
Induction chemotherapy reduces target volume drift in patients with locoregionally advanced nasopharyngeal carcinoma undergoing adaptive intensity-modulated radiotherapy: a retrospective cohort study
Introduction
Nasopharyngeal carcinoma (NPC) is a relatively common head and neck malignancy that is endemic in several provinces in southern China (1). Intensity-modulated radiation therapy (IMRT) can result in good local control and survival, alleviate radiation toxicity, and raise the quality of life for patients with NPC (2,3). However, the accuracy of the target range of IMRT is essential for the development of optimized IMRT plans. Marked geometric and volumetric changes caused by shrinkage of the primary tumor or metastatic cervical nodes and changes in body weight occur during the course of concurrent chemoradiotherapy (CCRT) (4). Adaptive radiotherapy (ART) corrects the target and dose in real time based on repeat computed tomography (CT) imaging, and replanning during IMRT in locoregionally advanced NPC is important (5-8). Indeed, compared to replanning during IMRT, hybrid IMRT plans have been shown to reduce doses of target volumes and increase doses to critical structures. Moreover, replanning during IMRT has been found to alleviate the late effects of radiotherapy and to improve local progression-free survival (PFS) and overall survival (OS) (9,10). However, repeated CT and replanning during IMRT are time-consuming, and this hinders its broader application. Moreover, the optimal replanning time is yet to be determined, and only patients deemed suitable can be selected for IMRT with replanning. Our previous study of 20 patients with locoregionally advanced NPC treated with CCRT without induction chemotherapy (IC) demonstrated the most appropriate replanning time was after 20 treatment fractions and that the pretreatment body mass index (BMI) and tumor apparent diffusion coefficient could predict the need for replanning during CCRT (11).
Currently, the usefulness of the addition of IC to CCRT for locoregionally advanced NPC is a controversial topic. Some retrospective studies have shown that IC is not associated with improved OS or PFS (12-15). However, many randomized controlled trials have demonstrated that IC can improve failure-free survival, recurrence-free survival (RFS), and OS (16-18). A recent phase III trial reported that IC with gemcitabine and cisplatin significantly improved RFS and OS among patients with locoregionally advanced NPC (18). However, none of the above studies mentioned repeat CT and replanning or ART. As NPC is a radiosensitive and chemosensitive carcinoma (19), most patients will experience significant changes in anatomic structures and clinical target volume (CTV) due to obvious shrinkage of the gross tumor volume (GTV) and/or weight loss during CCRT (4). Therefore, we speculated that the addition of IC to CCRT will improve survival by reducing target volume drift and increasing the accuracy of the treatment target of hybrid IMRT plans.
In this study, we analyzed the weekly changes in GTV and CTV on repeated CT scans during CCRT for locoregionally advanced NPC, and we compared these changes between patients treated with or without IC in order to determine whether IC improved target volume drift. We also attempted to identify factors that might influence the target-volume reduction observed in patients receiving IC. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-776/rc).
Methods
Study patients
In this retrospective cohort study, we collected the data of 50 patients with locoregionally advanced NPC treated in hospital from January to December 2012 and from January to December 2017 in the Department of Radiation Oncology in the First Affiliated Hospital, College of Medicine, Zhejiang University. The patients were followed up for more than 6 months.
Of the 50 patients, 25 consecutive patients received IC followed by concurrent cisplatin chemotherapy during radiotherapy (referred to as the IC + CCRT group) and were recruited from January to December 2017. The other consecutive 25 patients received the same CCRT regimen followed by adjuvant chemotherapy (referred to as the CCRT + AC group) and were recruited from January to December 2012. Some of the data of these patients were reported in our previous study (11). Both the CCRT + AC group and IC + CCRT group were enrolled with the same inclusion and exclusion criteria. The inclusion criteria were the following: diagnosis of locoregionally advanced NPC stage III or IVa [according to the seventh edition of the American Joint Committee on Cancer (AJCC) Staging Manual], age in the range of 18–70 years, normal cardiopulmonary function, normal routine blood test and biochemical index, and tolerance of cisplatin-based chemotherapy. The exclusion criteria were the following: pregnancy, refusal to undertake weekly CT scans, inability to cooperate with weight measurement, and incompletion of the whole radiotherapy.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Human Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University Hospital, and informed consent was acquired from all of the patients. All patients underwent nasopharyngoscopic examination of clinically suspected lesions in the nasopharynx and biopsy of the primary tumor. We excluded patients who were found to have distant metastases before treatment according to their staging work-up, which included chest CT, abdominal ultrasonography, and bone scintigraphy or positron emission tomography CT.
