
Optimization of hepatobiliary phase imaging in gadoxetic acid-enhanced magnetic resonance imaging: a narrative review
Introduction
Gadolinium ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA), a liver-specific contrast agent with the highest imaging efficiency of its kind, has been widely used in clinical practice. In addition to providing the same dynamic contrast enhancement as conventional extracellular space contrast agents, Gd-EOB-DTPA-enhanced magnetic resonance imaging (MRI) can provide an additional hepatobiliary phase (HBP) for the detection and identification of hepatic lesions and the evaluation of liver function and fibrosis (1-4). Hepatic cells begin to uptake Gd-EOB-DTPA at about 1.5 min after intravenous administration and then reach a peak of uptake around 20 min after administration (5,6). Therefore, an adequate HBP can be typically achieved with a delay of 20 min after intravenous injection. However, 20 min delay time is considered too drawn-out in patients with normal liver function and too short in patients with cirrhosis. Some studies demonstrated that liver uptake and excretion of Gd-EOB-DTPA are closely related to liver function (7,8). Therefore, researchers have employed personalized delay times for HBP acquisition based on patients’ liver function in order to optimize the HBP acquisition. These investigations and findings have contributed to the consensus on the HBP acquisition in Gd-EOB-DTPA-enhanced MRI from the International Forum for Liver Magnetic Resonance Imaging (IFFLMRI). However, liver function should be evaluated sufficiently before the MRI is performed according to the IFFLMRI consensus. Other investigations found that the initial visualization time of the intrahepatic bile duct (IHD) in Gd-EOB-DTPA-enhanced MRI was also related to liver enhancement and function (9). Therefore, initial visualization of the IHD is considered to be 1 of the 2 necessary conditions for adequate HBP in the consensus on the clinical application of Gd-EOB-DTPA from the European Society of Gastrointestinal and Abdominal Radiology (ESGAR) (10). Another necessary condition of the adequate HBP suggested by the ESGAR is a signal intensity (SI) of the hepatic parenchyma higher than that of the intrahepatic vessel, which depends on the observer’s personal judgment. Therefore, optimization of HBP imaging in Gd-EOB-DTPA-enhanced MRI is still a topic that needs to be reviewed and investigated further. We present the following article in accordance with the Narrative Review reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-916/rc).
Methods of the study
The electronic literature search was not restricted by publication date and was performed using the PubMed, MEDLINE, Cochrane, and EMBASE databases to identify published reports on optimizing HBP imaging in Gd-EOB-DTPA-enhanced MRI. The search terms we used in the investigation were “Gd-EOB-DTPA”, “gadoxetic acid disodium”, “gadolinium ethoxybenzyl-diethylenetriamine pentaacetic acid”, “hepatobiliary phase”, “hepatocyte phase”, and “consensus” (Table 1). The language of all publications was limited to English. Three authors, Chao Wang, Ning Wu, and Wei-Rong Sun, reviewed the above literature independently. The number of literatures retrieved from the above databases is 962, 380, 384, 132. We excluded articles that were not related to Gd-EOB-DTPA-enhanced MRI HBP acquisition, Finally, a total of 12 articles and the Liver Imaging Reporting and Data System (LI-RADS) version 2018 on the clinical application of liver-specific contrast agents Gd-EOB-DTPA were obtained (Tables 2,3) (10-22).
Table 1
| Items | Specification |
|---|---|
| Date of search | April 9, 2022 |
| Databases and other sources searched | PubMed, MEDLINE, Cochrane, Embase |
| Search terms used (including MeSH and free text search terms and filters) | (“Gd-EOB-DTPA” [MeSH]) AND “hepatobiliary phase” [MeSH] |
| (“gadoxetic acid disodium” [MeSH]) AND “hepatobiliary phase” [MeSH] | |
| (“gadolinium ethoxybenzyl-diethylenetriamine-pentaacetic acid” [MeSH]) AND “hepatobiliary phase” [MeSH] | |
| (“Gd-EOB-DTPA” [MeSH]) AND “hepatocyte phase” [MeSH] | |
| (“gadoxetic acid disodium” [MeSH]) AND “hepatocyte phase” [MeSH] | |
| (“gadolinium ethoxybenzyl-diethylenetriamine-pentaacetic acid” [MeSH]) AND “hepatocyte phase” [MeSH] | |
| (“Gd-EOB-DTPA” [MeSH]) AND “consensus” [MeSH] | |
| (“gadoxetic acid disodium” [MeSH]) AND “consensus” [MeSH] | |
| (“gadolinium ethoxybenzyl-diethylenetriamine-pentaacetic acid” [MeSH]) AND “consensus” [MeSH] | |
| Timeframe | January 1, 1999–April 9, 2022 |
| Inclusion and exclusion criteria | Inclusion criteria: original article, review, consensus statement. Exclusion criteria: animal trial, literature not published in English, and other literature not related to the topic |
| Selection process | Three authors (Chao Wang, Ning Wu, and Wei-Rong Sun) conducted an independent literature search and finally determined the relevant published reports |
| Any additional considerations, if applicable | The LI-RADS version 2018 was used in the review |
MeSH, Medical Subject Headings; Gd-EOB-DTPA, gadolinium ethoxybenzyl-diethylenetriamine pentaacetic acid; LI-RADS, Liver Imaging Reporting and Data System.
