
Primary malignant pericardial mesothelioma presenting as constrictive pericarditis with an atypical imaging appearance: a case description
Introduction
Primary malignant pericardial mesothelioma (PMPM) is a rare malignant tumor with a poor prognosis. Its atypical clinical symptoms and imaging characteristics lead to misdiagnoses and inconclusive diagnoses, and its final diagnosis mainly relies on pericardial histopathology, postoperative pathology, or even autopsy results (1-3). In this article, we report the case of a 50-year-old woman with PMPM that atypically presented as constrictive pericarditis to raise awareness of this easily neglected malignant pericardial tumor.
Case presentation
All the procedures in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and the accompanying images. A copy of the written consent form is available for review by the editorial office of this journal.
A 50-year-old woman with worsening chest distress and shortness of breath presented to the emergency department. Some 10 months before admission to our facility, clinicians at other hospitals had suspected that this patient had tuberculous pericarditis, as infectious lesions in the lung accompanied by recurrent pericardial effusion were observed, and an antituberculosis treatment was administered. However, the patient did not respond well to this treatment and gradually developed cardiac insufficiency.
The patient had no history of asbestos or radiation exposure or specific types of viral infections. At the time of her admission, the results of her physical examinations revealed a normal heart rate, blood pressure, cardiopulmonary auscultation, and moderate edema of the bilateral lower extremities. The laboratory examinations revealed increased N-terminal pro-brain natriuretic peptide (NT-proBNP; 2,266.6 pg/mL; normal range, 0–250 pg/mL), increased C-reactive protein (CRP; 14.3 mg/L; normal range, 0–8 mg/L), increased carbohydrate antigen (CA) 12-5 (113.14 U/mL; normal range, 0–35 U/mL), and decreased albumin (30.9 g/L; normal range, 40–55 g/L). The other hematologic and biochemical analyses were unremarkable. Electrocardiography showed sinus tachycardia, atrial premature beats, and flat T-waves in the limb leads and chest leads. The admitting diagnosis was cardiac insufficiency [New York Heart Association (NYHA) class I] and arrhythmia. Based on the patient’s medical history at the other hospitals combined with our findings of atrial arrhythmia and elevated CRP, it was not possible to directly diagnose tuberculous pericarditis, but the possibility of constrictive pericarditis was considered.
On admission, the patient underwent transthoracic echocardiography (TTE) as a preliminary screening test, which indicated pericardial thickening and adhesive and biatrial enlargement that presented as constrictive pericarditis, and recurrent pericardial effusion and pleural effusion thereafter (Figure 1). The patient underwent thoracentesis and pericardiocentesis multiple times to determine the nature of fluid, and 300–800 mL of pleural effusion and 100–120 mL of pericardial effusion were drained each time. The pericardial effusion was mostly a clear brick-red fluid but was sometimes light yellow according to the macroscopic appearance. The patient’s specific gravity was 1.022 to 1.026, Rivalta test had both positive and negative results, white blood cell count was 150 to 402 (×106/L), protein quantification was 2.34 to 2.74 g/dL, glucose was 5.91 to 6.44 mmol/L, and chlorine was 93.4 to 105.5 mmol/L, but no acid-fast bacilli or tumor cells were detected. The placement of a pericardial catheter was not considered because the patient presented with slight newly produced pericardial effusion at the time and did not have other indications. These results did not support a diagnosis of tuberculous pericarditis; however, a diagnosis of neoplastic pericarditis could not be excluded.
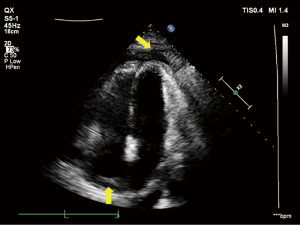
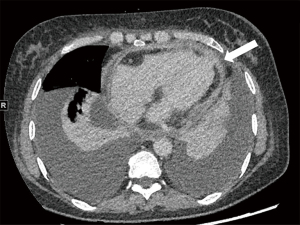
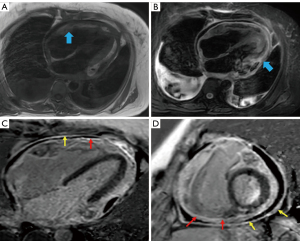
The patient underwent cardiac computed tomography (CT) and magnetic resonance imaging (MRI) to determine the progression of the pericardial disease further. Cardiac CT revealed visceral and parietal pericardial thickening enhancement and no calcification, slight pericardial effusion, and pleural effusion but no definite mass was found (Figure 2). Calcification is a finding highly suggestive of constrictive pericarditis; however, the absence of calcification does not rule out constriction. Based on our clinical experience, constrictive pericarditis should be considered as the primary diagnosis if pericardium thickening is >4 mm and if impaired diastolic filling and pleural effusion are observed; thus, an initial diagnosis of constrictive pericarditis was made. Cardiac MRI revealed thickening, adhesion, and delayed enhancement of the pericardium with an equal signal on T1-weighted imaging (T1WI) and a slightly high signal on T2-weighted imaging (T2WI), with limited biatrial enlargement and ventricle diastolic function. Thus, the MRI findings were consistent with a diagnosis of constrictive pericarditis (Figure 3).
