
Clinical and CT diagnosis of 50 cases of Chlamydia psittaci pneumonia
Introduction
Over 20 kinds of chlamydia have already been discovered, among which Chlamydia pneumoniae, Chlamydia trachomatis and Chlamydia psittaci are closely related to human diseases. Chlamydia psittaci, as a pathogen, may cause community-acquired pneumonia (CAP) among adults (1). Psittacosis, also known as bird fever, is mainly transmitted among a variety of birds with occasional transmission to humans by animals carrying the bacteria (2). When humans are infected with this pathogen and it results in lung inflammation, it is called Chlamydia psittaci pneumonia (CPP). Infection in humans often occurs via direct contact and respiratory tract inhalation (3). With advances in economic development, the number of pets, including birds, has greatly increased, and reports of CPP cases have also been on the rise year by year (4). CPP accounts for 1.03% of all CAP cases (1). However, these data may be biased due to the empiric treatment of CAP.
With the clinical application of metagenomics next-generation sequencing (mNGS), an increasing number of CPP cases have been reported (5). MNGS is a high-throughput sequencing technology that compares the microbial nucleic acid sequences in samples with the existing sequences in the database for analysis, to identify the suspected pathogenic microorganisms in the sample in an efficient and accurate manner. MNGS is widely used in identifying infectious diseases (6). The previous literature mainly includes individual cases, and lacks a summary of large samples. For example, Dai et al. published 2 cases of CPP (7). Zhao et al. reported a comparative study of 6 CPP cases with 31 COVID-19 cases (8). In the past two years, a large number of CPP cases have been confirmed by mNGS in China, and some articles have also been reported. For example, Yang et al. reported 27 patients in southwest China (9). Unfortunately, they only focused on the clinical manifestations. There is an urgent need to summarize and discuss the computed tomography (CT) features of this disease. This paper is the first work to analyze the CT manifestations of CPP in detail. By collecting the data of 50 confirmed CPP cases from multiple hospitals, this paper discusses its clinical manifestations, laboratory tests and imaging manifestations to strengthen radiologists’ familiarity with the disease and improve its early diagnosis. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-809/rc).
Methods
A retrospective analysis was conducted to analyze the clinical, laboratory and imaging data of 50 patients suspected of being infected with Chlamydia psittaci from multiple hospitals across China from January 20, 2019 to March 1, 2020. The present study was approved by the ethics committee of Nanjing Medical University. Written informed consent was obtained from all subjects. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Inclusion criteria: (I) Respiratory symptoms occurring within one month. (II) Pulmonary CT showed abnormal changes. (III) All cases tested positive for Chlamydia psittaci after applying mNGS to bronchoalveolar lavage fluid. (IV) Clinical and imaging improvement after targeted treatment.
Exclusion criteria: mNGS showed other pathogenic bacteria that may cause disease.
The cases in this study came from five hospitals: (I) Department of Radiology of Nanjing First Hospital; (II) Department of Imaging Centre for Tuberculosis Control of Guangdong Province; (III) Department of Respirology & Critical Care Medicine of the Third Xiangya Hospital of Central South University; (IV) Department of Respiratory Diseases of the Sixth Affiliated Hospital of Wenzhou Medical University; (V) Department of Respiratory and Critical Care Medicine, Inflammation & Allergic Diseases Research Unit of the Affiliated Hospital of Southwest Medical University. And the testing results were further confirmed after analyzing their clinical and imaging manifestations. After targeted treatment, the conditions of all of the patients improved, and they were discharged from the hospital.
General clinical information included age, sex, and poultry contact history. Information on clinical symptoms included general symptoms (fever, fatigue, night sweats, muscle soreness, skin rash); respiratory symptoms (cough, expectoration, dyspnea, chest pain); digestive tract symptoms (abdominal pain, diarrhea, poor appetite); neurological symptoms (dizziness, headache, unconsciousness, and gait instability); and urinary system symptoms (frequent micturition, urgent micturition, hematuria, changes in urine output). The laboratory tests included white blood cell count, procalcitonin, C-reactive protein, liver function, kidney function, cardiac function, electrolytes.