Table 2
| Sequence | Years | Venues | Technical optimization of HBP imaging (yes/no) | Consensus statements of HBP delay time |
|---|---|---|---|---|
| First (11) | 2007 | Dublin, Germany | Yes | Optimal time: 20 min |
| Some patients: 10 min | ||||
| Patients with diffuse liver disease: 40 min | ||||
| Second (12) | 2008 | Kyoto, Japan | Yes | Noncirrhotic patients: 10 min |
| Cirrhotic patients: 20 min or later | ||||
| Third (13) | 2009 | Frascati, Italy | Yes | Optimal time: 20 min |
| Noncirrhotic patients: 10–15 min | ||||
| The delay time of HBP may be influenced by bilirubin level; additional data are required to better understand this topic | ||||
| Fourth (14) | 2010 | Seoul, Korea | No | – |
| Fifth (15) | 2011 | Munich, Germany | Yes | The delay time of HBP should be selected approximately 10–20 min after intravenous administration, depending on liver function |
| Sixth (16) | 2012 | Vancouver, Canada | No | – |
| Seventh (17) | 2013 | Shanghai, China | Yes | While a 20-min delay is acceptable in most patients, a shorter delay time may be feasible for hepatic parenchymal enhancement in some patients. A longer delay time may be helpful for patients with impaired liver uptake and biliary system visualization |
| Eighth (18) | 2017 | Basel, Switzerland | Yes | The minimum protocol in noncirrhotic oncological patients: 10–15 min. The minimum protocol in cirrhotic patients for screening and presurgical evaluation/staging: 20 min |
| Ninth (19) | 2019 | Singapore | No | – |
MRI, magnetic resonance imaging; Gd-EOB-DTPA, gadolinium ethoxybenzyl-diethylenetriamine pentaacetic acid; HBP, hepatobiliary phase.
Table 3
| Other consensus | Technical optimization of HBP imaging (yes/no) | Consensus statements of HBP delay time |
|---|---|---|
| Consensus statements from a multidisciplinary expert panel [2012], (20) | Yes | Current recommendations: 20 min |
| However, there is increasing evidence supporting acquisition as soon as 10 min after injection in patients with normal liver function | ||
| Patients with cirrhosis are advised to have HPB collected longer than the conventional 20-min delay time | ||
| Chinese consensus [2019], (21) | Yes | The best HBP delay time: 20 min reach the peak absorption of the Gd-EOB-DTPA |
| Normal liver function: 10 min | ||
| Chronic liver disease: prolonged to 30 min | ||
| ESGAR [2016], (10) | Yes | Adequate HBP: |
| Gd-EOB-DTPA is detected in the bile ducts | ||
| SIliver >> SIintrahepatic vessels | ||
| However, depending on hepatic physiological and pathophysiological function, an optimal hypointense signal of the hepatic vessels cannot always be achieved | ||
| LI-RADS version [2018], (22) | Yes | Adequate HBP: |
| SIliver >> SIintrahepatic vessels. It is suboptimal otherwise | ||
| Drawback: visible excretion of Gd-EOB-DTPA into the bile duct does not indicate adequate HBP |
HBP, hepatobiliary phase; ESGAR, European Society of Gastrointestinal and Abdominal Radiology; LI-RADS, Liver Reporting and Data System; Gd-EOB-DTPA, gadolinium ethoxybenzyl-diethylenetriaminepentaacetic acid; SI, signal intensity; SIliver, the signal intensity of liver; SIintrahepatic vessels, the signal intensity of intrahepatic vessels.
Optimizing the delay time of HBP based on different liver functions
Gd-EOB-DTPA is specifically absorbed by the hepatocyte after intravenous injection. However, impaired liver function results in reduced specific absorption of Gd-EOB-DTPA by hepatocytes. Certain studies have shown that a 10-minute delay in detecting liver metastases is sufficient to obtain adequate HBP in patients without chronic liver disease (CLD) (23-25). For example, Caton et al. (26) recently verified that a 15-minute delay time is time-saving for patients with neuroendocrine tumors.
In reports on optimizing the HBP delay time for patients with different liver functions, most studies have concluded that a delay of 15 min is sufficient to obtain adequate HBP in patients with mildly impaired liver function (27). However, another report demonstrated that a 10-minute HBP delay time in patients with Child-Pugh (C-P) A grading is sufficient for identifying hepatocellular carcinomas (HCCs) (28). The suitable delay time of HBP for patients with severe liver function impairment is also controversial. It was reported that extending the delay to 30 min can improve the enhancement of liver parenchyma with cirrhosis, thus improving the conspicuity of hepatic focal lesions, but some investigators deem that further extending the acquisition time in patients with severe cirrhosis is meaningless for obtaining adequate HBP (20,27,29). Whether a delayed HBP acquisition will benefit patients with severe cirrhosis is also controversial. In clinical practice, we tend to postpone HBP collection time in patients with cirrhosis (21).