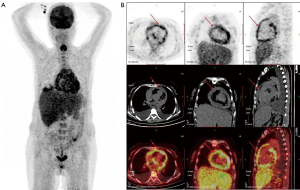
To clarify the metabolic activity of the entire pericardium, the patient also underwent positron emission tomography-CT (PET-CT). The results showed a thickening pericardium with a diffuse significant increased uptake of 18F-fluorodeoxyglucose (FDG) and a standard uptake value (SUV) of 4.3–6.7 (Figure 4). Thus, a malignancy, such as lymphoma, was considered, but its nature could not be determined.
As the patient experienced constant chest distress, shortness of breath, low blood pressure, and edema of the bilateral lower extremities during her hospitalization, she received repeated thoracentesis and routine anti-heart failure treatment, including diuretics, but her clinical symptoms did not improve. The patient had to undergo a pericardiectomy to clarify the diagnosis and relieve her symptoms. The cause of the disease was not identified until surgery. In the operation, the mediastinal tissue outside the parietal pericardium was observed to have severe edema. The visceral and parietal pericardium was thickened with unequal nodules and heavy congestion. The visceral pericardium and myocardial tissue were tightly connected without space. The histological examination revealed diffuse adenoid or epithelioid tumor cells in the wall of the pericardium. The tumor cells were of different sizes, round or oval, with vacuolar nuclei, obvious nucleoli, double nuclei, and rare pathological mitosis, and some of the cells had red-stained cytoplasm or vacuolar nuclei. The pathological diagnosis was epithelioid mesothelioma. The immunohistochemistry results were as follows: cytokeratin (CK)(+), CK7(+), calretinin(+), epithelial membrane antigen (EMA)(+), carcinoembryonic antigen (CEA)(–), thyroid transcription factor 1 (TTF-1)(–), chromogranin A (CgA)(–), and CD34(–) (Figure 5).
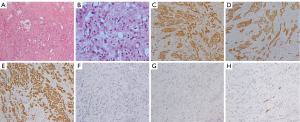
The patient received adjuvant cis-platinum-based chemotherapy postoperatively for 4 months and died of congestive heart failure. The period from the onset of the symptoms to death was 17 months.
Discussion
PMPM is a rare tumor. The largest autopsy study series reported that PMPM has an incidence rate of <0.0022%, and it accounts for only approximately 1% of malignant mesotheliomas (1,2,4). The pathogenesis of PMPM is not definite. A male predominance has been reported, with a male-to-female ratio of 2:1 to 3:1, especially in middle-aged people (5). Its systemic symptoms include fever, night sweats, cough, and weight loss, and patients frequently present with dyspnea, chest distress, or chest pain caused by pericardial constriction or cardiac tamponade (4,5). PMPM can be classified into the following 3 pathological types—epithelial, sarcomatoid, and biphasic—with epithelial being the most common.
Immunohistochemistry plays a fundamental role in the definitive diagnosis of PMPM. The commonly used positive markers are CK5/6, calretinin, and D2-40, while TTF-1, CEA, and CA19-9 are rarely expressed in mesothelioma (6). At present, there is still no effective treatment for this disease. Surgical treatment and postoperative adjuvant chemotherapy should be performed as soon as possible to prolong patients’ survival time (1,2). PMPM has a poor prognosis and a median survival time of 6 months (range, 2 months to 2 years), and heart failure or the involvement of visceral organs may lead to death (1,2,7).
The most common echocardiographic finding of PMPM is pericardial effusion (8,9). Hemorrhagic effusion has a positive likelihood ratio for malignancy; however, the reaccumulation of effusion alone is not a sign of a malignant etiology. As demonstrated in this case, a clear brick-red effusion may indicate a hemorrhage caused by the tumor involvement of the pericardium; thus, recurrent pericardial effusion and pleural effusion cannot be used to help to differentiate between PMPM and tuberculosis. In addition, the hemodynamic complications of cardiac tamponade and constrictive pericarditis in PMPM are quite common. According to previous reports, the presence of cardiac tamponade increases the likelihood of malignancy, but this case was not severe enough for the patient to have cardiac tamponade (10).
