
A magnetic resonance enterography index for assessing small bowel Crohn’s disease activity
Introduction
Crohn’s disease is a chronic autoimmune disorder characterized by intestinal inflammation and cyclical remission and relapse (1,2). Intestinal inflammation caused by Crohn’s disease relates to the disease duration and causes bowel damage that can change its phenotype such as intestinal stricture, fistulous, tumor, etc. (3-5). Evaluating inflammation caused by Crohn’s disease is essential to optimizing therapeutic management and testing the effects of management strategies. The Crohn’s disease activity index (CDAI) and Harvey-Bradshaw Index are widely used symptom-based scoring systems (6). However, clinical symptoms and intestinal inflammation are inconsistent; therefore, these scoring systems cannot accurately reflect the severity of Crohn’s disease (7). Blood markers, white blood cell count, and C-reactive protein (CRP) are sensitive to inflammatory reactions; however, infectious diseases and other chronic inflammation can also lead to an increase in their values (8,9). Fecal markers, such as calprotectin and lactoferrin, are sensitive to mucosal inflammation, but their diagnostic specificity is poor (10). Infectious and tumor diseases also lead to increased fecal markers (11).
Endoscopic scoring systems for Crohn’s disease can be used as a reference standard for assessing disease activity. The advantage of these scoring systems is that the intestinal mucosa can be directly observed. Existing research has developed the Crohn’s disease endoscopic index of severity (CDEIS) to monitor Crohn’s disease inflammation (12,13). However, enteroscopy is an invasive method that cannot be used repeatedly. Compared to colonoscopy and computed tomography (CT), magnetic resonance enterography (MRE) is a noninvasive and radiation-free method that can be repeated safely (14-16). MRE can better classify the disease phenotype and behavior by providing information on the bowel wall and extra enteric soft tissue pathology (17). MRE can be used to observe Crohn’s disease inflammation. The technique can also be used to monitor the inflammation of all layers, including those outside the sinus tract, to determine whether full-thickness healing has been achieved (18,19), thereby reducing hospitalization, surgery, and steroid treatment (20,21). Furthermore, we can use MRE in children to reduce intestinal damage caused by gastroenteroscopy, decrease the radiation dose delivered by CT, and effectively detect Crohn’s disease activity to assess the most suitable treatment (22,23). Although surgical histopathology was used in some studies, the colonoscopy activity score was selected as the reference standard in this study because it enabled a simple calculation of Crohn’s disease activity (24).
This study aimed to derive an MRE index from these findings to assess the inflammation activity of the small bowel with reference to the Simple Endoscopic Score for Crohn’s disease (SES-CD). Since a bowel afflicted with Crohn’s disease is often thickened and significantly strengthened, we prospectively tested the hypothesis that an evaluation model related to the Crohn’s disease bowel thickness and the ratio of enhancement can be applied clinically to rapidly assess the inflammatory activity of the small intestine in Crohn’s disease. We present the following article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1007/rc).
Methods
Patients and study protocol
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This prospective study was approved by the Ethics Committee of Wuxi No. 2 People’s Hospital. All patients provided written informed consent before enrollment. No participants experienced any adverse events.
Patients who were selected fulfilled the following criteria: (I) adults (≥16 years old) who were known to have Crohn’s disease or were suspected of having Crohn’s disease and required cross-image diagnosis of the small intestine, (II) no history of bowel resection, (III) no emergency care required, (IV) no pregnancy or renal insufficiency, and (V) no contraindications to MRE. Patients diagnosed with bacterial ulcerative colitis, ischemic enteritis, or neoplasm were excluded. A total of 34 consecutive patients who were clinically suspected of having Crohn’s disease that was confirmed by ileocolonoscopy between April 2014 and August 2015 at Jingling Hospital of Nanjing agreed to participate. After a colonoscopy, patients without any treatment underwent an MRE examination within 1 week. Three patients who were not diagnosed with Crohn’s disease were excluded. Two patients who would not cooperate and had poor image quality were excluded. Figure 1 shows the flowchart of the patient selection. The study population demographic characteristics and severity indexes are shown in Table 1.
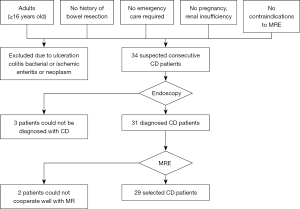
Table 1
| Characteristics | Value |
|---|---|
| No. of patients | 29 |
| Gender, n (%) | |
| Men | 21 (72.4) |
| Women | 8 (27.6) |
| Age at time of imaging (years), median [IQR] | 25 [19–35] |
| Days between ileocolonoscopy and MRE, median [IQR] | 3 [3–7] |
| The course of Crohn’s disease (years), median [IQR] | 1 [0.3–2] |
| <2 years, patients (%) | 21 (72.4) |
| 2–10 years, patients (%) | 7 (24.1) |
| >10 years, patients (%) | 1 (3.4) |
| Crohn’s disease activity index (CDEIS), median [IQR] | 101 [77–155] |
| >150, n (%) | 10 (34.5) |
| ≤150, n (%) | 19 (65.5) |
| Method of therapy, n (%) | |
| Mesalazine | 18 (62.1) |
| Sulfur | 2 (6.9) |
| Hormone | 1 (3.4) |
| Remicade | 6 (20.7) |
| Surgery | 2 (6.9) |
| Location of lesion, n (%) | |
| Upper gastrointestinal tract | 2 (6.9) |
| Small bowel | 23 (79.3) |
| Colon and/or rectum | 17 (58.6) |
| Anus | 6 (20.7) |
| Disease behavior, n (%) | |
| Nonstricturing and nonpenetrating | 14 (48.3) |
| Stricturing | 13 (44.8) |
| Penetrating | 2 (6.9) |
| Lesion in anorectum, n (%) | |
| No lesion | 16 (55.2) |
| Fistula | 9 (31.0) |
| Abscess | 2 (6.9) |
| Stricture | 1 (3.4) |
| Cutaneous tag | 1 (3.4) |
| C-reaction protein level (mg/L), median [IQR] | 9.9 [3.2–18.6] |
| Erythrocyte sedimentation rate (mm/min), median [IQR] | 14 [6–33] |
IQR, interquartile range; MRE, magnetic resonance enterography; CDEIS, Crohn’s disease Endoscopic Index of Severity.
