
A case of localized abdominal aortic dissection secondary to retroperitoneal fibrosis cured only with glucocorticoids and immunosuppressive agents
Introduction
Aortic dissection is a life-threatening disease resulting from an intimal tear in most cases or a rupture of the vasa vasorum and an intramural hematoma that may form an aortic dissection. Although the two main mechanisms are extracellular matrix degradation and inflammation, the precise trigger of aortic dissection remains unknown (1). Contributing factors are diverse, and arterial hypertension and known connective tissue diseases are the most common risk factors (2,3). Additionally, retroperitoneal fibrosis (RPF) is a rare condition whose central feature is the presence of a fibro-inflammatory tissue arising from the outer layers of the abdominal aorta and extending into the surrounding retroperitoneum (4). RPF has been deemed a manifestation of a systemic immune-mediated or inflammatory process rather than an exaggerated local reaction to atherosclerosis (5). The typical macroscopic appearance of RPF is a retroperitoneal plaque of varying thickness that surrounds the abdominal aorta, the iliac vessels, and, in most instances, the inferior vena cava and the ureters. The aortic wall also undergoes atherosclerotic degeneration of the intima, medial thinning, and pronounced adventitial inflammation. The inflammatory infiltrate is often centered on the adventitial vasa vasorum, which can show necrotizing vasculitis (6). The processes of degeneration and inflammation in the blood wall provide a pathological basis for the dissection formation.
Case description
A previously healthy 64-year-old man was admitted to Beijing Shijitan Hospital with intermitted abdominal pain that had developed 14 days prior. No significant abnormalities were observed during the physical examination. Laboratory tests performed at admission showed increased white blood cells (WBC; 13.57×109/L), C-reactive protein (CRP; 61.6 mg/L), and erythrocyte sedimentation rate (ESR; 82 mm/h) in addition to dysfunction of blood coagulation as evidenced by elevated D-dimer (D-D; 387 ng/mL), prothrombin time (PT; 12.7 s), fibrinogen (FIB; 7.92 g/L), and activated partial thromboplastin time (APTT; 38.8 s). Other laboratory tests showed that immunoglobulin G (IgG) was 17.0 g/L, immunoglobulin E (IgE) was 813 IU/mL, the peripheral eosinophil count was 0.81×109/L, total complement was 60 U/mL, and complement 3 (C3) was 1.46 g/L. The IgE, total complement, C3, and peripheral eosinophil counts were elevated.
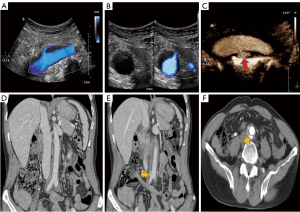
Ultrasound (US) showed that hypoechoic tissue surrounded the abdominal aorta between the origin of the renal arteries, the iliac artery appeared as a hypoechoic peri-aortic halo, and intimal dissection was observed at the distal abdominal aorta near the iliac artery bifurcation (Figure 1A,1B). Both color Doppler flow imaging (CDFI) and microscopic flow imaging (MFI) showed a single break of the aortic intima, and a located contrast agent (SonoVue) was observed in the false lumen (Figure 1C; Movies I–III). The patient underwent computed tomography angiography (CTA) of the abdomen. The examination findings revealed that low-density tissue surrounded the abdominal aorta and showed limited contrast filling in the wall near the bifurcation of the iliac artery (Figure 1D-1F). At this time, the patient was in an active inflammatory phase. Either open or laparoscopic surgery would have been invasive and could have worsened the patient’s condition, so the biopsy was not performed.
The diagnosis of abdominal aortic dissection secondary to RPF was confirmed based on the clinical presentation, laboratory findings, and imaging. In order to suppress the patient’s inflammatory response for the subsequent treatment of aortic dissection, glucocorticoids, and immunosuppressive agents were considered. Methylprednisolone was administered intravenously at 80 mg daily beginning on October, 27, 2021, and the dose was adjusted to 1 mg/kg/d after 3 days. Methylprednisolone was administered orally at 48 mg daily beginning on November 19, 2021; the dose was reduced to 40 mg after 1 week and to 32 mg after 2 weeks. From November 6, 2021, onward, 1 g of cyclophosphamide per month was administered via intravenous infusion.