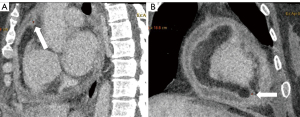
Cardiac CT is sensitive in evaluating the density of effusion and calcification and differentiating between malignancy and pericarditis by revealing solid nodules; it is also useful in assessing the involvement of pericardial layers, epicardial fat, and the myocardium. A definitive diagnosis was not made for our case, as no specific mass was observed on the cross-sectional images. However, the nodular thickening of the pericardium, local cardiac compression, and irregular linear soft tissue between the pericardium and myocardium should be noted (Figure 6A,6B), as these signs indicate the presence of a malignant tumor and the possibility of myocardial involvement and thus may be helpful in distinguishing between PMPM and inflammatory pericardial lesions (5,11). Pericardial calcification is less common in malignant tumors. Pericardial effusion and pleural effusion can present in both PMPM and inflammatory pericardial diseases; however, it is necessary to measure the CT attenuation of fluid, and the hemorrhagic density is highly suggestive of malignancy.
Cardiac MRI can be used to evaluate the abnormality of cardiac hemodynamics and ventricular wall motion caused by PMPM (7,8,11). A late gadolinium phase assessment can help to identify solid components, necrosis, and adjacent thrombi, and to distinguish a healthy myocardium from tumorous infiltration (8). In our case, cardiac MRI showed thickening, adhesion, delayed enhancement of the pericardium, and limited ventricle diastolic function, all of which are similar to the signs of constrictive pericarditis. More importantly, in addition to identifying the components of the tumor and the boundary between the tumor and the myocardium, diffuse PMPM has a high signal on T2WI, and isolated PMPM may interrupt the pericardial low signal.
We summarized the role of echocardiography, CT, and MRI in the examination of PMPM and the different imaging findings of PMPM (Table 1). Notably, the diffuse high uptake of inflammatory pericarditis on PET-CT made it difficult to determine the nature of lesions in this case. It can be difficult to distinguish between PMPM and non-neoplastic lesions using imaging features only if a diffuse thickening of the pericardium is observed (11). Thus, PMPM should only be considered after the exclusion of other lesions.
Table 1
| Approaches | Role of imaging examination | The main imaging findings of PMPM | The overlapping manifestations of PMPM and constrictive pericarditis |
|---|---|---|---|
| Echocardiography | Assessment for pericardial constriction and cardiac function | Cardiac tamponade | Early diastolic septal bounce |
| Inspiratory septal shift | |||
| High sensitivity in detecting pericardial effusion | Rapid reaccumulation of massive effusion | ||
| Limited ventricle diastolic function | |||
| CT | Identification of pericardial effusion density, evaluation of calcification | Hemorrhagic effusion | Pericardial effusion |
| Visualization of solid nodules | Enhanced nodules or pericardial masses | Pleural effusion | |
| Evaluation involvement of pericardial layers, epicardial fat, and myocardium | Local cardiac compression | Pericardial thickening and enhancement | |
| MRI | Determining the extent of the tumor location and evaluating cardiac hemodynamic changes | Hemorrhagic effusion | Pericardial-myocardial adherence |
| Assessment of definite solid components, necrosis, and adjacent thrombi in gadolinium phase | Enhanced nodules or pericardial masses | Late gadolinium enhancement | |
| Differentiating a healthy myocardium from tumorous infiltration | Discontinuous pericardial low signal in T2WI | Limited ventricle diastolic function |
PMPM, primary malignant pericardial mesothelioma; CT, computed tomography; MRI, magnetic resonance imaging; T2WI, T2-weighted imaging.
PMPM is often misdiagnosed because of its rarity. Indeed, previous studies have reported that it has a misdiagnosis rate of approximately 35%, and it is most commonly misdiagnosed as tuberculous pericarditis (6). Misdiagnoses and inconclusive diagnoses may be made for the following reasons: (I) there are no specific clinical symptoms and imaging findings for some diffuse lesions; (II) the symptoms are relieved by cardiac therapy, diuretics, or pericardiocentesis, which may mask the true conditions of the disease; and (III) a pericardial biopsy is relatively hard to achieve, and pericardial effusion cytology has a low positive rate <30% in the detection of malignant cells (2,3,6).
Our patient was suspected to have tuberculous pericarditis at the beginning of her visit, as she had atypical symptoms, such as chest distress and shortness of breath, and atypical imaging appearances, such as pleural effusion, pericardial effusion, and pericardium thickening. However, the diagnosis of tuberculous pericarditis was shortly overturned by other evidence, and we preferred the diagnosis of constrictive pericarditis; however, as no definite mass was found, the possibility of a tumor could not be ruled out and its nature could not be determined.