The types of CT equipment used by hospitals varied, including (I) SOMATOM Definition AS 128-slice spiral CT (Siemens, Germany); (II) NeuViz 128-slice Jing Rui CT (Neusoft, China); and (III) Light Speed V spiral CT (GE, USA). Each device adopts end-inspiratory scanning with a slice thickness of 5 mm and a reconstruction slice thickness of 1.5 mm. Through online collaboration, three doctors with over five years of work experience completed all of the work. The expert team consisted of 2 radiologists (attending physician) with over 8 and 10 years of experience in respiratory imaging and 1 pneumologist (associated chief physician). Three doctors reached a concern on the diagnosis of imaging features before diagnostics. When disagreements occurred, a meeting was held for discussion, and final decision was made by voting.
The CT appearance (unilateral lung/bilateral lung), involvement (single lobe/multiple lobes), distribution (subpleural distribution/secondary pulmonary lobule distribution/bronchial vascular bundle distribution/diffusion to all pulmonary lobes), and density (ground-glass shadows/consolidation) were all observed on the patients’ lung images. Statistical imaging signs included the number of cases and the proportion of patients with air bronchogram, bronchiectasis, “fine mesh sign”, “halo sign”, “reversed halo sign”, “tree-in-bud sign”, necrosis or cavity, pleural effusion, enlarged mediastinal lymph nodes, and enlarged spleen. A follow-up CT scan is usually performed about a week after the first CT scan. Patients completed approximately two to four times CT scans according to their respective conditions until they were discharged from the hospital. In terms of statistical methods, this study adopted the t-test to calculate the median and standard deviation of the patients’ ages.
Results
Among the 50 patients (Figure 1), there were 31 male patients (accounting for 62%, 95% CI: 0.47–0.75) and 19 female patients (accounting for 38%, 95% CI: 0.25–0.53) with a male-to-female ratio of 1.6:1. The onset age of CPP ranged from 33 to 80 (58.54±11.24) years old. In total, 80% (40/50, 95% CI: 0.66–0.90) of the patients had a history of poultry exposure, which included raising or coming into contact with birds such as parrots, chickens, and ducks and being exposed to poultry droppings.
The patients had several symptoms, including fever, night sweats, muscle soreness and fatigue. In addition, they might have experienced abnormalities in several systems, such as the respiratory system, digestive system, neurological symptoms, and urinary system. Among them, 74% (37/50, 95% CI: 0.60–0.85) of patients experienced respiratory abnormalities on admission: 70% (35/50, 95% CI: 0.55–0.82) had cough, 56% (28/50, 95% CI: 0.41–0.70) had expectoration, and 32% (16/50, 95% CI: 0.20–0.47) had short of breath. In addition, 96% (48/50, 95% CI: 0.85–0.99) of patients developed fever, 18% (9/50, 95% CI: 0.09–0.32) developed muscle soreness, 32% (16/50, 95% CI: 0.20–0.47) developed fatigue, and 4% (2/50, 95% CI: 0.01–0.15) developed night sweats. Moreover, 28% (14/50, 95% CI: 0.17–0.43) of patients had digestive symptoms, including 12% (6/50, 95% CI: 0.05–0.25) abdominal pain, 16% (8/50, 95% CI: 0.08–0.30) diarrhea and 18% (9/50, 95% CI: 0.09–0.32) poor appetite. Furthermore, 24% (12/50, 95% CI: 0.14–0.38) of the patients developed neurological symptoms, including dizziness and headache (22%, 11/50, 95% CI: 0.12–0.36), unconsciousness (2%, 1/50, 95% CI: 0.00–0.12) and gait instability (2%, 1/50, 95% CI: 0.00–0.12). Detailed information can be found in Table 1.