MRE
Patients were instructed to drink 2,000 mL of 2.5% mannitol solution in 45 minutes in 500 mL aliquots of the solution at 45, 30, 15, and 0 minutes before the scanning. For each patient, 20 mg of spasmolytic medicine (raceanisodamine; Minsheng Pharmaceutical Group Co., Ltd., Hangzhou, China) was intravenously injected before the MRE examination. Supine images were scanned using a 3-T static magnet (MR750; GE Healthcare, Chicago, IL, USA) with a receiver composed of phased-array body coils. MRE was performed according to the parameters shown in Table 2. For dynamic T1-weighted magnetic resonance imaging (MRI), patients were given 15 mL of gadopentetate dimeglumine (Magnevist; Beijing Beilu Pharmaceutical Co., Ltd., Beijing, China) at a rate of 3 mL/s using a power injector (Spectris Solaris; Liebel-Flarsheim, Mansfield, MA, USA). Multiphase MRI (for 0, 7, 15, 22, 39, 46, and 54 seconds) was performed. After the noncontrast phase was scanned the agent was injected immediately to get the enhanced images.
Table 2
| Parameters | Coronal SSFSE | Coronal fs-SSFSE | Axial fs-T2-PROPELLER | Axial DWI b value =0, 800 | Coronal 7-phase-LAVA |
|---|---|---|---|---|---|
| Flip angle | – | – | 110 | – | 12 |
| Echo time (ms) | 68.0 | 66.2 | 75.0 | 47.3 | 1.7 |
| Field of view (mm2) | 40×36 | 40×36 | 40×40 | 42×33.6 | 44×35.2 |
| Image matrix | 288×288 | 288×288 | 320×320 | 96×120 | 42×35.7 |
| Bandwidth (kHz) | 83.3 | 83.3 | 83.3 | 250.0 | 166.7 |
| TR (s) | 1,839.0 | 1,800.0 | 6,667.0 | 35,714.0 | 3.8 |
| TE (s) | 68.0 | 67.0 | 75.0 | 47.3 | 1.7 |
| Slice thickness (mm) | 3 | 3 | 4 | 4 | 2 |
| Intersection gap (mm) | 1 | 1 | 1 | 1 | 1 |
| Breath-hold time per stack (s) | 11–13 | 11–13 | – | – | 22 |
| NEX | 0.53 | 0.53 | 2.50 | 1.00 | 0.71 |
MRE, magnetic resonance enterography; fs, fat suppression; SSFSE, single-shot fast spin-echo; PROPELLER, periodically rotated overlapping parallel lines with enhanced reconstruction; DWI, diffusion-weighted imaging; TR, repetition time; TE, echo time; LAVA, liver acquisition with volume acceleration; NEX, number of excitations.
Image interpretation
Two board-certified gastrointestinal radiologists with 6 and 20 years of experience, respectively, reviewed all MRE images independently. The readers and assessors were blinded to the clinical information and reference standard results. There was no disagreement between the 2 radiologists. To obtain reliable data, the T1 and T2 signal intensities were measured 3 times, and the average was taken to reduce occasional errors. We selected the terminal ileum less than 5 cm above the ileocecal valves with more than 3 mm mural thickness and a high T2 signal intensity. The adjacent normal intestine and vertical muscle of a uniform signal without artifacts were chosen from the same scan level from which the terminal ileum was measured.
The inflammation ileum adjacent to the normal intestine and the vertical muscle T2 signal intensity were measured using the periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER) sequence. We calculated the ratio of ileal inflammation on T2 signal intensity to the adjacent normal intestine on T2 signal intensity (T2 ratio) and the ratio of ileal inflammation on T2 signal intensity to vertical muscle on T2 signal intensity (T2 ratio 2).
For the majority of patients, the fifth phase of imaging for the inflamed intestine and the sixth phase of imaging for the normal intestine were most obviously enhanced. The maximal enhancement signal gap of the inflamed intestine and the adjacent normal intestine was in the fifth phase. Consequently, the study selected phase 5 to calculate the T1 ratio.
The inflamed intestine and the adjacent normal intestinal T1 signal intensity were measured on the fifth phase of a 3-dimensional liver acquisition with volume acceleration (LAVA) dynamic contrast enhancement. We measured the intestine with the most distinctly enhanced inflammation and the adjacent normal intestine without artifacts. The noncontrast phase T1 signal intensity was measured from the same scan level and location. From this, we calculated the ratios of T1 signal intensity from the fifth phase images for the inflamed intestine and from the noncontrast phase images of the same lesion (T1 ratio 3) as well as T1 signal intensity from the fifth phase images for the inflamed intestine and the enhanced adjacent normal intestine (T1 ratio 4).