Image acquisition
Before radiotherapy, all patients underwent head and neck immobilization with thermoplastic masks (MedTec Inc., Orange City, IA, USA) and contrast-enhanced CT simulation. All CT scans were obtained on a Siemens Sensation Open 40-CT scanner (Siemens Healthineers, Erlangen, Germany) using 3-mm slice spacing. Thereafter, all patients underwent plain simulated CT scans weekly for 5 consecutive weeks (i.e., at the completion of 5, 10, 15, 20, and 25 fractions). Thus, 6 sets of CT images (CT0, CT1, CT2, CT3, CT4, and CT5) were collected and observed.
ART
All patients received simultaneous, integrated boost IMRT, which was designed to deliver a total GTV dose of 6,540–7,412 cGy/30–34 F to the involved lymph nodes (GTVnd) and the gross tumor target, the nasopharynx (GTVnx), with 6,016 cGy/32 F as the high-risk clinical target volume 1 (CTV1) and 5,344 cGy/32 F as the low risk clinical target volume 2 (CTV2). The CTV1 included the GTVnx with a 5–10 mm margin and high-risk structures. The CTV2 included regions of the nasopharyngeal cavity, maxillary sinus, pterygopalatine fossa, posterior ethmoid sinus, parapharyngeal space, skull base, anterior third of the clivus, inferior sphenoid sinus, and cavernous sinus. Using optimized CT images, 1 principal oncologist delineated and measured the target volume for all patients.
The pretreatment GTV and CTV were tagged as GTV0 and CTV0, respectively, and the subsequent GTVs and CTVs obtained weekly for 5 weeks were tagged as GTV1, GTV2, GTV3, GTV4, and GTV5, and CTV1, CTV2, CTV3, CTV4, and CTV5, respectively. After the weekly CT scan, CT–CT image fusions were performed between the pretreatment CT (CT0) and subsequent CT images (CT1, CT2, CT3, CT4, and CT5). The repeat CT images were aligned with the initial treatment simulation CT by using rigid bony coregistration, while accepting minor registration mismatch. The delineations of GTV0 and CTV0 were then copied onto the repeat CT images (CT1–5). The oncologist who was responsible for delineating GTV0 and CTV0 further modified and recorded the following weekly GTVs and CTVs according to anatomical changes and mass shrinkage observed. The baseline and weekly GTVs and CTVs were measured with the Varian system (Varian Medical Systems, Palo Alto, CA, USA) via “volume function,” which was generated automatically. The data were then recorded in 2 groups by the same oncologist.
Chemotherapy
During IMRT, all patients received concurrent cisplatin 80 mg/m2 as an intravenous infusion on days 1, 22, and 43. In the IC + CCRT group, patients received 2–3 cycles of docetaxel + cisplatin + 5-fluorouracil (TPF) chemotherapy before cisplatin-based CCRT. In the CCRT + AC group, patients were treated with CCRT plus 2–3 cycles of cisplatin + 5-fluorouracil adjuvant chemotherapy.
Statistical analysis
Statistical analysis was performed using the Statistical Analysis System (SAS; SAS Institute Inc., Cary, NC, USA) v. 9.0 software package. The chi-squared test was used to compare patient characteristics between the CCRT + AC group and the IC + CCRT group. Weight loss and the reductions in GTV and CTV at a particular time point as compared to the preceding week [e.g., week 1 vs. week 0 (pretreatment) and week 3 vs. week 2] were compared between the 2 groups using the paired t-test. Pearson correlation coefficients were used to determine whether CTV reduction was related to weight loss and GTV shrinkage rate and whether GTV shrinkage was related to the initial tumor mass volume. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
A total of 40 patients were analyzed in this study, including 20 patients in the IC + CCRT group and, 20 patients in the CCRT + AC group. Figure 1 summarizes the recruitment process. In the IC + CCRT group, the 20 patients included 15 men and 5 women with a median age of 50 years (range, 19–72 years). According to the AJCC Cancer Staging Manual (seventh edition) (20,21), 8 patients presented with stage IVa disease and 12 patients with stage III disease. Histological examination identified the following tumor types: well-differentiated nonkeratinizing cancer, 4 patients; poorly differentiated non-keratinizing cancer, 10 patients; and poorly differentiated squamous cell carcinoma, 6 patients. In the CCRT + AC group, as reported in our previous study, the 20 enrolled patients consisted of 13 men and 7 women with a median age of 49 years (range, 37–61 years). Of these, 17 patients presented with stage III disease and 3 patients with stage IVa disease. Histological examination revealed well-differentiated, nonkeratinizing NPC in 11 patients, poorly differentiated, nonkeratinizing NPC in 4 patients, poorly differentiated squamous cell NPC in 4 patients, and unclassified carcinoma in 1 patient. The initial median weight and BMI were 67 kg (range, 52–99 kg) and 23.1 kg/m2 (range, 19.1–35.4 kg/m2) in the IC + CCRT group and 60 kg (range, 50–86 kg) and 23.6 kg/m2 (range, 19.7–26.5 kg/m2) in the CCRT + AC group. The clinical characteristics of the patients were comparable in the 2 groups, except with respect to pathological type (P=0.04) and T stage (P=0.04; Table 1).