Table 1
| Characteristics | Number (case) | Percentage | 95% CI |
|---|---|---|---|
| Gender | |||
| Male | 31 | 62% | 0.4716–0.75 |
| Female | 19 | 38% | 0.25–0.5284 |
| Poultry exposure | 40 | 80% | 0.6586–0.895 |
| Clinical symptoms | |||
| Respiratory system | 37 | 74% | 0.5939–0.8492 |
| Cough | 35 | 70% | 0.5522–0.8171 |
| Expectoration | 28 | 56% | 0.4135–0.6973 |
| Chest pain | 7 | 14% | 0.0628–0.2736 |
| Short of breath | 16 | 32% | 0.1993–0.4683 |
| Constitutional symptoms | |||
| Fever | 48 | 96% | 0.8514–0.993 |
| Muscular soreness | 9 | 18% | 0.0905–0.3192 |
| Fatigue | 16 | 32% | 0.1993–0.4683 |
| Night sweats | 2 | 4% | 0.007–0.1486 |
| Digestive system | 14 | 28% | 0.1667–0.4271 |
| Abdominal pain | 6 | 12% | 0.0497–0.25 |
| Diarrhea | 8 | 16% | 0.0764–0.2966 |
| Poor appetite | 9 | .18% | 0.0905–0.3192 |
| Nervous system | 12 | 24% | 0.1352–0.3849 |
| Dizziness & headache | 11 | 22% | 0.1199–0.3633 |
| Gait instability | 1 | 2% | 0.001–0.1201 |
| Unconsciousness | 1 | 2% | 0.001–0.1201 |
| Urinary system | 4 | .8% | 0.0259–0.2011 |
| Frequent & urgent micturition | 3 | .6% | 0.0156–0.1754 |
| Hematuria | 2 | 4% | 0.007–0.1486 |
| Laboratory tests | |||
| Normal white blood cell count | 34 | 68% | 0.5317–0.8007 |
| Increased white blood cell count | 16 | 32% | 0.1993–0.4683 |
| Increased C-reactive protein | 49 | 98% | 0.8799–0.999 |
| Hyponatremia | 24 | 48% | 0.3388–0.6242 |
| Elevated procalcitonin | 31 | 62% | 0.4716–0.75 |
| Abnormal liver function | 25 | 50% | 0.3572–0.6428 |
| Abnormal renal function | 11 | 22% | 0.1199–0.3633 |
| Abnormal cardiac function | 12 | 24% | 0.1352–0.3849 |
A white blood cell count (4.0–10.0)×109/L was taken as the normal standard value. Among the patients, 68% (34/50, 95% CI: 0.53–0.80) had normal white blood cell numbers, and 32% (95% CI: 0.20–0.47) had increased white blood cell numbers, with the highest value being 16×109/L. In addition, 98% (95% CI: 0.88–1.0) of patients had increased C-reactive protein, 48% (95% CI: 0.34–0.62) of them had hyponatremia (blood sodium level <135 mmol/L), 50% (25/50, 95% CI: 0.36–0.64) of patients suffered from liver damage, and 22% (11/50, 95% CI: 0.12–0.36) and 24% (12/50, 95% CI: 0.14–0.38) of patients experienced damage to their renal and cardiac functions (abnormality of troponin and CK-MB), respectively. Detailed information can be found in Table 1.
The time from onset to the first CT examination was between 2–30 days, with an average of 7 days. Concerning the imaging appearance, distribution, involvement, density and signs, the following was noted:
- Imaging appearance and distribution: 62% (31/50) of the patients presented with a unilateral lung distribution, of which 28% (14/50, 95% CI: 0.17–0.43) with a left lung distribution, 34% (17/50, 95% CI: 0.22–0.49) with a right lung distribution, and 38% (19/50, 95% CI: 0.25–0.53) presented with a bilateral lung distribution. In addition, among all of the lesions on CT of 50 patients, 66% (33/50, 95% CI: 0.51–0.78) were located in the subpleural area, 50% (25/50, 95% CI: 0.36–0.64) were located in the secondary pulmonary lobules, 68% (34/50, 95% CI: 0.53–0.80) were located in the bronchovascular bundles, and 40% (20 /50, 95% CI: 0.27–0.55) spread to all lobes. Detailed information can be found in Figure 1.
- Involvement: 34% (17/50, 95% CI: 0.22–0.49) of the lesions were confined to one pulmonary lobe, while 66% (33/50, 95% CI: 0.51–0.78) of the lesions involved multiple lobes.
- Density: ground-glass shadows were observed in 100% (50/50, 95% CI: 0.91–1.00) of the patients, and consolidation accounted for 98% (49/50, 95% CI: 0.88–1.00).
- Air bronchogram sign was observed in 80% (40/50, 95% CI: 0.66–0.90) of the patients, 46% (23/50, 95% CI: 0.32–0.61) showed bronchial wall thickening, and 32% (16/50, 95% CI: 0.20–0.47) showed bronchiectasis sign.
- Signs: “Fine mesh sign”, “halo sign” and “reversed halo sign” were observed in 74% (37/50, 95% CI: 0.59–0.85), 10% (5/50, 95% CI: 0.04–0.23) and 6% (3/50, 95% CI: 0.02–0.18) of the cases, respectively. Detailed information can be found in Table 2.