Methods of optimizing the delay time for HBP and predicting adequate and reasonable HBP
A larger flip angle (FA) can improve the contrast-to-noise ratio, and further studies have indicated that a 5-minute transition phase with 30° FA or 10-minute HBP with 30° FA can replace the 20-minute HBP with a standard 10° FA (30-32). Despite this finding, the absorption of Gd-EOB-DTPA by liver parenchyma on HBP with a 5-minute delay was not sufficient in our clinical practice. Liver enhancement is influenced by clinical laboratory indicators (33-35). In a multicenter study, Okada et al. (36) showed that the enhancement of liver parenchyma is significantly correlated with biochemical indexes. Then, the delay time could be shortened to obtain adequate HBP when prothrombin (PT) activity was at least 86.9%. In order to avoid giving more expensive drugs to patients who cannot benefit from HBP, Kobi et al. (37) showed that a model for end-stage liver disease score, direct bilirubin, and total bilirubin could accurately predict suboptimal HBP to optimize HBP acquisition. Another report showed that suboptimal HBP could be predicted by combining the albumin-bilirubin grading with the liver-to-spleen ratio (LSR) during the 3-minute transition phase (38). The combination of MR elastography and serological examination of liver function using the Bayesian method can also predict applicable HBP to help radiologists and MRI technicians assess the benefits of GD-EOB-DTPA and prevent diagnostic pitfalls (39).
Subsequently, other studies have proven that the liver-portal vein contrast ratio can be used as a surrogate for LSR to evaluate HBP; these studies provided a cutoff value of 1.5, which assisted us in obtaining sufficient HBP to improve the detection of hepatic lesions (40-42). The subjective visual criterion based on the portal vein is also applicable to the estimate of the HBP image and can replace LSR, enriching the means of HBP evaluation (43). In addition, the liver-inferior vena cava ratio (LVCR) can also be used as an objective indicator to evaluate the adequacy of HBP in patients without CLD; when the LVCR reaches 2.0, the delay time can be reduced in 38% of patients without CLD (44). However, most of the above-mentioned methods for predicting HBP are still based on liver function. Some techniques for predicting HBP adequacy based on the MRI still need to be performed manually.
With the rise of artificial intelligence, a convolutional neural network (CNN) has been used to automatically evaluate the adequacy of HBP and optimize the acquisition time. The application of the CNN algorithm may shorten the delay time of HBP and help radiologists identify technically unsatisfactory images and avoid diagnostic pitfalls, shortening HBP acquisition time in almost 48% of patients with liver disease (45). The method based on CNN to evaluate the adequacy of HBP is automated and time-saving. However, CNN, as an emerging technology, still faces great challenges in its generalization because of the requirements on professional knowledge and low robustness in clinical settings (46,47).
Consensus on optimizing HBP delay time
Consensus statements and reports of the IFFLMRI
The consensus statement of the First International Primovist User Meeting held in Dublin on September 22–23, 2007, recommended a delay time of 20 min for HBP. For some patients, a delay of 10 min is sufficient; however, for patients with CLD, an extension of 40 min is necessary (11). Subsequently, optimization of the Gd-EOB-DTPA-enhanced protocol in the Second and Third International Forum for Liver MRI both recommended that a delay time of 10 to 15 min is sufficient for patients without cirrhosis, while the delay time may be extended to 20 min for patients with cirrhosis (12,13). In the Consensus Report of the Fourth International Forum for Gd-EOB-DTPA-enhanced MRI, the role of HBP in detecting small liver lesions was emphasized, and the progress of organic anion-transporting polypeptide 8 transporters was also discussed, without much elaboration on the technical aspects of optimizing HBP (14). Standardization of the Gd-EOB-DTPA-enhanced MRI protocol in the Consensus Report of the Fifth International Forum for Liver MRI recommended an appropriate HBP delay time between 10 and 20 min, depending on liver function (15). However, T1 mapping demonstrated that the T1 relaxation time in liver parenchyma is not shortened after a 13-minute delay time in patients with and without liver function damage, which may indicate that an excessive delay time is meaningless (48). Since the SI of liver parenchyma is related to the shortening of the T1 relaxation time by Gd-EOB-DTPA, a consensus statement was reached on the use of 40° FA in HBP to improve liver lesion conspicuity in the Consensus Report From the Sixth International Forum for Liver MRI Using Gd-EOB-DTPA held in Vancouver, Canada in September 2012, however, the optimal HBP delay time was not explained (16). The consensus report of the Seventh Liver International Forum on the technical optimization of HBP acquisition also suggests that a larger FA would increase the visibility of liver lesions (17,49). If the HBP acquisition time is prolonged appropriately, the visualization of the intrahepatic lesions can be optimized, however reducing patient comfort. The principle for determining when to collect HBP in Gd-EOB-DTPA-enhanced MRI is a trade-off between patient comfort and visualization of the intrahepatic lesions. The 20-min delay time for HBP is often suitable for most individuals. A delay time of less than 20 min may be feasible for some patients, and a delay time greater than the traditional 20 min may be significant for improving liver parenchyma enhancement in patients with cirrhosis (17). The shortened MRI protocols of Gd-EOB-DTPA-enhanced MRI in the Consensus Report from the Eighth International Forum, held in October 2017 in Basel, Switzerland, advised that the minimum delay time of HBP is 20 min in cirrhotic patients, while the minimum delay time of HBP for a patient with normal liver function is 10–15 min (18). Recently, at the Ninth International Forum for Liver MRI held in Singapore in 2019, the significance of abbreviated MRI (AMRI) sequences for detecting HCCs and liver metastases was mentioned, in which HBP in the AMRI sequences were all obtained at a 20-min delay time (19). Based on the comprehensive analysis of the previous consensus reports from the International Forum for Liver MRI, we can conclude that a delay of 10 to 15 min is sufficient for patients with normal liver function, while a conventional delay of 20 min is required for patients with cirrhosis, and a longer delay time is beneficial for the visualization of the biliary and liver parenchyma enhancement in severely cirrhotic patients (Table 2; Figure 1).