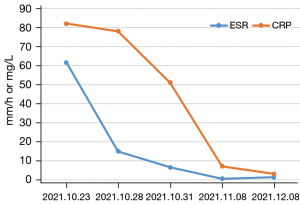
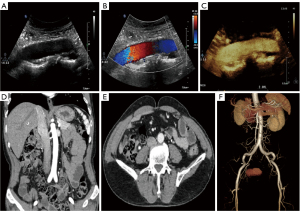
During follow-up, the CRP and ESR significantly decreased and eventually fell to normal levels (Figure 2). In addition, the CTA and US suggested that the wall of the abdominal aorta had become smooth, and no significant hypoechoic tissue around the artery was observed. The aortic dissection had completely disappeared (Figure 3). Based on the imaging and laboratory test results, the patient’s RPF and aortic dissection seemed to have resolved only due to the glucocorticoids and immunosuppressive agents.
All procedures performed in this study were conducted in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
RPF is divided into idiopathic retroperitoneal fibrosis (iRPF) and secondary retroperitoneal fibrosis (sRPF), which are often secondary to malignancy, infection, drugs, radiation therapy, and connective tissue disease. A proportion of previously diagnosed iRPF cases should be classified as another group of diseases with a broader spectrum of disease, immunoglobulin G4-related disease (IgG4-RD). In a study by Khosroshahi et al. (7), 57% of patients with RPF were diagnosed with IgG4-RPF, and IgG4+ plasma cell infiltration was observed in the pathological tissues. The patient in our case had no significant involvement of the characteristic organs susceptible to IgG4-RD, such as in the pancreas, salivary glands, lungs, kidneys, or thyroid, and there was no history of malignancy or other autoimmune diseases.
Differential diagnosis between RPF and the two main large-vessel vasculitis (LVV) is challenging. Serum IgG4 measurement might aid in the differential diagnosis, but the IgG4 was negative in the patient. However, the value of IgG4 was 1,395 mg/L, which was very close to the upper limit of the normal value.
Imaging may detect luminal stenosis and concentric mural thickening, which is a characteristic finding of LVV (8,9). The patient lacked typical manifestations of giant-cell arteritis-like cranial manifestations (e.g., headache, jaw claudication, scalp tenderness, and temporal artery tenderness) or clinical features indicative of ischemia of the upper limbs (e.g., claudication of the upper extremities, bruits, and decreased or absent pulses), which are frequently observed in Takayasu arteritis.
The most common complication of RPF is compressed obstruction of the ureter. The patient in this case did not show this corresponding manifestation, possibly due to the prompt detection of the patient’s disease and its relatively short duration. Aortic dissection is a rare complication of RPF, but it is most likely life-threatening. Open surgical repair and thoracic endovascular aortic repair may be optimal for treating acute aortic dissection, but glucocorticoids are currently among of the most effective drugs considered for the treatment of RPF by virtue of the ability to inhibit the inflammatory response in the early stage of the disease. Due to the relative limitation of the dissection in the patient in this study, only glucocorticoids and immunosuppressive agents were used to control the active inflammation.
As to why the aortic dissection resolved spontaneously, we speculate that it was due to the patient’s idiosyncratic hemodynamics. This patient had no underlying diseases, such as hypertension or diabetes, and the dissection rupture was localized with no significant rupture at the distal end of the dissection. The blood flow in the false lumen was low, and the blood flow velocity was slow. Thrombosis gradually occurred in the distal end of the false lumen and filled it up, allowing the lumen to close. Alleviating the primary cause of RPF prevented further aggravation of the aortic dissection. The false lumen gradually closed, and the wall was reshaped under this unique combination of multiple factors.
Localized aortic dissection is a risk of further progression in the treatment of RPF, so close testing should be performed during treatment to guard against the rupture of the aortic dissection. Imaging might also provide incremental information to identify patients at high risk of adverse events and help to select patients for more aggressive treatment. Imaging is usually performed at onset: it may detect both aneurysmal aortic dilatation and periaortitis. Due to the convenience and affordability, some patients are first detected by US.