- No case showed necrosis, cavities and "tree-in-bud sign”.
- Other findings: 66% (33/50, 95% CI: 0.51–0.78) of the patients had pleural effusion, of whom 46% (23/50, 95% CI: 0.32–0.61) had unilateral pleural effusion, and 20% (10/50, 95% CI: 0.11–0.34) had bilateral pleural effusion. In addition, 36% (18/50, 95% CI: 0.23–0.51) of the patients experienced enlarged mediastinal lymph nodes, and 30% (15/50, 95% CI: 0.18–0.45) had enlarged spleens. Detailed information can be found in Table 2.
Table 2
| Characteristics | Number (case) | Percentage | 95% CI |
|---|---|---|---|
| Range | |||
| Single left lung | 14 | 28% | 0.1667–0.4271 |
| Single right lung | 17 | 34% | 0.2159–0.4886 |
| Bilateral lungs | 19 | 38% | 0.25–0.5284 |
| Number | |||
| Single pulmonary lobe | 17 | 34% | 0.2159–0.4886 |
| Multiple pulmonary lobes | .33 | 66% | 0.5114–0.7841 |
| Distribution | |||
| Subpleural | 33 | 66% | 0.5114–0.7841 |
| Bronchial vascular bundle | .34 | 68% | 0.5317–0.8007 |
| Secondary pulmonary lobule . | 25 | 50% | 0.3572–0.6428 |
| Diffusion to all pulmonary lobe | 20 | 40% | 0.2673–0.548 |
| Density | |||
| Ground glass . | 50 | 100% | 0.9111–1 |
| Consolidation | 49 | 98% | 0.8799–0.999 |
| Other signs | |||
| Bronchial wall thickening | 23 | 46% | 0.3206–0.6055 |
| Bronchiectasis | 16 | 32% | 0.1993–0.4683 |
| Air bronchogram | 40 | 80% | 0.6586–0.895 |
| Tree-in-bud sign | 0 | 0% | 0–0.0889 |
| Necrosis and cavity . | 0 | 0% | 0–0.0889 |
| Fine mesh sign | 37 | 74% | 0.5939–0.8492 |
| Halo sign | 5 | 10% | 0.0374–0.2259 |
| Reversed halo sign | 3 | 6% | 0.0156–0.1754 |
| Concomitant signs | |||
| Pleural effusion | 33 | 66% | 0.5114–0.7841 |
| Unilateral pleural effusion | 23 | 46% | 0.3206–0.6055 |
| Bilateral pleural effusion | 10 | 20% | 0.105–0.3414 |
| Enlarged spleen | 15 | 30% | 0.1829–0.4478 |
| Enlarged lymph nodes | 18 | 36% | 0.2329–0.5086 |
Discussion
Psittacosis pneumonia is an uncommon disease caused by Chlamydia psittaci, which accounts for approximately 1% of CAP cases worldwide (1), but recently, it has been increasingly found in China. In a recent study (10), only a few cases of CPP have been reported, and no conclusion on its imaging manifestations has been shared. This article summarizes the clinical characteristics and laboratory results, focuses on a detailed analysis of the disease's imaging and represents the first work related to the imaging manifestations of CPP.
Clinical manifestations
In this study, 80% of the cases had a history of poultry exposure, which is consistent with the previous report (11). The contact history was of great value for the diagnosis of psittacosis. Almost all patients develop fever, usually lasting for 10 to 14 days, while in severe cases, it may last for 3 to 7 weeks (12). Chlamydia psittaci can cause constitutional symptoms, and respiratory symptoms are the most common, accounting for 74% of cases. Chlamydia psittaci mainly infects the respiratory tract through inhalation and may subsequently infect multiple systems including the spleen, liver, kidneys and neurological system (13). Multisystem involvement is an important feature that can be used to differentiate from other common pathogens.
Laboratory tests
In this study, most of the patients had normal or mildly elevated white blood cell counts (14). Interestingly, in this study, 98% of patients had increased C-reactive protein. In addition, hyponatremia and liver, renal and cardiac damage may occur. These may be indicators of an atypical pathogen infection and can be used as important markers to distinguish the pathogen from other bacterial infections except for Legionella which behaves very similarly to Chlamydia psittaci, requiring a combination of exposure history for its identification. In addition, patients with psittacosis were found to have different procalcitonin levels. Procalcitonin is not specific to Chlamydia psittaci and thus cannot be used for its differentiation from other pathogens (15,16).
