Diagnostic accuracy of contrast-enhanced dual-energy computed tomography for detecting metastatic lymph nodes in patients with malignant tumors: a systematic review and meta-analysis
Introduction
In most countries, cancer is the primary cause of mortality before the age of 70 years and a major impediment to extending life expectancy. According to figures from the International Agency for Research on Cancer (IARC), 19.3 million new cancer cases and approximately 10 million deaths were estimated to occur globally in 2020 (1). The main purpose of lymph node assessment is the staging of the cancer, which is important in the choice of treatment options. Lymph nodes serve as conduits for the spread of cancer to other organs in many different forms of cancer. Lymph node metastasis is an essential prognostic characteristic and one of the most important determinants impacting patient survival (2). Therefore, accurate detection of lymph node metastases is critical for cancer staging and treatment planning (3).
In recent years, dual-energy computed tomography (DECT) has been widely used for the prediction of various types of cancer and other diseases such as lung cancer (4), gastrointestinal tumors (5), breast cancer (6), biliary tract cancer (7), adherent perinephric fat (8), microthrombosis associated with COVID-19 pneumonia (9), pulmonary emboli (10), and lumbar disk herniation (11). DECT is a CT technique that uses two different X-ray energies, and it can accurately determine the composition of objects, thereby substantially increasing the capabilities of traditional CT single-energy scanning (12).
The difference between contrast-enhanced CT and traditional CT is that contrast-enhanced CT uses intravenous iodine contrast to assess whether there is blood perfusion. When the contrast is injected in the blood stream and shows perfusion, this is crucial since perfusion is a hallmark of cancer. Contrast-enhanced DECT can provide an iodine map to assess the iodine content of the tissue and indirectly reflect the blood supply to the tissue (13). In addition to traditional CT images, DECT can provide monochromatic images at 40–200 keV energy levels (14), iodine concentration (IC) mapping, effective atomic number (effc-z) images, and many other quantitative parameters, making it a significant milestone for CT diagnosis (4-11,15). DECT has shown promise in the diagnosis of preoperative metastatic lymph nodes in recent years (16), and DECT characteristics may help to distinguish metastatic from benign lymph nodes (17).
Different manufacturers often apply different DECT imaging techniques, which can be broadly divided into two categories: (I) one based on the detector side of the approach, such as detector-based DECT, which has 2 layers of detectors that detect low versus high energy photons and (II) another based on the tube sphere side of the approach, such as dual-source DECT, single-source DECT with rapid kilovoltage switching, and split-beam DECT (18). The differences of these technologies mainly stem from the product characteristics of different manufacturers and the different implementation forms of energy scanning (19). Each of these techniques has its own characteristics. To date, there is no consensus on which manufacturer and which technique provides the best diagnostic performance (20).
Furthermore, no systematic assessments or meta-analyses have been conducted on the usefulness of DECT in the detection of metastatic lymph nodes. Therefore, this review includes a large number of studies relevant to the topic. This allowed for an assessment of the sensitivity and specificity of DECT for lymph node metastasis. The following article is presented in accordance with the PRISMA-DTA reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-527/rc).
Methods
The PRISMA-DTA standards were used to conduct this systematic review and meta-analysis, which follows current best practices (21). Prior to the start of the review, the systematic review was prospectively registered and submitted to the PROSPERO database (CRD42022303023). This meta-analysis was not subject to ethical approval.
Search strategy
A comprehensive search of the PubMed, Embase, and Cochrane Library databases was performed for literature published in the English language from the inception of each database to September 30, 2022. Preliminary keywords and medical subject headings (MeSH) terms, including lymph nodes, metastatic lymph nodes, spectral CT, dual energy, and CT, were combined to generate a list of studies. The search strategy is shown in Appendix 1.
Selection of studies
After eliminating duplicates, the abstract and title of the remaining articles were independently screened by two investigators according to the inclusion and exclusion criteria. When a study was deemed eligible, the full text was obtained, and further screening was performed. Once agreement between the two investigators (ZKX and ZC) was reached, the final list of studies underwent full-text analysis and data extraction. When there was disagreement between raters, a consensus was reached through discussion or consultation with a third investigator (KDR).
Full-text articles were thoroughly selected according to the following inclusion criteria: (I) inclusion of patients with malignant neoplasms; (II) the application of DECT in the detection of metastatic lymph nodes; (III) surgical removal of metastatic lymph nodes for pathological confirmation; and (IV) the assessment of the diagnostic accuracy of lymphatic metastasis with DECT, with the data permitting the construction of a 2×2 table for calculating the diagnostic accuracy of DECT, including true positive (TP), false positive (FP), false negative (FN), and true negative (TN) results. Publications were excluded if they met any of the following criteria: (I) a lack of blood supply for the primary tumor; (II) inability to obtain the full text and extract data or the diagnostic indicators for individual parameters; (III) lack of reporting for the outcome of tumor recurrence; (IV) non-English language literature; and (V) reviews, retrospective studies, conference abstracts, case report/case series, and meta-analyses.
Data extraction
Two investigators (KDR and CXY) independently extracted data from the identified studies. When the data information in the original articles was unclear or the two investigators had different opinions, differences were resolved through discussion.