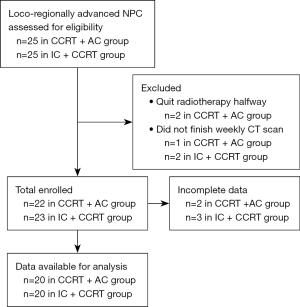
Table 1
| Characteristics | IC + CCRT group (n=20) | CCRT + AC group (n=20) | P value |
|---|---|---|---|
| Gender, n | 0.49 | ||
| Male | 15 | 13 | |
| Female | 5 | 7 | |
| Age (years) | 0.07 | ||
| Median | 50 | 49 | |
| Range | 19–72 | 37–61 | |
| Pathological type*, n | 0.04 | ||
| 1 | 6 | 4 | |
| 2 | 10 | 4 | |
| 3 | 4 | 11 | |
| 4 | 0 | 1 | |
| Clinical tumor stage, n | 0.24 | ||
| IVa | 8 | 3 | |
| III | 12 | 17 | |
| T stage, n | 0.04 | ||
| T1 | 1 | 5 | |
| T2 | 10 | 9 | |
| T3 | 5 | 5 | |
| T4 | 4 | 1 | |
| N stage, n | 0.77 | ||
| N1 | 0 | 4 | |
| N2 | 13 | 14 | |
| N3 | 7 | 2 | |
| Weight (kg) | 0.11 | ||
| Median | 67 | 60 | |
| Range | 52–99 | 50–86 | |
| BMI, kg/m2 | 0.37 | ||
| Median | 23.1 | 23.6 | |
| Range | 19.1–35.4 | 19.7–26.5 | |
*, type 1, poorly differentiated squamous cell carcinoma; type 2, poorly differentiated nonkeratinizing carcinoma; type 3, well-differentiated nonkeratinizing carcinoma; type 4: unclassified carcinoma. IC + CCRT, induction chemotherapy plus concurrent chemoradiotherapy; CCRT + AC, concurrent chemoradiotherapy plus adjuvant chemotherapy; BMI, body mass index.
Changes in body weight
In the IC + CCRT group, the initial (before IC) median weight and BMI were 67 kg (range, 52–99 kg) and 23.1 kg/m2 (range, 19.1–35.4 kg/m2), respectively. Before IMRT, 16 of the 20 patients experienced weight loss and 4 patients gained weight; the mean weight loss was 1.28 kg (range, −1.1–5.5 kg; Figure 2), post 2–3 cycles of TPF IC.
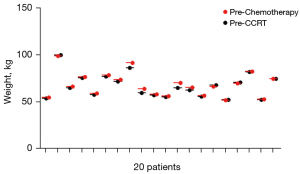
Almost all the patients experienced significant weight loss during IMRT, both in the CCRT + AC and IC + CCRT groups. In the IC + CCRT group, the mean weight loss after 25 fractions was 5.7 kg compared with the baseline, which corresponded to an 8.3% (range, 3.6–20%) reduction of the initial body weight. In the CCRT + AC group, the mean weight loss was 7.0 kg, corresponding to a 13.6% (range, 3.9–25.5%) reduction of the initial body weight, which was a much bigger reduction than that in the IC + CCRT groups (P=0.01; Table 2). The weight loss in percentage reduction observed at the end of the fifth week compared with the preceding week was significantly greater in the CCRT + AC group than in the IC + CCRT group (P=0.015; Table 2).
Table 2
| Comparison | Absolute reduction (mean + SD, kg) | Percentage reduction (mean, %) | |||||
|---|---|---|---|---|---|---|---|
| IC + CCRT | CCRT + AC | P value | IC + CCRT | CCRT + AC | P value | ||
| W0 vs. W1 | 1.30 | 0.52 | 0.12 | 1.9 | 0.7 | 0.11 | |
| W1 vs. W2 | 1.29 | 1.38 | 0.86 | 2.0 | 2.0 | 0.63 | |
| W2 vs. W3 | 1.05 | 1.25 | 0.52 | 1.6 | 2.0 | 0.38 | |
| W3 vs. W4 | 1.09 | 1.28 | 0.50 | 1.7 | 2.1 | 0.34 | |
| W4 vs. W5 | 1.19 | 2.35 | 0.01 | 1.6 | 3.9 | 0.015 | |
| W0 vs. W5 | 5.7 | 7.0 | 0.07 | 8.3 | 13.6 | 0.01 | |
IC + CCRT, induction chemotherapy plus concurrent chemoradiotherapy; CCRT + AC, concurrent chemoradiotherapy plus adjuvant chemotherapy.