Imaging manifestations
The focus of CPP may be located in unilateral lung or bilateral lung. According to the follow-up observation of patients, most of the lesions originated in the unilateral lung; If the corresponding treatment is not carried out in time, it can rapidly progress to biliary lung. In addition, the lesions can be distributed under the pleura, around the bronchovascular bundle, secondary pulmonary lobule and diffusion to all pulmonary lobes (Figure 2). The above distribution mode can be observed in the CT of one patient. According to our observation, the lesion first occurs in the center of the secondary pulmonary lobule, causing interstitial changes around small blood vessels (17). Then it spreads to the surrounding alveoli/lung parenchyma, rapidly forming a large amount of exudation in the alveoli. The image thus shows a large area of consolidation and ground glass opacity (18,19). When the disease is not treated in time, multiple lesions may fuse with each other and eventually spread to all lobes (Figure 2).
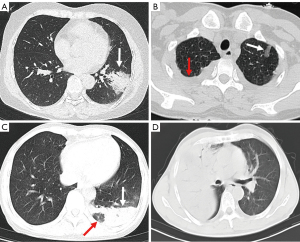
Among the imaging signs, a “fine mesh sign” (Figure 3A) appeared most frequently (74%). The main reason was that the lesions were distributed in the interstitium, and the fine mesh corresponded to the thickening of the interlobular septum. This sign was viewed as an important one that distinguished CPP from general bacterial infections. Some negative signs were also highly critical in disease identification. For example, the “tree-in-bud sign” did not appear in any of the 50 cases of this study, which is helpful for distinguishing it from other pathogens such as tuberculosis and mycoplasma pneumonia. Similarly, the appearance of necrosis and cavities could rule out the possibility of CPP infection, which can be found in pyogenic bacterial infections. Furthermore, the bronchus of the CPP was unobstructed overall, and an air bronchogram was commonly seen. When the disease enters the recovery period and becomes organized, bronchiectasis may be seen (Figure 3B). In addition, CPP often has several important concomitant signs, such as pleural effusion, enlarged lymph nodes and spleen. Interestingly, 30% of patients experienced splenomegaly, which is consistent with the previous study (11). It is believed that Chlamydia psittaci is an intracellular parasitic bacterium that can affect the human reticuloendothelial system and proliferate within lymphatic tissue (5). Figure 4 shows the complete disease course of a patient.
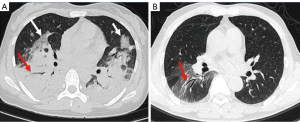
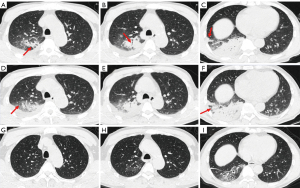
Our results showed that chest CT has an important role in diagnosis and could help in differentiating Chlamydia psittaci from other infections. The distribution of the interstitium and parenchyma on the image is an important point of identification and can be used for virus identification (20). There is no tree-in-bud sign, necrosis or cavity, which may help to differentiate against tuberculosis and other purulent bacteria, such as Staphylococcus aureus and Klebsiella pneumoniae. Of course, the combination of clinical and laboratory tests is also particularly important.
Treatment
Traditional methods to detect Chlamydia psittaci infection are mainly based on etiological culturing, serological testing or polymerase chain reaction (PCR). The etiological culturing method is highly dangerous to laboratory personnel thus difficult to use in clinical practice. Although the cost of adopting such a method is inexpensive, the sensitivity and specificity of serological testing are both unsatisfactory. Using PCR-probe may be more sensitive and rapid, but the identification of rare Chlamydia psittaci serotypes is usually unreliable. With the widespread use of mNGS in clinical practice, an increasing number of Chlamydia psittaci cases have been detected (6,21). However, although mNGS is very important for diagnosis, it also has some limitations. First, it is very expensive and will not be considered for routine cases. This examination is recommended only when the disease is highly suspected by clinical and imaging (22). Second, mNGS often does not detect a single patient, which also needs to be combined with clinical and imaging diagnostics.