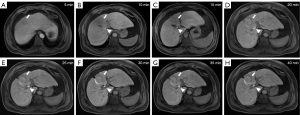
Consensus statements on the delay time of HBP from a multidisciplinary expert panel
A multidisciplinary expert panel established in June 2012 recommended an appropriate HBP delay of 20 min. A delay time of more than 20 min was recommended for patients with cirrhosis. However, there is increasing evidence that a delay of 10 min is sufficient for patients with normal liver function (20,50,51).
Chinese consensus on the delay time of HBP
Gd-EOB-DTPA was launched in China in early 2011; however, it is not yet widely used in Chinese hospitals. Moreover, there is a lack of consensus on the use of Gd-EOB-DTPA. Based on this background, a consensus on the clinical application of Gd-EOB-DTPA was proposed in China in 2019. The Chinese consensus on the clinical application of Gd-EOB-DTPA mentioned the scanning protocol to optimize HBP imaging; that is, the general delay time for HBP is 20 min after intravenous injection. Specifically, HBP can be obtained at a 10-min delay time in patients with normal liver function. A longer delay time can increase liver parenchyma enhancement. In patients with cirrhosis, the delay time can be extended to 30 min to further satisfy the enhancement of liver parenchyma (21,52).
Both the multidisciplinary expert panel and Chinese consensus statements determine the reasonable HBP delay time based on liver function. A 10-min delay time is considered feasible for patients with normal liver function, and the HBP collection time can be appropriately extended for patients with cirrhosis (Table 3).
ESGAR consensus statement on the delay time of HBP
ESGAR provides consensus and up-to-date advice on liver MRI and the clinical use of Gd-EOB-DTPA. ESGAR defines an adequate HBP as when the intrahepatic vascular SI is lower than the hepatic parenchyma SI during IHD opacity (10).
Adequate HBP in the LI-RADS version 2018
The technical section of the LI-RADS version 2018 describes how to judge the adequacy of HBP. There is a misconception that biliary imaging represents the adequate enhancement of the hepatic parenchyma (22). LI-RADS version 2018 emphasized the importance of liver enhancement over intrahepatic vessels to obtain an adequate HBP.
Compared with the delay time of individualized adequate HBP based on liver function, if we use ESGAR or LI-RADS version 2018 to determine the adequacy of HBP, we often need to track the changes of multiple structural SIs in the liver for multiple phases (Table 3; Figure 1).
The delay time of HBP acquisition for the evaluation of the liver reserve function
As a liver-specific contrast agent, Gd-EOB-DTPA could be taken up by hepatocytes through the same passageway as bilirubin, resulting in unique HBP imaging. Therefore, Gd-EOB-DTPA-enhanced MRI has been used for hepatic lesion detection and also for liver function reserve evaluation. Reports demonstrated that hepatocytes begin to uptake Gd-EOB-DTPA at about 1.5 min after intravenous administration. However, the early-stage imaging of its liver enhancement, such as the arterial phase, the portal vein phase, and even the transitional phase, is not suitable for evaluating hepatic functional reserve. This is because, at this stage, a large amount of the agents is distributed among the extracellular spaces, which contributes much more to the SI of MRI than do the agents from the intracellular spaces. Theoretically, the amount of the agents accumulated in the intracellular spaces of hepatocytes reflects the hepatic functional reserve. The HBP acquired between 15 and 20 min after intravenous administration has demonstrated good contrast between liver parenchyma and intrahepatic vessels, suggesting that there is more agent in the intracellular spaces than in the extracellular spaces. Therefore, a 15–20-min HBP may be suitable for evaluating the hepatic functional reserve. However, a recent investigation demonstrated the liver-spleen contrast on a 60-min delay HBP has a strong positive correlation with the receptor index (LHL15) calculated from 99mTc-GSA liver scintigraphy (53). Therefore, a much longer delayed acquisition time should be recommended for hepatic functional reserve evaluation than that for hepatic lesion detection.