Changes in GTV
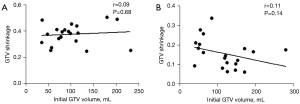
The weekly absolute and percentage reductions in GTV are listed in Table 2, which are compared to those of our previous study (11). Compared with the pre-IMRT GTV, the mean GTV shrinkages after 25 fractions were 16.55 mL (15.7%; range, 6.1–23.7%) and 39.86 mL (38.79%; range, 25.3–50.7%) in the IC + CCRT group and CCRT + AC group, respectively. The weekly reductions in GTV in the IC + CCRT group were relatively consistent throughout the course of IMRT (Figure 3). The greatest percentage reductions in GTV in the CCRT + AC group occurred in week 1 (10.3%) and week 4 (10.5%). Significantly greater GTV shrinkages were observed in each week compared with the preceding week in the CCRT + AC group than in the IC + CCRT group, both in absolute and percentage terms (Table 3 and Figure 3A). In both groups, no significant correlation was observed between GTV reduction and the pre-IMRT GTV (IC + CCRT: P=0.14; CCRT + AC: P=0.68; Figure 4A,4B).
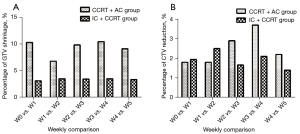
Table 3
| Comparison | Absolute reduction (mean + SD, mL) | Percentage reduction (mean, %) | |||||
|---|---|---|---|---|---|---|---|
| IC + CCRT | CCRT + AC | P value | IC + CCRT | CCRT + AC | P value | ||
| GTV0 vs. GTV1 | 3.85 | 8.80 | 0.02* | 3.1 | 10.3 | <0.001* | |
| GTV1 vs. GTV2 | 3.52 | 5.98 | 0.21 | 3.47 | 6.8 | 0.01* | |
| GTV2 vs. GTV3 | 3.45 | 8.79 | 0.052 | 3.44 | 9.9 | 0.01* | |
| GTV3 vs. GTV4 | 2.89 | 8.01 | 0.10 | 3.5 | 10.5 | 0.01* | |
| GTV4 vs. GTV5 | 2.83 | 6.20 | 0.008* | 3.37 | 9.1 | <0.001* | |
| GTV0 vs. GTV5 | 16.55 | 39.86 | 0.003* | 15.7 | 38.79 | <0.001* | |
| CTV0 vs. CTV1 | 13.37 | 10.95 | 0.53 | 1.94 | 1.8 | 0.64 | |
| CTV1 vs. CTV 2 | 17.21 | 10.91 | 0.26 | 2.5 | 1.8 | 0.22 | |
| CTV2 vs. CTV 3 | 10.04 | 12.27 | 0.07 | 1.66 | 2.9 | 0.75 | |
| CTV3 vs. CTV 4 | 12.27 | 23.63 | 0.03* | 2.1 | 3.7 | 0.046* | |
| CTV4 vs. CTV 5 | 8.15 | 13.69 | 0.04* | 1.4 | 2.2 | 0.03* | |
| CTV0 vs. CTV5 | 61.25 | 87.72 | 0.17 | 9.33 | 12.7 | 0.048* | |
*, P<0.05. GTV, gross tumor volume; CTV, clinical target volume; IC + CCRT, induction chemotherapy plus concurrent chemoradiotherapy; CCRT + AC, concurrent chemoradiotherapy plus adjuvant chemotherapy.
Changes in CTV
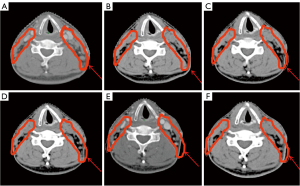
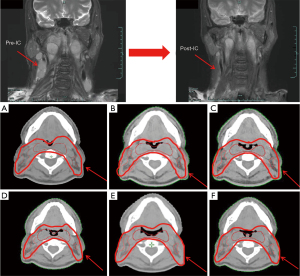
Compared with the baseline, the mean CTV reduction after 25 fractions was 61.25 mL (9.33%; range, 4.4–17.0%) and 87.72 mL (12.7%; range, 6.7–22.9%) in the IC + CCRT group and the CCRT + AC group, respectively. Analysis of the weekly changes in the CTV relative to the CTV in the preceding week showed that the greatest reduction in the CTV occurred from week 1 to week 2 (17.21 mL; 2.5%) in the IC + CCRT group and from week 3 to week 4 (23.63 mL; 3.7%) in the CCRT + AC group (Table 3, Figure 3B). The CTV reductions observed at the end of the fourth and fifth weeks compared with the CTVs in the preceding weeks were significantly greater in the CCRT + AC group than in the IC + CCRT group in both absolute (P=0.03 and 0.04, respectively) and percentage (P=0.046 and 0.03, respectively) terms (Table 2). Superimposition of CTV0 on CT1–5 (Figures 5A-5F,6A-6F) was used to analyze the weekly changes in the anatomical structures. The analysis of these cervical slices showed that the CTV target drift was less in the IC + CCRT group (Figure 6A-6F) than in the CCRT + AC group (Figure 5A-5F).