Chlamydia psittaci is an intracellular pathogen without a typical cell wall and is inherently resistant to wall-breaking antibiotics (such as β-lactams). Doxycycline and macrolides such as azithromycin can be used to treat psittacosis, but doxycycline can penetrate chlamydia in a rapid manner with a high cure rate and mild adverse reactions. Therefore, it is the first choice for the treatment of psittacosis for adults (23). Psittacosis is very sensitive to treatment, and the fever of most psittacosis patients can be abated within 48 hours after initiation of doxycycline (24). Therefore, for patients whose symptoms have not been improved within 72 hours after treatment, the possibility of other infections should be considered (25).
Limitations
Our retrospective study has several limitations. First, the number of cases in this study was small, which might lead to statistical deviations. We will continue to increase the sample size for more in-depth research. Second, the lack of histopathology in this study makes it impossible to explore the relationship between various imaging findings and pathology. Third, the selection bias of patient recruitment may also exist. Fourth, the authors found that it may still be challenging to distinguish CPP from other bacteria on the basis of clinical infections, laboratory tests and imaging. Therefore, we will introduce a control group in the future to conduct comparative studies.
Conclusions
Patients affected with Chlamydia psittaci often involve multiple systems, the respiratory system is the most common. Untreated severe Chlamydia psittaci can rapidly progress to ARDS. CT is of great value for the early diagnosis of this disease. The lesions were usually distributed in the secondary pulmonary lobules, around the bronchovascular bundles, under the pleura or diffusely. Both the lung parenchyma and the interstitium are involved. Therefore, consolidation and ground-glass opacities are always visible, and the fine mesh sign is a common sign of importance. Necrosis, cavitation, and tree-in-bud signs were not observed. With these evocative images, supported by the contact history, clinical symptoms, and laboratory findings, mNGS examination should be carried out to provide appropriate early treatment.
Acknowledgments
Funding: This work was funded by the Natural Science Foundation of China (Nos. 82102012, and 82102006) and Medical Science and Technology Development Project Fund of Nanjing (No. ZKX21041).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-809/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-809/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study was approved by the ethics committee of Nanjing Medical University. Written informed consent was obtained from all subjects. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hogerwerf L. DE Gier B, Baan B, VAN DER Hoek W. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect 2017;145:3096-105. [Crossref] [PubMed]
- Shaw KA, Szablewski CM, Kellner S, Kornegay L, Bair P, Brennan S, Kunkes A, Davis M, McGovern OL, Winchell J, Kobayashi M, Burton N, de Perio MA, Gabel J, Drenzek C, Murphy J, Holsinger C, Forlano L. Psittacosis Outbreak among Workers at Chicken Slaughter Plants, Virginia and Georgia, USA, 2018. Emerg Infect Dis 2019;25:2143-5. [Crossref] [PubMed]
- Wallensten A, Fredlund H, Runehagen A. Multiple human-to-human transmission from a severe case of psittacosis, Sweden, January-February 2013. Euro Surveill 2014; [Crossref] [PubMed]
- Harkinezhad T, Geens T, Vanrompay D. Chlamydophila psittaci infections in birds: a review with emphasis on zoonotic consequences. Vet Microbiol 2009;135:68-77. [Crossref] [PubMed]
- Yang M, Yang DH, Yang H, Ding SZ, Liu CH, Yin HM, Liu D, Chen P, Luo H. Clinical Characteristics of Chlamydia psittaci Pneumonia Infection in Central South China. Infect Dis Ther 2022;11:1631-47. [Crossref] [PubMed]