Conclusions
The value of imaging is not confined to image interpretation. Every initiative that helps the imaging process benefit patients will maintain value beyond image interpretation (54). The image quality, time requirement, and the patients’ comfort in the MRI protocol are all important considerations in the strategies for optimizing HBP acquisition. The importance of the former is self-evident, while the latter two represent a value beyond image interpretation. Due to technical limitations, MRI examination takes a long time, and the noise during the examination is relatively high. The patient’s tolerance to an MRI examination is not very good. Therefore, if the study time is to be extended, especially in Gd-EOB-DTPA-enhanced MRI, we should inform and comfort the examinee to ensure cooperation in the examination. With the in-depth investigation of adequate HBP acquisition, several HBP acquisition protocols have been proposed for different clinical settings. If the relevant laboratory indicators are accessible and liver function grading is achieved prior to the MRI examination, the IFFLMRI consensus can be used to formulate the adequate HBP acquisition protocol. Otherwise, the ESGAR consensus can be used to determine the adequate HBP during the MRI process, which will be the end of the examination. However, it could be time-consuming and laborious for radiologists to judge whether an HBP is adequate.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 81671680).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-916/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-916/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Huppertz A, Balzer T, Blakeborough A, Breuer J, Giovagnoni A, Heinz-Peer G, Laniado M, Manfredi RM, Mathieu DG, Mueller D, Reimer P, Robinson PJ, Strotzer M, Taupitz M, Vogl TJEuropean EOB Study Group. Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology 2004;230:266-75. [Crossref] [PubMed]
- Brismar TB, Dahlstrom N, Edsborg N, Persson A, Smedby O, Albiin N. Liver vessel enhancement by Gd-BOPTA and Gd-EOB-DTPA: a comparison in healthy volunteers. Acta Radiol 2009;50:709-15. [Crossref] [PubMed]
- Verloh N, Fuhrmann I, Fellner C, Nickel D, Zeman F, Kandulski A, Hornung M, Stroszczynski C, Wiggermann P, Haimerl M. Quantitative analysis of liver function: 3D variable-flip-angle versus Look-Locker T1 relaxometry in hepatocyte-specific contrast-enhanced liver MRI. Quant Imaging Med Surg 2022;12:2509-22. [Crossref] [PubMed]
- Zhou N, Hu A, Shi Z, Wang X, Zhu Q, Zhou Q, Ma J, Zhao F, Kong W, He J. Inter-observer agreement of computed tomography and magnetic resonance imaging on gross tumor volume delineation of intrahepatic cholangiocarcinoma: an initial study. Quant Imaging Med Surg 2021;11:579-85. [Crossref] [PubMed]
- Pascolo L, Cupelli F, Anelli PL, Lorusso V, Visigalli M, Uggeri F, Tiribelli C. Molecular mechanisms for the hepatic uptake of magnetic resonance imaging contrast agents. Biochem Biophys Res Commun 1999;257:746-52. [Crossref] [PubMed]
- Akimoto S, Mori H, Fujii T, Furuya K. Optimal scan timing for Gd-EOB-DTPA enhanced liver dynamic MR imaging. Nihon Hoshasen Gijutsu Gakkai Zasshi 2009;65:626-30. [Crossref] [PubMed]
- Nassif A, Jia J, Keiser M, Oswald S, Modess C, Nagel S, Weitschies W, Hosten N, Siegmund W, Kühn JP. Visualization of hepatic uptake transporter function in healthy subjects by using gadoxetic acid-enhanced MR imaging. Radiology 2012;264:741-50. [Crossref] [PubMed]
- Feng ST, Wu L, Chan T, Cai H, Luo Y, Zheng K, Tang D, Li ZP. Functional magnetic resonance cholangiography enhanced with Gd-EOB-DTPA: effect of liver function on biliary system visualization. J Magn Reson Imaging 2014;39:1254-8. [Crossref] [PubMed]
- Ringe KI, Husarik DB, Gupta RT, Boll DT, Merkle EM. Hepatobiliary transit times of gadoxetate disodium (Primovist®) for protocol optimization of comprehensive MR imaging of the biliary system--what is normal? Eur J Radiol 2011;79:201-5. [Crossref] [PubMed]
- Neri E, Bali MA, Ba-Ssalamah A, Boraschi P, Brancatelli G, Alves FC, Grazioli L, Helmberger T, Lee JM, Manfredi R, Martì-Bonmatì L, Matos C, Merkle EM, Op De Beeck B, Schima W, Skehan S, Vilgrain V, Zech C, Bartolozzi C. ESGAR consensus statement on liver MR imaging and clinical use of liver-specific contrast agents. Eur Radiol 2016;26:921-31. [Crossref] [PubMed]
- Malone D, Zech CJ, Ayuso C, Bartolozzi C, Jonas E, Tanimoto A, 1st International Primovist User Meeting Attendees. Magnetic resonance imaging of the liver: consensus statement from the 1st International Primovist User Meeting. Eur Radiol 2008;Suppl 18:849-64.