A patient’s tuberculosis history, as well as their pericardial effusion adenosine deaminase, lactate dehydrogenase, hyaluronic acid, CA125, and tuberculin test results are useful for differential diagnosis of PMPM. Noneffective antituberculosis therapy and the persistence or worsening of symptoms, such as severe cardiac insufficiency and effusions, should draw the attention of clinicians (6). In addition, flow cytometry is critical in basic immunology study and clinical diagnosis, and the application of flow cytometry could improve the sensitivity of diagnostic cytology.
Due to the feasibility of next-generation sequencing, the use of liquid biopsies in early cancer detection and treatment response monitoring have been extensively studied in recent years. Liquid biopsy samples [blood cell-free DNA (cfDNA)] and the targeted sequencing of mesothelioma cancer gene panels or pan-cancer gene panels could be used to assist clinicians to make diagnoses in the future. More importantly, some imaging features, such as a lack of calcification in the pericardium, nodular thickening of the pericardium, and myocardium involvement, can be used to differentiate between diffuse PMPM and inflammatory lesions. The density of the pericardial effusion, pericardial thickness, the degree of pericardial enhancement, and radioactive uptake of the pericardium are important imaging features that are easily ignored. It can also be difficult to definitely distinguish between neoplastic pericardial lesions and inflammatory pericardial lesions.
Conclusions
PMPM with an atypical imaging appearance is very rare; however, it should be noted that some of its imaging features differ to those of constrictive pericarditis. Combining clinical information with imaging can help provide more diagnostic information before surgery. Thus, the possibility of PMPM should be considered when diagnosing constrictive pericarditis of an unknown cause.
Acknowledgments
We are grateful to Dr. Xiaoxin Sun, Dr. Jinghui Li, and Dr. Siqi Liu for providing the images for this article.
Funding: This work was supported by the Ministry of Science and Technology of China, National Key Research and Development Project (No. 2016YFC1300400).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-556/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All the procedures in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and the accompanying images. A copy of the written consent form is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Istomin V, Blondheim DS, Meisel SR, Frimerman A, Lapidot M, Rachmilevitch R. Pericardial Effusion due to Primary Malignant Pericardial Mesothelioma: A Common Finding but an Uncommon Cause. Case Rep Med 2016;2016:4810901. [Crossref] [PubMed]
- Godar M, Liu J, Zhang P, Xia Y, Yuan Q. Primary pericardial mesothelioma: a rare entity. Case Rep Oncol Med 2013;2013:283601. [Crossref] [PubMed]
- Kurosawa T, Sugino K, Isobe K, Hata Y, Fukasawa Y, Homma S. Primary malignant pericardial mesothelioma with increased serum mesothelin diagnosed by surgical pericardial resection: A case report. Mol Clin Oncol 2016;5:553-6. [Crossref] [PubMed]
- Lingamfelter DC, Cavuoti D, Gruszecki AC. Fatal hemopericardial tamponade due to primary pericardial mesothelioma: a case report. Diagn Pathol 2009;4:44. [Crossref] [PubMed]
- Raeside MC, Gormly K, Neuhaus SJ, Kotasek D, James C. Primary pericardial mesothelioma presenting as multiple pericardial masses on CT. BJR Case Rep 2016;2:20150295. [Crossref] [PubMed]
- Cao S, Jin S, Cao J, Shen J, Zhang H, Meng Q, Pan B, Yu Y. Malignant pericardial mesothelioma: A systematic review of current practice. Herz 2018;43:61-8. [Crossref] [PubMed]
- Vavalle J, Bashore TM, Klem I. Surprising finding of a primary pericardial mesothelioma. Int J Cardiovasc Imaging 2010;26:625-7. [Crossref] [PubMed]
- Banišauskaitė A, Jankauskas A, Šarauskas V, Aržanauskaitė M. A case report of malignant primary pericardial mesothelioma with atypical imaging appearance: multimodality imaging with histopathological correlation. Eur Heart J Case Rep 2020;4:1-5. [Crossref] [PubMed]
- Sardar MR, Kuntz C, Patel T, Saeed W, Gnall E, Imaizumi S, Lande L. Primary pericardial mesothelioma unique case and literature review. Tex Heart Inst J 2012;39:261-4. [PubMed]
- Kong L, Li Z, Wang J, Lv X. Echocardiographic characteristics of primary malignant pericardial mesothelioma and outcomes analysis: a retrospective study. Cardiovasc Ultrasound 2018;16:7. [Crossref] [PubMed]
- Kobayashi Y, Murakami R, Ogura J, Yamamoto K, Ichikawa T, Nagasawa K, Hosone M, Kumazaki T. Primary pericardial mesothelioma: a case report. Eur Radiol 2001;11:2258-61. [Crossref] [PubMed]