The perimural T2 signal, ulceration, edema, “comb” sign, and lymph nodes with different number, size, and enhancement were treated as categorical variables, which were classified by 2 radiologists using a discrete data scoring system (Table 3; Figures 2-4). The T2 signal ratio of the inflamed intestine (T2 ratio 1–2), inflamed intestine enhancement (T1 ratio 3–4), and wall thickness were graded with 0–3 score (Table 3; Figures 2-4).
Table 3
| Score | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Mural thickness | 1–3 mm | >3–5 mm | >5–7 mm | >7 mm |
| T2 ratio 1 | 0–1.0 | >1.0–1.2 | >1.2–1.8 | >1.8 |
| T2 ratio 2 | 1.0–1.5 | >1.5–2.0 | >2.0–2.5 | >2.5 |
| T1 ratio 3 | 1.0–1.5 | >1.5–2.5 | >2.5–3.5 | >4.0 |
| T1 ratio 4 | 1.0–1.2 | >1.2–1.5 | >1.5–1.8 | >1.8 |
| Perineural T2 signal | Equivalent to normal mesentery | Increase in mesenteric signal but no fluid | Small fluid rim (≤2 mm) | Larger fluid rim (>2 mm) |
| Ulceration | Absent | Mucosal lesions or irregularities | Linear high-signal-intensity protrusions | Deep ulcerations and a cobblestone mucosa |
| Edema | Equivalent to a normal bowel wall | Minor increase in fat on the saturated images | Moderate increase in fat on the saturated images | Marked increase in fat on the saturated images |
| Comb sign | Absent | Mild enhancement | Moderate enhancement | Marked enhancement |
| Lymph nodes increasing in number | 0–5 | >5–10 | >10–25 | >25 |
| Number of enlarged lymph nodes (>1 cm) | 0 | 1–5 | >5 | – |
| Lymph node enhancement | Absent | Mild enhancementd | Moderate enhancemente | Marked enhancementf |
| Small bowel distortion | Absent | Only 1 section | 2 sections | 3 and above |
T2 ratio 1 = terminal ileal inflammation on T2 signal intensity/adjacent normal intestine on T2 signal intensity; T2 ratio 2 = terminal ileal inflammation on T2 signal intensity/vertical muscle on T2 signal intensity; T1 ratio 3 = fifth phase of the inflamed intestine on T1 signal/baseline (no contrast) on T1 signal; T1 ratio 4 = fifth phase of the inflamed intestine on T1 signal intensity/adjacent normal intestinal on T1 signal intensity. d, mild enhancement = enhancement was milder than the intestinal enhancement; e, moderate enhancement = enhancement was more severe than that of the intestinal but milder than that of the vessel; f, marked enhancement = enhancement equal to the vessel. MRE, magnetic resonance enterography.
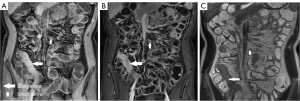
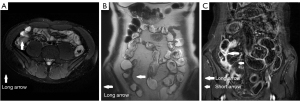
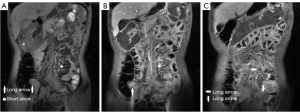
Reference standards (SES-CD)
Colonoscopy preparation
Patients were forbidden from eating vegetables or fruit and ate a semiliquid diet without residue for 3 days before the examination. Patients with glucopenia or poor physical condition were given infusion therapy. Patients were forbidden from eating on the day of the examination. Patients were instructed to drink 250 mL of 20% mannitol and 500 ml of 10% glucose solution within 20 minutes and drink 2,000–3,000 mL of water 6 hours before their endoscopy. After the preparation, patients were permitted to undertake appropriate activities to stimulate the intestine.
Endoscopy examination
The gastroenterologists were blinded to the MRE results and reference standard results. When the gastroenterologists disagreed, they reread the lesion photos to reach a concordant score. Patients underwent the enteroscopy while under the anesthesia of propofol or awake. The enteroscopy was inserted into the terminal ileum, and mucous was observed when the enteroscopy returned. The examination was taken nearly 2PM. For the peroral enteroscopy, patients were prohibited from eating after 8AM before the day of the examination and prohibited from drinking after midnight. If there was no luminal stricture, the endoscopy entered 3 cm below the pylorus with the assistance of the outer sleeve. For the colonoscopy, the preparation was completed as described above. If there was no luminal stenosis, the colonoscopy entered 3 m above the ileocecal valves with the assistance of the outer sleeve. The intestine was observed when enteroscopy returned to terminal ileum within 5 cm. We recorded the ulcer size, the involved intestinal area of the ulcer, the involved intestinal area and segment of the lesions, and luminal stenosis from the terminal ileum within 5 cm. The intestine was divided into 8 segments: the rectum, sigmoid and left colon, transverse colon, right colon, ileum, terminal ileum within 5 cm, ileum 5 cm above ileocecal valves, and jejunum (Figure 5).
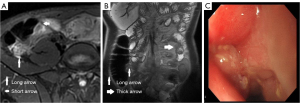
Simple endoscopic score in Crohn’s disease
Two board-certified gastrointestinal doctors classified the ulcer size (diameter >0.1 and <0.5, 0.5–2, or >2 cm), the proportion of the ulcerated surface (<10%, 10–30%, or >30%), the proportion of the surface area affected by any disease lesion (<50%, 50–75%, or >75%), and stenosis (single, multiple, and whether the colonoscopy passed through the narrowing). Each item was scored from 0 with no lesion to 3 with 2-cm ulcer, 30% ulcerated surface, 75% affected area or stenosis that could not be passed, and the scores were tallied (Figure 5; Table 4).