- Tanimoto A, Lee JM, Murakami T, Huppertz A, Kudo M, Grazioli L. Consensus report of the 2nd International Forum for Liver MRI. Eur Radiol 2009;19:S975-89. [Crossref] [PubMed]
- Grazioli L, Lee JM, Malfertheiner P, Zech CJ, Blomqvist L, Merkle EM. Consensus Report of the Third International Forum for Liver Magnetic Resonance Imaging. Investigative Radiology 2010;45:S1-S10. [Crossref]
- Lee JM, Zech CJ, Bolondi L, Jonas E, Kim MJ, Matsui O, Merkle EM, Sakamoto M, Choi BI. Consensus report of the 4th International Forum for Gadolinium-Ethoxybenzyl-Diethylenetriamine Pentaacetic Acid Magnetic Resonance Imaging. Korean J Radiol 2011;12:403-15. [Crossref] [PubMed]
- Zech CJ, Bartolozzi C, Bioulac-Sage P, Chow PK, Forner A, Grazioli L, Huppertz A, Laumonier H, Min Lee J, Murakami T, Ricke J, Sirlin CB. Consensus report of the Fifth International Forum for Liver MRI. AJR Am J Roentgenol 2013;201:97-107. [Crossref] [PubMed]
- Sirlin CB, Hussain HK, Jonas E, Kanematsu M, Min Lee J, Merkle EM, Peck-Radosavljevic M, Reeder SB, Ricke J, Sakamoto M. Consensus report from the 6th International forum for liver MRI using gadoxetic acid. J Magn Reson Imaging 2014;40:516-29. [Crossref] [PubMed]
- Merkle EM, Zech CJ, Bartolozzi C, Bashir MR, Ba-Ssalamah A, Huppertz A, Lee JM, Ricke J, Sakamoto M, Sirlin CB, Ye SL, Zeng M. Consensus report from the 7th International Forum for Liver Magnetic Resonance Imaging. Eur Radiol 2016;26:674-82. [Crossref] [PubMed]
- Zech CJ, Ba-Ssalamah A, Berg T, Chandarana H, Chau GY, Grazioli L, Kim MJ, Lee JM, Merkle EM, Murakami T, Ricke J. B Sirlin C, Song B, Taouli B, Yoshimitsu K, Koh DM. Consensus report from the 8th International Forum for Liver Magnetic Resonance Imaging. Eur Radiol 2020;30:370-82. [Crossref] [PubMed]
- Koh DM, Ba-Ssalamah A, Brancatelli G, Fananapazir G, Fiel MI, Goshima S, Ju SH, Kartalis N, Kudo M, Lee JM, Murakami T, Seidensticker M, Sirlin CB, Tan CH, Wang J, Yoon JH, Zeng M, Zhou J, Taouli B. Consensus report from the 9(th) International Forum for Liver Magnetic Resonance Imaging: applications of gadoxetic acid-enhanced imaging. Eur Radiol 2021;31:5615-28. [Crossref] [PubMed]
- Jhaveri K, Cleary S, Audet P, Balaa F, Bhayana D, Burak K, Chang S, Dixon E, Haider M, Molinari M, Reinhold C, Sherman M. Consensus statements from a multidisciplinary expert panel on the utilization and application of a liver-specific MRI contrast agent (gadoxetic acid). AJR Am J Roentgenol 2015;204:498-509. [Crossref] [PubMed]
- Rao SX, Wang J, Wang J, Jiang XQ, Long LL, Li ZP, Li ZL, Shen W, Zhao XM, Hu DY, Zhang HM, Zhang L, Huan Y, Liang CH, Song B, Zeng MS. Chinese consensus on the clinical application of hepatobiliary magnetic resonance imaging contrast agent: Gadoxetic acid disodium. J Dig Dis 2019;20:54-61. [Crossref] [PubMed]
- LI-RADS® v2018 Core [Internet]. American College of Radiology; v2018 [cited 2022 April 2]. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/LI-RADS-CT-MRI-v2018
- Jeong HT, Kim MJ, Park MS, Choi JY, Choi JS, Kim KS, Choi GH, Shin SJ. Detection of liver metastases using gadoxetic-enhanced dynamic and 10- and 20-minute delayed phase MR imaging. J Magn Reson Imaging 2012;35:635-43. [Crossref] [PubMed]
- van Kessel CS, Veldhuis WB, van den Bosch MA, van Leeuwen MS. MR liver imaging with Gd-EOB-DTPA: a delay time of 10 minutes is sufficient for lesion characterisation. Eur Radiol 2012;22:2153-60. [Crossref] [PubMed]
- Sofue K, Tsurusaki M, Tokue H, Arai Y, Sugimura K. Gd-EOB-DTPA-enhanced 3.0 T MR imaging: quantitative and qualitative comparison of hepatocyte-phase images obtained 10 min and 20 min after injection for the detection of liver metastases from colorectal carcinoma. Eur Radiol 2011;21:2336-43. [Crossref] [PubMed]