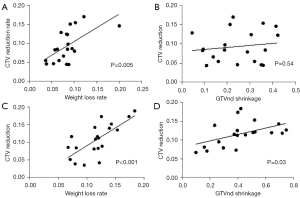
In the IC + CCRT group, the CTV reduction only correlated with weight loss (P=0.005), and no significant correlation was observed with GTV (P=0.06) or GTVnd shrinkage (P=0.54; Figure 7A,7B). However, the CTV reduction was related to both weight loss (P<0.001) and GTVnd shrinkage (P=0.03; Figure 7C,7D) in the CCRT + AC group.
Discussion
CCRT and IMRT can improve survival and locoregional control in patients with locally advanced NPC (22,23). However, even with aggressive CCRT schedules, the rate of distant metastases remains high (approximately 30%) (24,25). The addition of further chemotherapy to CCRT can reduce the distant failure rate, but its benefit needs to be demonstrated by rigorous clinical evidence. Both IC + CCRT and CCRT + AC are standard treatments for advanced NPC (26). However, CCRT + AC appears to cause more severe toxicities, leaving many patients unable to complete the assigned cycles, which diminishes its therapeutic effect and restricts its wider clinical application (26). In contrast, IC + CCRT is an effective and promising alternative which shows better compliance rates and may eliminate early micrometastases (27). Therefore, IC + CCRT seems to be a better clinical option for advanced NPC.
However, in head and neck cancers other than NPC, IC + CCRT has shown no survival superiority over CCRT (28,29). It remains to be determined whether this treatment strategy has the same results for NPC. Recent studies have investigated the addition of IC to CCRT for the treatment of advanced NPC (12,13,30,31). Most of these studies found that IC did not improve survival, which is consistent with the results obtained for other studies of head and neck cancers. However, some studies reported that IC added to CCRT might be ineffective for T3–4N0–1 NPC patients, indicating that only high-risk patients, including patients with N2–3 tumors and overall stage IVA disease, may benefit from IC (30,31). Some other studies demonstrated that IC followed by CCRT provided long-term DFS (27,32,33) and OS benefits in locoregionally advanced NPC (27,32). In the latest research, IC + CCRT was reported to significantly improve the locoregional RFS (LRFS) and OS (17,18,34,35). Another interesting finding is that the TPF regimen seems to be more active regarding the LRFS, which is potentially related to the more obvious effect of this chemotherapy regimen on tumor volume shrinkage (35). In the present study paper, the TPF regimen was used as the IC scheme in enrolled patients.
The most important reason for the differences between the results obtained for NPC and those obtained for other head and neck cancers is that IC can measurably reduce tumor volume before CCRT in patients with N2–3 or stage IVA NPC, thereby reducing target drift during CCRT and improving LRFS and OS. In the present study, to confirm this hypothesis, weight loss and GTV and CTV shrinkage were compared between 2 common treatment strategies for NPC, namely, IC + CCRT and CCRT + AC. After 2 or 3 cycles of IC with the TPF regimen, most patients experienced obvious weight loss, as IC induces digestive side effects, including decreased appetite and food intake. Weekly CT scanning was then performed for 5 consecutive weeks during 32-fraction IMRT. Weight loss was apparent in both groups after five weeks of radiation therapy. This is consistent with the literature, which indicates that weight loss is inevitable during radiotherapy, even with early nutritional intervention (5,7,36). Lu et al. reported that the mean weight loss during treatment was 13.2% (±6.0%) of the initial body weight and that this correlated with a reduction in GTVnd (5). Height et al. reported that the median weight loss after 40–50 Gy radiotherapy was 3% even when all patients received early nutritional intervention via tube feeding to minimize weight loss during CCRT (7).
During the long course of radical chemoradiotherapy, patients develop nausea and vomiting due to the radiotherapy or chemotherapy and acute oral mucosal reactions due to the radiotherapy. All of these reactions can lead to reduced intake and varying degrees of weight loss. In this study, the weight loss in the IC + CCRT group was relatively stable week by week and was less than the weight loss observed in the CCRT + AC group. The weight loss after 25 fractions was significantly greater in the CCRT + AC group than in the IC + CCRT group [7.0 kg (13.6%) vs. 5.7 kg (8.3%)]. This may be because IC leads to some weight decline before CCRT. Thus, one of the advantages of IC is that it can reduce the degree of weight loss during CCRT, thereby decreasing target drift.