Table 4
| Variables | Score |
|---|---|
| Size of ulceration | |
| None | 0 |
| Aphthous ulcer (<7 mm) | 1 |
| Liner ulcer | 2 |
| Confluent, large ulcer | 3 |
| The surface area of ulceration | |
| None | 0 |
| <10% | 1 |
| 10–30% | 2 |
| >30% | 3 |
| Influence scope of the lesion | |
| None | 0 |
| <50% | 1 |
| 50–75% | 2 |
| >75% | 3 |
| Stricture of the lesion | |
| None | 0 |
| Single stricture that can be passed through | 1 |
| Multistrictures which can be passed through | 2 |
| Strictures that cannot be passed through | 3 |
SES-CD, simple endoscopic score for Crohn’s disease.
Statistical analysis
For the index construction, the Crohn’s disease activity parameters were first compared with the SES-CD score in pairs using the Pearson correlation coefficient. The different calculation of ratio on T1 and T2 which has better correlation with the SES-CD score was chosen to construct a magnetic resonance (MR) activity index with the other MR parameters using multiple linear regression and the least squares method. A variable needed a sample size of at least 10 to be included in the clinical prediction model. However, due to time constraints, the sample size obtained in this study was small, and only 29 patients were included. The distribution of characteristics for the patients and disease enrolled are described as sample size, frequency, percentage, and median or interquartile range (IQR) of the whole sample.
In this model, the dependent variable was the SES-CD score system from 0 to 12, which was the sum of the intestinal damage. The independent variables of the MR model were selected from the MRE activity parameters manually using backward selection and the likelihood ratio test.
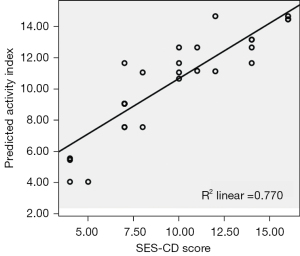
First, the quality of the MRE activity index was assessed as follows: first, the proportion of the variance of the dependent variable explained by the multiple linear regression model was estimated by adjusted R2 (adjusted R2>0.75); second, residuals (the difference between predicted SES-CD value and the rue SES-CD value) were tested for equal variances and normality through the scatterplot and normal probability plot; and third, there was no collinearity between MRE index variables. The correlation between dependent and independent variables was tested using a partial correlation scatterplot (Figure 6).
The ability of the derived multivariate analysis MR model to predict active disease was assessed by calculating the receiver operating characteristic (ROC) curves. Confidence intervals (95%) around the area under the curve (AUC) estimate were calculated using the DeLong variance estimate. In the interobserver variation study, the intra-class correlation coefficient (ICC) was used to assess the reproducibility level of the MRE index.
The influence of disease duration and CDAI on the MRE index distribution was tested using the Kruskal-Wallis method. The influence of disease CRP and the erythrocyte sedimentation rate (ESR) on the MRE index distribution was tested using a t-test. The statistical analyses were partially carried out with MATLAB 7 (MathWorks, Natick, MA, USA) and partially carried out with SPSS 20.0 (IBM Corp., Armonk, NY, USA).
Results
Constructing the MRE index
The SES-CD score of the whole sample ranged from 0 to 12, with a median score of 6. Correlations between multiple variables are shown in Table 5. Ulceration and the perineural T2 signal both had a significant correlation with the endoscopic activity score. Mural thickness, T1 ratio, T2 ratio, edema, and comb sign were moderately associated with the endoscopic activity score. Intestinal distortion, enlargement, and enhancement of lymph nodes were weakly associated with endoscopy. T2 ratio 1 and T1 ratio 4 had better associations with the SES-CD score than did T2 ratio 2 and T1 ratio 3. Therefore, we selected T2 ratio 1 and T1 ratio 4 to construct the MRE index. Ulceration, perimural T2 signal, edema, and comb sign had a significant correlation with the SES-CD score. Therefore, these parameters were selected to derive the MRE index. The multivariate analysis and backward selection eliminated 3 parameters and left an index of T1 ratio 4 (coefficient 1.5, 95% CI: 0.48–2.56; P=0.006) and ulceration (coefficient 2, 95% CI: 0.76–3.31; P=0.003; Table 6).
Table 5
| MRE index/SES-CD | SES-CD | Mural thickness | T1 ratio 3 | T1 ratio 4 | T2 ratio 1 | T2 ratio 2 | Perineural T2 signal | Ulceration | Edema | Comb sign |
|---|---|---|---|---|---|---|---|---|---|---|
| SES-CD | 1 | |||||||||
| Mural thickness | 0.57 | 1 | ||||||||
| T1 ratio 3 | 0.61 | 0.58 | 1 | |||||||
| T1 ratio 4 | 0.68 | 0.51 | 0.57 | 1 | ||||||
| T2 ratio 1 | 0.52 | 0.63 | 0.70 | 0.58 | 1 | |||||
| T2 ratio 2 | 0.44 | 0.66 | 0.65 | 0.47 | 0.83 | 1 | ||||
| Perineural T2 signal | 0.81 | 0.59 | 0.58 | 0.52 | 0.54 | 0.50 | 1 | |||
| Ulceration | 0.85 | 0.65 | 0.64 | 0.51 | 0.63 | 0.58 | 0.80 | 1 | ||
| Edema | 0.76 | 0.71 | 0.53 | 0.56 | 0.60 | 0.59 | 0.81 | 0.82 | 1 | |
| Comb sign | 0.74 | 0.68 | 0.65 | 0.70 | 0.68 | 0.70 | 0.82 | 0.71 | 0.80 | 1 |
| Enlargement and enhancement of lymph nodes | 0.29 | |||||||||
| Small bowel distortion | 0.25 |
MRE, magnetic resonance enterography; SES-CD, simple endoscopic score for Crohn’s disease.