- Caton MT Jr, Shinagare AB, Lee B, Tirumani SH. Optimization of timing of hepatocellular phase imaging after gadoxetate disodium injection for evaluation of patients with neuroendocrine tumor. Abdom Radiol (NY) 2020;45:2358-69. [Crossref] [PubMed]
- Liang M, Zhao J, Xie B, Li C, Yin X, Cheng L, Wang J, Zhang L. MR liver imaging with Gd-EOB-DTPA: The need for different delay times of the hepatobiliary phase in patients with different liver function. Eur J Radiol 2016;85:546-52. [Crossref] [PubMed]
- Wu JW, Yu YC, Qu XL, Zhang Y, Gao H. Optimization of hepatobiliary phase delay time of Gd-EOB-DTPA-enhanced magnetic resonance imaging for identification of hepatocellular carcinoma in patients with cirrhosis of different degrees of severity. World J Gastroenterol 2018;24:415-23. [Crossref] [PubMed]
- Esterson YB, Flusberg M, Oh S, Mazzariol F, Rozenblit AM, Chernyak V. Improved parenchymal liver enhancement with extended delay on Gd-EOB-DTPA-enhanced MRI in patients with parenchymal liver disease: associated clinical and imaging factors. Clin Radiol 2015;70:723-9. [Crossref] [PubMed]
- Lee D, Cho ES, Kim DJ, Kim JH, Yu JS, Chung JJ. Validation of 10-Minute Delayed Hepatocyte Phase Imaging with 30° Flip Angle in Gadoxetic Acid-Enhanced MRI for the Detection of Liver Metastasis. PLoS One 2015;10:e0139863. [Crossref] [PubMed]
- Cho ES, Yu JS, Park AY, Woo S, Kim JH, Chung JJ. Feasibility of 5-minute delayed transition phase imaging with 30° flip angle in gadoxetic acid-enhanced 3D gradient-echo MRI of liver, compared with 20-minute delayed hepatocyte phase MRI with standard 10° flip angle. AJR Am J Roentgenol 2015;204:69-75. [Crossref] [PubMed]
- Jeon I, Cho ES, Kim JH, Kim DJ, Yu JS, Chung JJ. Feasibility of 10-Minute Delayed Hepatocyte Phase Imaging Using a 30° Flip Angle in Gd-EOB-DTPA-Enhanced Liver MRI for the Detection of Hepatocellular Carcinoma in Patients with Chronic Hepatitis or Cirrhosis. PLoS One 2016;11:e0167701. [Crossref] [PubMed]
- Higaki A, Tamada T, Sone T, Kanki A, Sato T, Tanimoto D, Higashi H, Ito K. Potential clinical factors affecting hepatobiliary enhancement at Gd-EOB-DTPA-enhanced MR imaging. Magn Reson Imaging 2012;30:689-93. [Crossref] [PubMed]
- Motosugi U, Ichikawa T, Sou H, Sano K, Tominaga L, Kitamura T, Araki T. Liver parenchymal enhancement of hepatocyte-phase images in Gd-EOB-DTPA-enhanced MR imaging: which biological markers of the liver function affect the enhancement? J Magn Reson Imaging 2009;30:1042-6. [Crossref] [PubMed]
- Chernyak V, Kim J, Rozenblit AM, Mazzoriol F, Ricci Z. Hepatic enhancement during the hepatobiliary phase after gadoxetate disodium administration in patients with chronic liver disease: the role of laboratory factors. J Magn Reson Imaging 2011;34:301-9. [Crossref] [PubMed]
- Okada M, Murakami T, Kuwatsuru R, Nakamura Y, Isoda H, Goshima S, Hanaoka R, Haradome H, Shinagawa Y, Kitao A, Fujinaga Y, Marugami N, Yuki M, Ichikawa T, Higaki A, Hori M, Fujii S, Matsui O. Biochemical and Clinical Predictive Approach and Time Point Analysis of Hepatobiliary Phase Liver Enhancement on Gd-EOB-DTPA-enhanced MR Images: A Multicenter Study. Radiology 2016;281:474-83. [Crossref] [PubMed]
- Kobi M, Paroder V, Flusberg M, Rozenblit AM, Chernyak V. Limitations of GD-EOB-DTPA-enhanced MRI: can clinical parameters predict suboptimal hepatobiliary phase? Clin Radiol 2017;72:55-62. [Crossref] [PubMed]
- Takatsu Y, Nakamura M, Kobayashi S, Miyati T. Prediction of Sufficient Liver Enhancement on the Gadoxetate Disodium-enhanced Hepatobiliary Phase Imaging Using Transitional Phase Images and Albumin-bilirubin Grade. Magn Reson Med Sci 2021;20:152-9. [Crossref] [PubMed]