- Gu L, Liu W, Ru M, Lin J, Yu G, Ye J, Zhu ZA, Liu Y, Chen J, Lai G, Wen W. The application of metagenomic next-generation sequencing in diagnosing Chlamydia psittaci pneumonia: a report of five cases. BMC Pulm Med 2020;20:65. [Crossref] [PubMed]
- Dai N, Li Q, Geng J, Guo W, Yan W. Severe pneumonia caused by Chlamydia psittaci: report of two cases and literature review. J Infect Dev Ctries 2022;16:1101-12. [Crossref] [PubMed]
- Zhao W, He L, Xie XZ, Liao X, Tong DJ, Wu SJ, Liu J. Clustering cases of Chlamydia psittaci pneumonia mimicking COVID-19 pneumonia. World J Clin Cases 2021;9:11237-47. [Crossref] [PubMed]
- Yang F, Li J, Qi B, Zou L, Shi Z, Lei Y, Li J, Luo X, Zeng F, Lu S, Huang X, Liu R, Lan Y. Clinical Symptoms and Outcomes of Severe Pneumonia Caused by Chlamydia psittaci in Southwest China. Front Cell Infect Microbiol 2022;11:727594. [Crossref] [PubMed]
- Stewardson AJ, Grayson ML. Psittacosis. Infect Dis Clin North Am 2010;24:7-25. [Crossref] [PubMed]
- Yung AP, Grayson ML. Psittacosis--a review of 135 cases. Med J Aust 1988;148:228-33. [Crossref] [PubMed]
- Radomski N, Einenkel R, Müller A, Knittler MR. Chlamydia-host cell interaction not only from a bird's eye view: some lessons from Chlamydia psittaci. FEBS Lett 2016;590:3920-40. [Crossref] [PubMed]
- Fraeyman A, Boel A, Van Vaerenbergh K, De Beenhouwer H. Atypical pneumonia due to Chlamydophila psittaci: 3 case reports and review of literature. Acta Clin Belg 2010;65:192-6. [Crossref] [PubMed]
- Crosse BA. Psittacosis: a clinical review. J Infect 1990;21:251-9. [Crossref] [PubMed]
- Dandona P, Nix D, Wilson MF, Aljada A, Love J, Assicot M, Bohuon C. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab 1994;79:1605-8. [PubMed]
- van Langevelde P, Joop K, van Loon J, Frölich M, Groeneveld PH, Westendorp RG, van Dissel JT. Endotoxin, cytokines, and procalcitonin in febrile patients admitted to the hospital: identification of subjects at high risk of mortality. Clin Infect Dis 2000;31:1343-8. [Crossref] [PubMed]
- Heine H, Müller-Loennies S, Brade L, Lindner B, Brade H. Endotoxic activity and chemical structure of lipopolysaccharides from Chlamydia trachomatis serotypes E and L2 and Chlamydophila psittaci 6BC. Eur J Biochem 2003;270:440-50. [Crossref] [PubMed]
- Chu J, Li X, Qu G, Wang Y, Li Q, Guo Y, Hou L, Liu J, Eko FO, He C. Chlamydia psittaci PmpD-N Exacerbated Chicken Macrophage Function by Triggering Th2 Polarization and the TLR2/MyD88/NF-κB Signaling Pathway. Int J Mol Sci 2020;
- He SY, Nomura K, Whittam TS. Type III protein secretion mechanism in mammalian and plant pathogens. Biochim Biophys Acta 2004;1694:181-206. [Crossref] [PubMed]
- Greffier J, Hoballah A, Sadate A, de Oliveira F, Claret PG, de Forges H, Loubet P, Mauboussin JM, Hamard A, Beregi JP, Frandon J. Ultra-low-dose chest CT performance for the detection of viral pneumonia patterns during the COVID-19 outbreak period: a monocentric experience. Quant Imaging Med Surg 2021;11:3190-9. [Crossref] [PubMed]
- Chen X, Cao K, Wei Y, Qian Y, Liang J, Dong D, Tang J, Zhu Z, Gu Q, Yu W. Metagenomic next-generation sequencing in the diagnosis of severe pneumonias caused by Chlamydia psittaci. Infection 2020;48:535-42. [Crossref] [PubMed]
- Li N, Li S, Tan W, Wang H, Xu H, Wang D. Metagenomic next-generation sequencing in the family outbreak of psittacosis: the first reported family outbreak of psittacosis in China under COVID-19. Emerg Microbes Infect 2021;10:1418-28. [Crossref] [PubMed]
- Lee H, Yun KW, Lee HJ, Choi EH. Antimicrobial therapy of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. Expert Rev Anti Infect Ther 2018;16:23-34. [Crossref] [PubMed]
- Bradley JS, Peacock G, Krug SE, Bower WA, Cohn AC, Meaney-Delman D, Pavia ATAAP Committee on Infectious Diseases and Disaster Preparedness Advisory Council. Pediatric anthrax clinical management. Pediatrics 2014;133:e1411-36. [Crossref] [PubMed]
- Khatib R, Thirumoorthi MC, Kelly B, Grady KJ. Severe psittacosis during pregnancy and suppression of antibody response with early therapy. Scand J Infect Dis 1995;27:519-21. [Crossref] [PubMed]