Table 6
| MRE index | Coefficient | P value | Final coefficienta | |
|---|---|---|---|---|
| Estimate | SE | |||
| Intercept | 0.89 | 1.72 | 0.61 | 0.9 |
| Mural thickness | –0.14 | 0.53 | 0.80 | –0.1 |
| T1 ratio 4 | 1.52 | 0.50 | 0.006 | 1.5 |
| T2 ratio 1 | –0.86 | 0.66 | 0.21 | –0.9 |
| Perimural T2 signal | 0.94 | 0.64 | 0.16 | 0.9 |
| Ulceration | 2.04 | 0.61 | 0.003 | 2.0 |
| Edema | 0.02 | 0.60 | 0.98 | 0.0 |
| Comb sign | –0.14 | 0.72 | 0.85 | –0.1 |
a, final coefficients for the organ indexes were obtained by rounding the estimated coefficients. MRE, magnetic resonance enterography; SE, standard error.
The final MRE index that best predicted the Crohn’s disease activity was as follows: 1.52 × T1 ratio 4 score + 2.03 × ulceration score.
Multiple analyses of the MRE index
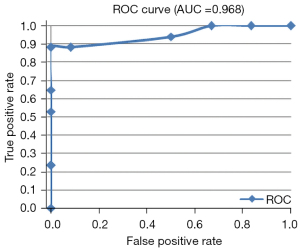
The mean MRE index (1.52 T1 ratio 4 score + 2.03 ulceration score) was 5.9 (range, 0–10.2). The percentage of the SES-CD score variance explained by the model was 77%. The scatterplot showed that the MRE index had a good fit with the SES-CD score (Figure 6). The optimum cutoff of the MRE index for the presence of acute endoscopic inflammation was 1. The ROC curves of the MRE index (1.52 T1 ratio 4 score + 2.03 ulceration score) to diagnose inflammation had a sensitivity of 0.962, a specificity of 0.667, and an AUC of 0.968 (95% CI: 0.921–1.000; Figure 7). The ICC for the interobserver agreement of the MRE index was 0.921 (95% CI: 0.833–0.963).
MRE index by disease duration and clinical activity
The median MRE index for disease duration of fewer than 2 years was 10.7 (IQR, 7.1–11.2), that between 2 and 10 years was 10.2 (IQR, 5.1–12.7), and that for >10 years was 14.2. The MRE index increased with the disease duration.
Clinical disease activity at enrollment according to CDAI had no significant influence on the distribution of the MRE index (Kruskal-Wallis test; P=0.24). When disease duration was taken into account, MRE index of patients with median values of CDAI ≤150 and CDAI >150 were 7.1 and 11.2, respectively for disease duration <2.0 years. MRE index of patients with median values of CDAI ≤150 and CDAI >150 were 8.6 and 10.5, respectively for disease duration between 2 and 10 years. MRE index of patients with median values of CDAI >150 was 14.2 for disease duration ≥10 years.
CRP and ESR had no significant influence on the distribution of the MRE index (t-test, P=0.544 and P=0.236). Median MRE index values of patients with CRP ≤8 mg/L and CRP >8 mg/L were 8.6 and 10.7, respectively. Median MRE index values of patients with ESR ≤20 mm/h and ESR >20 mm/h were 10.7 and 11.2, respectively.
Discussion
Historically, Crohn’s disease treatment regimens have been guided by clinical symptoms. However, recently, treatment regimens have focused on achieving mucosal healing of the inflamed bowel, which has been shown to have better long-term outcomes (25,26). Ileocolonoscopy is considered the gold standard for assessing Crohn’s disease activity (27). Endoscopic scoring systems, such as CDEIS, have been developed to classify disease activity and have been validated to enable a consistent and reproducible assessment of Crohn’s disease activity. The inherent complexity of the CDEIS led to the development of the SES-CD, which has a high level of agreement with CDEIS of 0.994 (95% CI: 0.976–1.000) (28). The SES-CD index is frequently used because of its qualified outcome. Therefore, his study selected SES-CD as a standard reference for the MRE index derivation.
Some studies have obtained and validated the Crohn’s disease magnetic resonance index of activity (MaRIA), and other studies have investigated the effectiveness of MRE in diagnosing patients with complex Crohn’s disease (29). However, these MRE characteristic parameters were measured by comparing them with the example images from a database of MRE studies, which may be subjective and possibly led to erroneous activity assessment. Our study measured MR parameters qualitatively and derived a more objective MRE index that can assess Crohn’s disease inflammation activity quickly. This derivation cohort also has a better diagnostic performance than other MR activity indexes, such as that described in the study by Steward et al. (30).
To assess the activity of the inflamed bowel in patients with Crohn’s disease, we assessed the MRE features. Measurements of ulceration, wall thickness, T2 signal intensity ratio compared to the adjacent normal bowel, T1 enhancement ratio compared to the adjacent normal bowel, perineural T2 signal, comb sign, and edema were combined to obtain a Crohn’s disease activity system. This system can improve the accuracy of the assessment of inflammatory activity in patients with Crohn’s disease.