Contrast US can visualize new microvessels in the lesion and help to indirectly assess the activity of the disease. However, due to the dependence on technicians and the lack of homogeneity, identifying this disease with US imaging is still challenging. CTA and magnetic resonance imaging (MRI) are superior in diagnosing RPF, and 18F-fluorodeoxyglucose (18F-FD) positron emission tomography (PET) can be used to evaluate the activity of RPF and the metabolism of residual lesions after treatment. Imaging studies can help differentiate idiopathic RPF from retroperitoneal neoplasms that appear inhomogeneous and lobulated, displace the aorta anteriorly, and have infiltrated muscles and bones. These neoplasms can also often extend above the origin of the renal arteries (10,11), and the RPF tissue usually develops between the origin of the renal arteries and the pelvic brim.
In this case, through use of conservative drug treatment alone, aortic remodeling occurred in a short time; that is, the false lumen was completely closed, and there were no obvious abnormalities in the morphology of the artery. Patients with aortic dissection secondary to RPF rarely recover on their own after the treatment of the only primary disease of RPF. The patient in this study is the first such case to be reported in the literature.
The rarity of this case makes our case report a valuable reference for devising clinical treatment plans for this type of disease. However, the question of whether conservative treatment can be used for patients with limited abdominal aortic coarctation secondary to RPF requires greater clinical case accumulation and more extensive follow-up.
Acknowledgments
Funding: This work was partially supported by funds from Beijing Hospitals Authority Clinical Medicine Development of Special Funding (No. XMLX202113) and the Beijing Municipal Science and Technology Commission (No. z191100006619051).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1036/coif). The authors report that this work was partially supported by funds from Beijing Hospitals Authority Clinical Medicine Development of Special Funding (No. XMLX202113) and Beijing Municipal Science and Technology Commission (No. z191100006619051). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were conducted in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nienaber CA, Clough RE, Sakalihasan N, Suzuki T, Gibbs R, Mussa F, Jenkins MP, Thompson MM, Evangelista A, Yeh JSM, Cheshire N, Rosendahl U, Pepper J. Aortic dissection. Nat Rev Dis Primers 2016;2:16053. [Crossref] [PubMed]
- Nienaber CA, Clough RE. Management of acute aortic dissection. Lancet 2015;385:800-11. [Crossref] [PubMed]
- Goldfinger JZ, Halperin JL, Marin ML, Stewart AS, Eagle KA, Fuster V. Thoracic aortic aneurysm and dissection. J Am Coll Cardiol 2014;64:1725-39. [Crossref] [PubMed]
- Vaglio A, Salvarani C, Buzio C. Retroperitoneal fibrosis. Lancet 2006;367:241-51. [Crossref] [PubMed]
- Vaglio A, Greco P, Corradi D, Palmisano A, Martorana D, Ronda N, Buzio C. Autoimmune aspects of chronic periaortitis. Autoimmun Rev 2006;5:458-64. [Crossref] [PubMed]
- Mitchinson MJ. Aortic disease in idiopathic retroperitoneal and mediastinal fibrosis. J Clin Pathol 1972;25:287-93. [Crossref] [PubMed]
- Khosroshahi A, Carruthers MN, Stone JH, Shinagare S, Sainani N, Hasserjian RP, Deshpande V. Rethinking Ormond's disease: "idiopathic" retroperitoneal fibrosis in the era of IgG4-related disease. Medicine (Baltimore) 2013;92:82-91. [Crossref] [PubMed]
- Mason JC. Takayasu arteritis--advances in diagnosis and management. Nat Rev Rheumatol 2010;6:406-15. [Crossref] [PubMed]
- Prieto-González S, Arguis P, Cid MC. Imaging in systemic vasculitis. Curr Opin Rheumatol 2015;27:53-62. [Crossref] [PubMed]
- Cronin CG, Lohan DG, Blake MA, Roche C, McCarthy P, Murphy JM. Retroperitoneal fibrosis: a review of clinical features and imaging findings. AJR Am J Roentgenol 2008;191:423-31. [Crossref] [PubMed]
- Urban ML, Palmisano A, Nicastro M, Corradi D, Buzio C, Vaglio A. Idiopathic and secondary forms of retroperitoneal fibrosis: a diagnostic approach. Rev Med Interne 2015;36:15-21. [Crossref] [PubMed]


