak L, Nabeel MM, Hassan E, Omran D, Zakaria Z. Real-time elastography as a noninvasive assessment of liver fibrosis in chronic hepatitis C Egyptian patients: a prospective study. Ann Gastroenterol 2016;29:358-62. [Crossref] [PubMed]
- Patel K, Sebastiani G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep 2020;2:100067. [Crossref] [PubMed]
- Ferraioli G, Wong VW, Castera L, Berzigotti A, Sporea I, Dietrich CF, Choi BI, Wilson SR, Kudo M, Barr RG. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med Biol 2018;44:2419-40. [Crossref] [PubMed]
- Wu M, Wu L, Jin J, Wang J, Li S, Zeng J, Guo H, Zheng J, Chen S, Zheng R. Liver Stiffness Measured with Two-dimensional Shear-Wave Elastography Is Predictive of Liver-related Events in Patients with Chronic Liver Disease Due to Hepatis B Viral Infection. Radiology 2020;295:353-60. [Crossref] [PubMed]
- Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560-99. [Crossref] [PubMed]
- Bota S, Sporea I, Peck-Radosavljevic M, Sirli R, Tanaka H, Iijima H, et al. The influence of aminotransferase levels on liver stiffness assessed by Acoustic Radiation Force Impulse Elastography: a retrospective multicentre study. Dig Liver Dis 2013;45:762-8. [Crossref] [PubMed]
- Zhou J, Yan F, Xu J, Lu Q, Zhu X, Gao B, Zhang H, Yang R, Luo Y. Diagnosis of steatohepatitis and fibrosis in biopsy-proven nonalcoholic fatty liver diseases: including two-dimension real-time shear wave elastography and noninvasive fibrotic biomarker scores. Quant Imaging Med Surg 2022;12:1800-14. [Crossref] [PubMed]