Pearson correlation coefficients were used to analyze the correlation between MRE parameters and endoscopic activity scores. Ulcers were strongly correlated with perineural T2 signal, edema, and comb sign. The reason why these 4 parameters were significantly correlated with each other may be that the greater severity of the inflammation, congestion, edema, and inflammatory exudation of the intestinal wall resulted in changes in the MRE parameters. The results of this study showed that measurement of 4 factors, namely T1 contrast enhancement ratio, wall thickness, tooth comb sign, and T2 signal around the bowel, can better predict acute inflammation of the bowel wall. Multivariate analysis and reverse selection eliminated the other 3 parameters, leaving only the ulcer. Wall thickness, T1 ratio, and T2 ratio were moderately consistent with SES-CD. Hyperemia and edema led to the thickening of the inflammatory bowel wall and elevated T2 signal. Inflammation of the intestinal and perimesenteric vessels caused by hyperemia was greater, resulting in changes in the T1 ratio. Our study did not find that bowel deformation, lymphadenopathy, or enhancement correlated with SES-CD scores, which was the same as findings from other studies (31).
A multiple linear regression model was used to derive the MRE index. The least squares method leaves weighted independent variables with the smallest deviation from the dependent variable and eliminates insignificant independent variables. The partial correlation coefficients of the multiple linear regression models that removed the effects of the remaining independent variables reflected the most valuable variables, T1 ratio, and ulcers. The results were slightly different from the hypothesis, as the model was associated with ulcers and enhancement ratios rather than with thickness and enhancement ratios. Crohn’s disease is characterized by abnormal neovascularization in the intestinal wall, and both the density of microvessels in the wall and the chronicity of the disease have also been shown to be significantly associated with intestinal contrast absorption (32). In addition, acute intestinal inflammatory disease is characterized by superficial and deep ulcers. MR T2 sequences allowed us to visualize intestinal ulcers in transverse sections clearly and provided a way to detect small mucosal lesions or irregularities. The ulcer could be seen on MR images as a hyperintense lesion surrounded by a border of moderate signal intensity. Deep ulcers and cobblestone mucosal lesions are considered to indicate advanced intestinal inflammation and manifest as linear, high-intensity bowel walls or moderate muscular protrusions. SES-CD grades the ulcer size, ulcer surface proportion, and surface area proportion affected by lesions and stenosis, and it primarily evaluates intestinal ulcers and the changes involved. This may be why MR ulceration changes are most often associated with SES-CD (33). Although the perineural T2 signal change and comb sign had a significant Pearson correlation with SES-CD, they were excluded from the MR model. Other dependent variables affecting the association of these 2 variables with SES-CD scores might have contributed to this result.
The increased T2 signal and wall thickness were mainly caused by transmural inflammatory edema, which may be less pronounced than is the congestion in Crohn’s disease. In addition, T2 signal detection is easily interfered with by various factors, and it is difficult to measure the change of the T2 signal accurately.
The MRE index derived from the multiple linear regression model showed a good agreement with the SES-CD assessment, and the percentage of the SES-CD variance explained by the model was 77%. To obtain an unbiased estimate of the diagnostic value of the MRE index, the ROC curves were derived, which had a sensitivity of 0.962, a specificity of 0.667, and an AUC of 0.968 (95% CI: 0.921–1.000) with the optimum cutoff of 1 for the presence of acute endoscopic inflammation. Reproducibility assessment of Crohn’s disease activity is an important quality for the MRE index; therefore, it should be validated with an interobserver variation study. The ICC for the interobserver agreement of the MRE index was 0.921 (95% CI: 0.833–0.963), which indicated a good agreement. The MRE index increased with the disease duration, which is a similar finding to that reported in a study using the Lémann index (34). CRP, ESR, and CDAI had no significant influence on the distribution of the MRE index.
Our study has several limitations. First, due to time constraints, the sample size obtained in this study was small, and the diagnostic efficacy of the derived cohort may not be sufficiently accurate. Future studies and validation with large sample sizes will be carried out. Second, the T2 ratio is limited by a dynamic range because of the high T2 signal of the bowel content. In order to obtain the exact ratio, the ratio of the inflammatory bowel wall and normal intestinal wall with the best display on the same plane were selected and measured 3 times to obtain the average. With this method, the dynamic range was reduced. Third, the use of SES-CD as a reference for assessing inflammation intestine activity relies heavily on estimates of the surface area involved. The lack of correlation between symptom scores and serum inflammatory markers may need to be further confirmed. However, mucosal healing as defined by endoscopy may differ from mucosal healing as defined by histologic assessment, and the predictive validity of histologic mucosal healing on subsequent clinical outcomes remains to be determined.
Furthermore, we could not estimate global intestinal inflammation due to endoscopic limitations. MR activity scores did not consider the extra-luminal complications and surgery history. This group of patients was the most frequently encountered clinically. In the future, we can improve the measurement of endoscopic activity to study global MRE index assessments (35). Finally, we could not be sure that our endoscopic site exactly matched the site for which the imaging score was obtained. However, range errors were reduced by using the last 5 cm of the terminal ileum.
Distinguishing between inflammatory and fibrotic bowel strictures is critical for developing treatment plans for patients with Crohn’s disease. Fibrotic strictures require surgery or colonoscopy, and acute inflammation requires drug therapy. Special techniques for MR bowel imaging, such as diffusion-weighted imaging (DWI), dynamic contrast-enhanced MR perfusion (36), MR motion imaging (37), and feasibility of intestinal MR elastography (38,39), may provide superior diagnostic value. Future research may take these variables into account. Other types of detection, such as ultrasound, are also valuable for the diagnosis of Crohn’s disease (40).