The dynamic changes in GTV were also analyzed in these 2 study groups. The weekly shrinkage of GTV was significantly greater in the CCRT + AC group than in the IC + CCRT group. This is because IC greatly reduces tumor volume. The initial GTV before CCRT was smaller in the IC + CCRT group than in the CCRT + AC group, but no significant correlation was observed between GTV shrinkage during CCRT and the pre-CCRT tumor volume in either group.
Finally, weekly CTV reduction was compared in the 2 study groups. CTV reductions at the end of the fourth and fifth weeks as compared with the CTVs in the preceding weeks were significantly greater in the CCRT + AC group than in the IC + CCRT group. These reductions in CTV were consistent with the reductions observed in body weight. Following this, the relationship of CTV reduction with GTV reduction and weight loss was assessed, and a positive correlation between CTV reduction and weight loss in both groups was found. In the CCRT + AC group, the CTV reduction was found to obviously correlate with GTVnd reduction, but not with GTV reduction (including GTVnd and GTVnx). In the IC + CCRT group, neither GTV nor GTVnd reduction correlated with CTV reduction. These results showed that changes in the cervical metastatic lymph nodes were the main contributors to cervical anatomical drift in patients with locoregionally advanced NPC. After IC, most patients experienced large tumor volume reductions, especially in the metastatic lymph nodes, with complete responses. Therefore, shrinkage of the tumor masses (which had caused changes in the target anatomical structures) was partially eliminated by IC.
In the CCRT + AC group, CTV reduction was related to weight loss and GTVnd reduction (i.e., cervical lymph node mass volume). In the IC + CCRT group, CTV reduction was only related to weight loss. Furthermore, the weight loss in the IC + CCRT group was significantly less than that in the CCRT + AC group. Accordingly, the CTV drift was also smaller than that in the CCRT + AC group. Thus, we postulate that if nutritional support is given to minimize weight loss during CCRT among patients who receive IC, changes in anatomical structures and CTV drift would be greatly reduced and the accuracy of IMRT would consequently improve.
Some studies have provided evidence that individual patients with advanced N stage NPC can benefit from the addition of IC to CCRT (13,27). These results are consistent with this study, which showed that CTV reduction obviously correlated with GTVnd reduction among patients who did not receive IC. The volume of the cervical lymph nodes and the CTV were significantly decreased after IC in patients with N2–3 disease. Therefore, the most probable factor contributing to the survival benefit observed among these patients might have been the decrease of weight loss and shrinkage of the CTV during CCRT after IC.
We acknowledge that this study has limitations. First, volume comparison is only one parameter for monitoring target drift. Other parameters, such as center of mass, volume edge analysis, and concordance index, will be analyzed in our further studies. Second, the study cohort size was modest and the study took place in a single center. In fact, in this study, almost all patient records of weekly GTV and CTV shrinkage were more obvious in the CCRT + AC group than in the IC + CCRT group; hence, the further recruitment of patients did not occur.
Conclusions
This study found that weight loss and GTV and CTV reductions during CCRT were much lower in patients who received IC than in those who directly received CCRT. Moreover, CTV reduction was closely related to weight loss and GTVnd shrinkage among patients who received CCRT without IC. However, in the IC + CCRT group, CTV reduction was associated with only weight loss. These findings suggest that, as long as the body weight of patients who receive IC does not decrease significantly during IMRT, target drift can be reduced and replanning can be avoided.
Therefore, to some degree, IC can improve the accuracy of treatment by decreasing anatomical and target drift, and thereby reduce the likelihood of the recurrence of locoregionally advanced NPC, especially among patients with large metastatic cervical lymph nodes. This result provides evidence indicating that IC improves the LRFS and OS of patients with locoregionally advanced NPC.