- Mori Y, Motosugi U, Shimizu T, Ichikawa S, Kromrey ML, Onishi H. Predicting Patients With Insufficient Liver Enhancement in the Hepatobiliary Phase Before the Injection of Gadoxetic Acid: A Practical Approach Using the Bayesian Method. J Magn Reson Imaging 2020;51:62-9. [Crossref] [PubMed]
- Takatsu Y, Kobayashi S, Miyati T, Shiozaki T. A novel method for evaluating enhancement using gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid in the hepatobiliary phase of magnetic resonance imaging. Clin Imaging 2016;40:1112-7. [Crossref] [PubMed]
- Takatsu Y, Nakamura M, Shiozaki T, Narukami S, Yoshimaru D, Miyati T, Kobayashi S. Assessment of the cut-off value of quantitative liver-portal vein contrast ratio in the hepatobiliary phase of liver MRI. Clin Radiol 2021;76:551.e17-24. [Crossref] [PubMed]
- Motosugi U, Ichikawa T, Tominaga L, Sou H, Sano K, Ichikawa S, Araki T. Delay before the hepatocyte phase of Gd-EOB-DTPA-enhanced MR imaging: is it possible to shorten the examination time? Eur Radiol 2009;19:2623-9. [Crossref] [PubMed]
- Takatsu Y, Nakamura M, Kobayashi S, Miyati T. Visual criterion for evaluating hepatobiliary phase image acquisition of gadolinium-ethoxybenzyl-diethylenetriaminepentaacetic acid-enhanced MRI. Clin Radiol 2018;73:760.e1-6. [Crossref] [PubMed]
- Bashir MR, Breault SR, Braun R, Do RK, Nelson RC, Reeder SB. Optimal timing and diagnostic adequacy of hepatocyte phase imaging with gadoxetate-enhanced liver MRI. Acad Radiol 2014;21:726-32. [Crossref] [PubMed]
- Cunha GM, Hasenstab KA, Higaki A, Wang K, Delgado T, Brunsing RL, Schlein A, Schwartzman A, Hsiao A, Sirlin CB, Fowler KJ. Convolutional neural network-automated hepatobiliary phase adequacy evaluation may optimize examination time. Eur J Radiol 2020;124:108837. [Crossref] [PubMed]
- Chan HP, Samala RK, Hadjiiski LM, Zhou C. Deep Learning in Medical Image Analysis. Adv Exp Med Biol 2020;1213:3-21. [Crossref] [PubMed]
- Sanaat A, Shiri I, Ferdowsi S, Arabi H, Zaidi H. Robust-Deep: A Method for Increasing Brain Imaging Datasets to Improve Deep Learning Models' Performance and Robustness. J Digit Imaging 2022;35:469-81. [Crossref] [PubMed]
- Katsube T, Okada M, Kumano S, Hori M, Imaoka I, Ishii K, Kudo M, Kitagaki H, Murakami T. Estimation of liver function using T1 mapping on Gd-EOB-DTPA-enhanced magnetic resonance imaging. Invest Radiol 2011;46:277-83. [Crossref] [PubMed]
- Bashir MR, Merkle EM. Improved liver lesion conspicuity by increasing the flip angle during hepatocyte phase MR imaging. Eur Radiol 2011;21:291-4. [Crossref] [PubMed]
- Cruite I, Schroeder M, Merkle EM, Sirlin CB. Gadoxetate disodium-enhanced MRI of the liver: part 2, protocol optimization and lesion appearance in the cirrhotic liver. AJR Am J Roentgenol 2010;195:29-41. [Crossref] [PubMed]
- Ringe KI, Husarik DB, Sirlin CB, Merkle EM. Gadoxetate disodium-enhanced MRI of the liver: part 1, protocol optimization and lesion appearance in the noncirrhotic liver. AJR Am J Roentgenol 2010;195:13-28. [Crossref] [PubMed]
- Kukuk GM, Schaefer SG, Fimmers R, Hadizadeh DR, Ezziddin S, Spengler U, Schild HH, Willinek WA. Hepatobiliary magnetic resonance imaging in patients with liver disease: correlation of liver enhancement with biochemical liver function tests. Eur Radiol 2014;24:2482-90. [Crossref] [PubMed]
- Mori H, Machimura H, Iwaya A, Baba M, Furuya K. Comparison of liver scintigraphy and the liver-spleen contrast in Gd-EOB-DTPA-enhanced MRI on liver function tests. Sci Rep 2021;11:22472. [Crossref] [PubMed]
- Duong PA, Pastel DA, Sadigh G, Ballard D, Sullivan JC, Bresnahan B, Buch K, Duszak R Jr. The Value of Imaging Part II: Value beyond Image Interpretation. Acad Radiol 2016;23:23-9. [Crossref] [PubMed]