In conclusion, a more objective MRE index for Crohn’s disease based on the T1 contrast ratio and ulceration was derived. This index could assess intestinal inflammation activity, measure the impact of different therapeutic strategies, and observe lesion healing associated with clinical remission.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-1007/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1007/coif). The authors report that they receive consulting fees from AMCA. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This prospective study was approved by the Ethics Committee of Wuxi No. 2 People’s Hospital. All patients provided written informed consent before enrollment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol 2010;105:289-97. [Crossref] [PubMed]
- Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011;140:1785-94. [Crossref] [PubMed]
- Burisch J, Kiudelis G, Kupcinskas L, Kievit HAL, Andersen KW, Andersen V, et al. Natural disease course of Crohn's disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study. Gut 2019;68:423-33. [Crossref] [PubMed]
- Fumery M, Singh S, Dulai PS, Gower-Rousseau C, Peyrin-Biroulet L, Sandborn WJ. Natural History of Adult Ulcerative Colitis in Population-based Cohorts: A Systematic Review. Clin Gastroenterol Hepatol 2018;16:343-356.e3. [Crossref] [PubMed]
- Torres J, Billioud V, Sachar DB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis as a progressive disease: the forgotten evidence. Inflamm Bowel Dis 2012;18:1356-63. [Crossref] [PubMed]
- Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology 1976;70:439-44. [Crossref] [PubMed]
- Sandborn WJ, Feagan BG, Hanauer SB, Lochs H, Löfberg R, Modigliani R, Present DH, Rutgeerts P, Schölmerich J, Stange EF, Sutherland LR. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology 2002;122:512-30. [Crossref] [PubMed]
- Voudoukis E, Karmiris K, Oustamanolakis P, Theodoropoulou A, Sfiridaki A, Paspatis GA, Koutroubakis IE. Association between thrombocytosis and iron deficiency anemia in inflammatory bowel disease. Eur J Gastroenterol Hepatol 2013;25:1212-6. [Crossref] [PubMed]
- Menees SB, Powell C, Kurlander J, Goel A, Chey WD. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am J Gastroenterol 2015;110:444-54. [Crossref] [PubMed]
- Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut 2006;55:426-31. [Crossref] [PubMed]
- Røseth AG, Schmidt PN, Fagerhol MK. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol 1999;34:50-4. [Crossref] [PubMed]
- Shergill AK, Lightdale JR, Bruining DH, Acosta RD, Chandrasekhara V, et al. The role of endoscopy in inflammatory bowel disease. Gastrointest Endosc 2015;81:1101-21.e1-13.
- Limdi JK, Picco M, Farraye FA. A review of endoscopic scoring systems and their importance in a treat-to-target approach in inflammatory bowel disease (with videos). Gastrointest Endosc 2020;91:733-45. [Crossref] [PubMed]
- Terheggen G, Lanyi B, Schanz S, Hoffmann RM, Böhm SK, Leifeld L, Pohl C, Kruis W. Safety, feasibility, and tolerability of ileocolonoscopy in inflammatory bowel disease. Endoscopy 2008;40:656-63. [Crossref] [PubMed]
- Butcher RO, Nixon E, Sapundzieski M, Filobbos R, Limdi JK. Radiation exposure in patients with inflammatory bowel disease--primum non nocere? Scand J Gastroenterol 2012;47:1192-9. [Crossref] [PubMed]
- Desmond AN, O'Regan K, Curran C, McWilliams S, Fitzgerald T, Maher MM, Shanahan F. Crohn's disease: factors associated with exposure to high levels of diagnostic radiation. Gut 2008;57:1524-9. [Crossref] [PubMed]
- Taylor SA, Avni F, Cronin CG, Hoeffel C, Kim SH, Laghi A, Napolitano M, Petit P, Rimola J, Tolan DJ, Torkzad MR, Zappa M, Bhatnagar G, Puylaert CAJ, Stoker J. The first joint ESGAR/ ESPR consensus statement on the technical performance of cross-sectional small bowel and colonic imaging. Eur Radiol 2017;27:2570-82. [Crossref] [PubMed]
- Horsthuis K, Bipat S, Bennink RJ, Stoker J. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology 2008;247:64-79. [Crossref] [PubMed]
- Panés J, Bouzas R, Chaparro M, García-Sánchez V, Gisbert JP, Martínez de Guereñu B, Mendoza JL, Paredes JM, Quiroga S, Ripollés T, Rimola J. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn's disease. Aliment Pharmacol Ther 2011;34:125-45. [Crossref] [PubMed]
- Messaris E, Chandolias N, Grand D, Pricolo V. Role of magnetic resonance enterography in the management of Crohn disease. Arch Surg 2010;145:471-5. [Crossref] [PubMed]
- Hafeez R, Punwani S, Boulos P, Bloom S, McCartney S, Halligan S, Taylor SA. Diagnostic and therapeutic impact of MR enterography in Crohn's disease. Clin Radiol 2011;66:1148-58. [Crossref] [PubMed]
- He L, Sun Y, Hu X, Yao Q. Diagnostic performance of magnetic resonance enterography and ultrasound in children with inflammatory bowel diseases: a diagnostic test accuracy meta-analysis. Eur Radiol 2022;32:1330-41. [Crossref] [PubMed]