Acknowledgments
Funding: This work was supported by the Natural Science Foundation of the Zhejiang Province of China (No. LSY19H160004) and the National Key R&D Program of China (No. 2018AAA0102102).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-776/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-776/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Human Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University Hospital, and informed consent was acquired from all of the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Huang TL, Chien CY, Tsai WL, Liao KC, Chou SY, Lin HC, Dean Luo S, Lee TF, Lee CH, Fang FM. Long-term late toxicities and quality of life for survivors of nasopharyngeal carcinoma treated with intensity-modulated radiotherapy versus non-intensity-modulated radiotherapy. Head Neck 2016;38:E1026-32. [Crossref] [PubMed]
- Song T, Fang M, Zhang XB, Zhang P, Xie RF, Wu SX. Sustained improvement of quality of life for nasopharyngeal carcinoma treated by intensity modulated radiation therapy in long-term survivors. Int J Clin Exp Med 2015;8:5658-66. [PubMed]
- Bhide SA, Davies M, Burke K, McNair HA, Hansen V, Barbachano Y, El-Hariry IA, Newbold K, Harrington KJ, Nutting CM. Weekly volume and dosimetric changes during chemoradiotherapy with intensity-modulated radiation therapy for head and neck cancer: a prospective observational study. Int J Radiat Oncol Biol Phys 2010;76:1360-8. [Crossref] [PubMed]
- Lu N, Feng LC, Cai BN, Hou J, Wang YL, Xie CB. Clinical study on the changes of the tumor target volume and organs at risk in helical tomotherapy for nasopharyngeal carcinoma. Chin Med J (Engl) 2012;125:87-90. [PubMed]
- Lam SK, Zhang Y, Zhang J, Li B, Sun JC, Liu CY, et al. Multi-Organ Omics-Based Prediction for Adaptive Radiation Therapy Eligibility in Nasopharyngeal Carcinoma Patients Undergoing Concurrent Chemoradiotherapy. Front Oncol 2022;11:792024. [Crossref] [PubMed]
- Height R, Khoo V, Lawford C, Cox J, Joon DL, Rolfo A, Wada M. The dosimetric consequences of anatomic changes in head and neck radiotherapy patients. J Med Imaging Radiat Oncol 2010;54:497-504. [Crossref] [PubMed]
- Tan W, Wang Y, Yang M, Amos RA, Li W, Ye J, Gary R, Shen W, Hu D. Analysis of geometric variation of neck node levels during image-guided radiotherapy for nasopharyngeal carcinoma: recommended planning margins. Quant Imaging Med Surg 2018;8:637-47. [Crossref] [PubMed]
- Zhao L, Wan Q, Zhou Y, Deng X, Xie C, Wu S. The role of replanning in fractionated intensity modulated radiotherapy for nasopharyngeal carcinoma. Radiother Oncol 2011;98:23-7. [Crossref] [PubMed]
- Nishimura Y, Shibata T, Nakamatsu K, Kanamori S, Koike R, Okubo M, Nishikawa T, Tachibana I, Tamura M, Okumura M. A two-step intensity-modulated radiation therapy method for nasopharyngeal cancer: the Kinki University experience. Jpn J Clin Oncol 2010;40:130-8. [Crossref] [PubMed]
- Yan D, Yan S, Wang Q, Liao X, Lu Z, Wang Y. Predictors for replanning in loco-regionally advanced nasopharyngeal carcinoma patients undergoing intensity-modulated radiation therapy: a prospective observational study. BMC Cancer 2013;13:548. [Crossref] [PubMed]
- Gabani P, Barnes J, Lin AJ, Rudra S, Oppelt P, Adkins D, Rich JT, Zevallos JP, Daly MD, Gay HA, Thorstad WL. Induction chemotherapy in the treatment of nasopharyngeal carcinoma: Clinical outcomes and patterns of care. Cancer Med 2018;7:3592-603. [Crossref] [PubMed]
- Liu L, Fei Z, Chen M, Zhao L, Su H, Gu D, Lin B, Cai X, Lu L, Gao M, Ye X, Jin X, Xie C. Induction chemotherapy plus concurrent chemoradiotherapy versus induction chemotherapy plus volumetric modulated arc therapy alone in the treatment of stage II-IVB nasopharyngeal carcinoma patients: a retrospective controlled study. Radiat Oncol 2018;13:148. [Crossref] [PubMed]
- Tan T, Lim WT, Fong KW, Cheah SL, Soong YL, Ang MK, Ng QS, Tan D, Ong WS, Tan SH, Yip C, Quah D, Soo KC, Wee J. Concurrent chemo-radiation with or without induction gemcitabine, Carboplatin, and Paclitaxel: a randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2015;91:952-60. [Crossref] [PubMed]
- Li Y, Tang LQ, Liu LT, Guo SS, Liang YJ, Sun XS, Tang QN, Bei JX, Tan J, Chen S, Ma J, Zhao C, Chen QY, Mai HQ. Induction Chemotherapy Plus Concurrent Chemoradiotherapy Versus Concurrent Chemoradiotherapy Alone in Locoregionally Advanced Nasopharyngeal Carcinoma in Children and Adolescents: A Matched Cohort Analysis. Cancer Res Treat 2018;50:1304-15. [Crossref] [PubMed]
- Cao SM, Yang Q, Guo L, Mai HQ, Mo HY, Cao KJ, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase III multicentre randomised controlled trial. Eur J Cancer 2017;75:14-23. [Crossref] [PubMed]
- Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 2016;17:1509-20. [Crossref] [PubMed]
- Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. N Engl J Med 2019;381:1124-35. [Crossref] [PubMed]
- Taamma A, Fandi A, Azli N, Wibault P, Chouaki N, Hasbini A, Couteau C, Armand JP, Cvitkovic E. Phase II trial of chemotherapy with 5-fluorouracil, bleomycin, epirubicin, and cisplatin for patients with locally advanced, metastatic, or recurrent undifferentiated carcinoma of the nasopharyngeal type. Cancer 1999;86:1101-8. [Crossref] [PubMed]
- Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed. Philadelphia: Lippincott-Raven, 2009.
- Pan J, Xu Y, Qiu S, Zong J, Guo Q, Zhang Y, Lin S, Lu JJ. A comparison between the Chinese 2008 and the 7th edition AJCC staging systems for nasopharyngeal carcinoma. Am J Clin Oncol 2015;38:189-96.
- Baujat B, Audry H, Bourhis J, Chan AT, Onat H, Chua DT, Kwong DL, Al-Sarraf M, Chi KH, Hareyama M, Leung SF, Thephamongkhol K, Pignon JP. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys 2006;64:47-56. [Crossref] [PubMed]
- Langendijk JA, Leemans CR, Buter J, Berkhof J, Slotman BJ. The additional value of chemotherapy to radiotherapy in locally advanced nasopharyngeal carcinoma: a meta-analysis of the published literature. J Clin Oncol 2004;22:4604-12. [Crossref] [PubMed]
- Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol 2003;21:631-7. [Crossref] [PubMed]
- Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, Bosch W, Morrison WH, Quivey J, Thorstad W, Jones C, Ang KK. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol 2009;27:3684-90. [Crossref] [PubMed]
- Liu M, You W, Song YB, Miao JD, Zhong XB, Cai DK, Xu L, Xie LF, Gao Y. The Changing Role of Chemotherapy in Locoregionally Advanced Nasopharyngeal Carcinoma: A Updated Systemic Review and Network Meta-Analysis. Front Oncol 2018;8:597. [Crossref] [PubMed]
- Frikha M, Auperin A, Tao Y, Elloumi F, Toumi N, Blanchard P, Lang P, Sun S, Racadot S, Thariat J, Alfonsi M, Tuchais C, Cornely A, Moussa A, Guigay J, Daoud J, Bourhis J. GORTEC. A randomized trial of induction docetaxel-cisplatin-5FU followed by concomitant cisplatin-RT versus concomitant cisplatin-RT in nasopharyngeal carcinoma (GORTEC 2006-02). Ann Oncol 2018;29:731-6. [Crossref] [PubMed]
- Vidal L, Ben Aharon I, Limon D, Cohen E, Popovtzer A. Role of Induction Chemotherapy Prior to Chemoradiation in Head and Neck Squamous Cell Cancer-Systematic Review and Meta-analysis. Cancer J 2017;23:79-83. [Crossref] [PubMed]
- Cohen EE, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol 2014;32:2735-43. [Crossref] [PubMed]
- Lan XW, Xiao Y, Zou XB, Zhang XM, OuYang PY, Xie FY. Outcomes of adding induction chemotherapy to concurrent chemoradiotherapy for stage T3N0-1 nasopharyngeal carcinoma: a propensity-matched study. Onco Targets Ther 2017;10:3853-60. [Crossref] [PubMed]
- Wu LR, Yu HL, Jiang N, Jiang XS, Zong D, Wen J, Huang L, Xie P, Chen W, Wang TT, Gu DY, Yan PW, Yin L, He X. Prognostic value of chemotherapy in addition to concurrent chemoradiotherapy in T3-4N0-1 nasopharyngeal carcinoma: a propensity score matching study. Oncotarget 2017;8:76807-15. [Crossref] [PubMed]
- Yang Q, Cao SM, Guo L, Hua YJ, Huang PY, Zhang XL, et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur J Cancer 2019;119:87-96. [Crossref] [PubMed]
- Hong RL, Hsiao CF, Ting LL, Ko JY, Wang CW, Chang JTC, Lou PJ, Wang HM, Tsai MH, Lai SC, Liu TW. Final results of a randomized phase III trial of induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in patients with stage IVA and IVB nasopharyngeal carcinoma-Taiwan Cooperative Oncology Group (TCOG) 1303 Study. Ann Oncol 2018;29:1972-9. [Crossref] [PubMed]
- Wang P, Zhang M, Ke C, Cai C. The efficacy and toxicity of induction chemotherapy plus concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2020;99:e19360. [Crossref] [PubMed]
- Bongiovanni A, Vagheggini A, Fausti V, Mercatali L, Calpona S, Di Menna G, Miserocchi G, Ibrahim T. Induction chemotherapy plus concomitant chemoradiotherapy in nasopharyngeal carcinoma: An updated network meta-analysis. Crit Rev Oncol Hematol 2021;160:103244. [Crossref] [PubMed]
- Cheng HC, Wu VW, Ngan RK, Tang KW, Chan CC, Wong KH, Au SK, Kwong DL. A prospective study on volumetric and dosimetric changes during intensity-modulated radiotherapy for nasopharyngeal carcinoma patients. Radiother Oncol 2012;104:317-23. [Crossref] [PubMed]