- Herman RB, Dumnicka P, Fyderek K. A review of magnetic resonance enterography based Crohn's disease activity indices in paediatric patients. Prz Gastroenterol 2022;17:190-5. [Crossref] [PubMed]
- Servais L, Boschetti G, Meunier C, Gay C, Cotte E, François Y, Rozieres A, Fontaine J, Cuminal L, Chauvenet M, Charlois AL, Isaac S, Traverse-Glehen A, Roblin X, Flourié B, Valette PJ, Nancey S. Intestinal Conventional Ultrasonography, Contrast-Enhanced Ultrasonography and Magnetic Resonance Enterography in Assessment of Crohn's Disease Activity: A Comparison with Surgical Histopathology Analysis. Dig Dis Sci 2022;67:2492-502. [Crossref] [PubMed]
- Arnott ID, Watts D, Ghosh S. Review article: is clinical remission the optimum therapeutic goal in the treatment of Crohn's disease? Aliment Pharmacol Ther 2002;16:857-67. [Crossref] [PubMed]
- Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Van Assche G, Hoffman I, Van Steen K, Vermeire S, Rutgeerts P. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn's disease. Inflamm Bowel Dis 2009;15:1295-301. [Crossref] [PubMed]
- Swoger JM, Regueiro M. Evaluation for postoperative recurrence of Crohn disease. Gastroenterol Clin North Am 2012;41:303-14. [Crossref] [PubMed]
- Sipponen T, Nuutinen H, Turunen U, Färkkilä M. Endoscopic evaluation of Crohn's disease activity: comparison of the CDEIS and the SES-CD. Inflamm Bowel Dis 2010;16:2131-6. [Crossref] [PubMed]
- Sanli DET, Sanli AN, Kandemirli SG, Esmerer E, Kayadibi Y, Demiryas S, Korman MU. The mutually complementary role of magnetic resonance enterography and conventional enteroclysis in patients with complicated and/or advanced stage of Crohn's disease. Bratisl Lek Listy 2021;122:270-6. [PubMed]
- Steward MJ, Punwani S, Proctor I, Adjei-Gyamfi Y, Chatterjee F, Bloom S, Novelli M, Halligan S, Rodriguez-Justo M, Taylor SA. Non-perforating small bowel Crohn's disease assessed by MRI enterography: derivation and histopathological validation of an MR-based activity index. Eur J Radiol 2012;81:2080-8. [Crossref] [PubMed]
- Tielbeek JA, Makanyanga JC, Bipat S, Pendsé DA, Nio CY, Vos FM, Taylor SA, Stoker J. Grading Crohn disease activity with MRI: interobserver variability of MRI features, MRI scoring of severity, and correlation with Crohn disease endoscopic index of severity. AJR Am J Roentgenol 2013;201:1220-8. [Crossref] [PubMed]
- Taylor SA, Punwani S, Rodriguez-Justo M, Bainbridge A, Greenhalgh R, De Vita E, Forbes A, Cohen R, Windsor A, Obichere A, Hansmann A, Rajan J, Novelli M, Halligan S. Mural Crohn disease: correlation of dynamic contrast-enhanced MR imaging findings with angiogenesis and inflammation at histologic examination--pilot study. Radiology 2009;251:369-79. [Crossref] [PubMed]
- Daperno M, D'Haens G, Van Assche G, Baert F, Bulois P, Maunoury V, Sostegni R, Rocca R, Pera A, Gevers A, Mary JY, Colombel JF, Rutgeerts P. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc 2004;60:505-12. [Crossref] [PubMed]
- Pariente B, Mary JY, Danese S, Chowers Y, De Cruz P, D'Haens G, et al. Development of the Lémann index to assess digestive tract damage in patients with Crohn's disease. Gastroenterology 2015;148:52-63.e3. [Crossref] [PubMed]
- Minordi LM, Bevere A, Papa A, Larosa L, Manfredi R CT. Acad Radiol 2022;29:1206-27. [Crossref] [PubMed]
- Taylor SA, Punwani S, Rodriguez-Justo M, Bainbridge A, Greenhalgh R, De Vita E, Forbes A, Cohen R, Windsor A, Obichere A, Hansmann A, Rajan J, Novelli M, Halligan S. Mural Crohn disease: correlation of dynamic contrast-enhanced MR imaging findings with angiogenesis and inflammation at histologic examination--pilot study. Radiology 2009;251:369-79. [Crossref] [PubMed]
- Cullmann JL, Bickelhaupt S, Froehlich JM, Szucs-Farkas Z, Tutuian R, Patuto N, Dawson H, Patak MA. MR imaging in Crohn's disease: correlation of MR motility measurement with histopathology in the terminal ileum. Neurogastroenterol Motil 2013;25:749-e577. [Crossref] [PubMed]
- Reiter R, Loch FN, Kamphues C, Bayerl C, Marticorena Garcia SR, Siegmund B, Kühl AA, Hamm B, Braun J, Sack I, Asbach P. Feasibility of Intestinal MR Elastography in Inflammatory Bowel Disease. J Magn Reson Imaging 2022;55:815-22. [Crossref] [PubMed]
- Mazza S, Conforti FS, Forzenigo LV, Piazza N, Bertè R, Costantino A, Fraquelli M, Coletta M, Rimola J, Vecchi M, Caprioli F. Agreement between real-time elastography and delayed enhancement magnetic resonance enterography on quantifying bowel wall fibrosis in Crohn's disease. Dig Liver Dis 2022;54:69-75. [Crossref] [PubMed]
- Ma L, Wang M, Li W, Liu W, Yang H, Jiang Y, Zhu Q. Pilot case-control study to explore the value of intestinal ultrasound in the differentiation of two common diseases involving the ileocecal region: intestinal Behçet's disease and Crohn's disease. Quant Imaging Med Surg 2021;11:3200-8. [Crossref] [PubMed]