The following characteristics were extracted: study characteristics, including first author, year of publication, prospective versus retrospective study design, total number of patients, mean age, number and percentage of males, type of disease, total number of lymph nodes, and number of metastatic lymph nodes; and DECT characteristics, including machine brand, DECT type, tube voltage, tube current, slice thickness (mm), collimation (mm), rotation time (s), and contrast time (s). Finally, the true-positive, false-positive, true-negative, and false-negative rates (%), as well as sensitivity and specificity of identifying metastatic lymph nodes were reported. If they were not explicitly stated in the original research, data on TPs, FPs, TNs, and FNs were estimated based on the number, sensitivity, and specificity of lymph nodes described in the literature.
Assessment of methodological quality
Two investigators (GPH and ZHW) independently assessed the methodological quality of the included studies using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool (22), and differences were addressed by consensus. If no consensus was achieved between the 2 raters, a third rater (KDR) was consulted.
Statistical analysis and data synthesis
If the parameters included in this analysis had different degrees of heterogeneity, different cutoff values could have led to different sensitivities and specificities of diagnostic tests, and a threshold effect would be generated. Therefore, we first tested whether there was a threshold effect for the diagnostic test. To determine whether there was a threshold effect, Spearman correlation coefficient and summary receiver operating characteristic (SROC) curves were used. If there was a positive correlation between sensitivity and specificity (P<0.05) or the scatter points in the SROC curve showed a “shoulder-arm” distribution, heterogeneity caused by a threshold effect was considered. Conversely, if there was no positive correlation between sensitivity and specificity (P>0.05) or the scatter points in the SROC curve showed a “non-shoulder arm” distribution, there was considered to be was no threshold effect leading to heterogeneity. If there was a threshold effect, the best method to merge data was to fit the SROC curve and to calculate the area under the ROC curve, or not to merge the data. If there was no threshold effect, the effect values were combined. The effect model depended on whether there was heterogeneity between the studies.
The I2 test [I2=100% × (Q-df)/Q] was used to assess heterogeneity of the studies that were included in the meta-analysis. Each parameter was statistically assessed using Stata 15.0 (StataCorp LLC, College Station, TX, USA) and Meta-Disc 14.0 (https://meta-disc.software.informer.com/1.4/). The effect size included sensitivity, specificity, positive likelihood ratio, negative likelihood ratio (NLR), diagnostic advantage ratio, and area under the SROC curve (AUC). If I2≤50% or P>0.05 showed that there was no substantial heterogeneity, the fixed effects model was employed to combine the effect indicators. If I2>50% or P<0.05, a random effects model was used to combine effect indicators, and sensitivity and heterogeneity tests were performed. A forest plot was also generated to show the results of the data synthesis.
To perform sensitivity analyses, Stata software was used. The software examined the effect of a single study on the pooled effect size by observing whether the results changed significantly after removal of a particular study. Finally, Deeks test was employed to examine publication bias, with P<0.05 indicating the presence of publication bias.
Results
Literature search
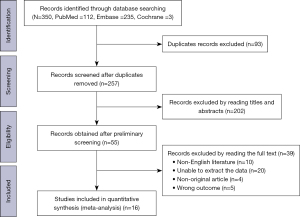
A total of 350 relevant papers were obtained by searching the databases, and after deduplication, 257 original studies were identified. After a reading of the titles and abstracts of these 257 original studies, 202 papers were excluded. Then, full texts were read in detail, and a rescreening was performed based on the inclusion and exclusion criteria, resulting in a final sample of 16 eligible studies for inclusion in this meta-analysis (16,23-37). The flowchart outlining the screening process is shown in Figure 1. The specific reasons for exclusion at the full-text screening stage are described in the Appendix 2.
Study characteristics
This meta-analysis included 16 original investigations, totaling 2,577 lymph nodes from 984 individuals. The papers included were published between 2015 and 2022, with 11 studies having been published in the last 5 years (16,23-32). The mean age of patients in the 16 included studies varied from 34.0±7.8 to 59.5±8.7 years, while the percentage of males ranged from around 0.0% to 70.9%. Moreover, 7 of the 16 studies included patients with thyroid cancer (16,23,24,29,30,33,36), 4 studies included patients with colorectal cancer (25,28,35,37), and the remaining 5 studies included patients with esophageal cancer, liver cancer, breast cancer, squamous cell carcinoma of the oropharynx, or lung cancer (26,27,31,32,34). Table 1 details the patient characteristics of the included studies. Table 2 details the DECT characteristics of the listed studies. Regarding manufacturer type, 6 studies used Siemens equipment, 9 nine studies used General Electric Company equipment. The majority of studies used dual-energy X-rays at energies of 80 to 140 kV. Of the studies included, 4 used single-energy DECT while 9 used dual-energy DECT, and the technology employed in the remaining 3 studies was not specified.
Table 1
| Reference | Year | Study type | Number of patients | Age (years) | Sex (% male) | Cancer type | Total number of LNs | Total number of MLNs |
|---|---|---|---|---|---|---|---|---|
| Zou et al. 2021 | 2021 | Retrospective study | 52 | 43.00±15.22 | 11 (21.2) | Papillary thyroid carcinoma | 359 | 139 |
| Zhuo et al. 2021 | 2021 | Retrospective study | 74 | N | 40 (54.1) | Papillary thyroid carcinoma | 216 | 92 |
| Wu et al. 2021 | 2021 | Controlled study | 35 | 39.79±13.58 | 6 (17.1) | Papillary thyroid carcinoma | 206 | 80 |
| Qiu et al. 2021 | 2021 | Prospective study | 71 | 59.3±14.1 | 41 (57.7) | Colorectal cancer | 150 | 84 |
| Sun et al. 2020 | 2020 | Retrospective study | 26 | N | N | Esophageal cancer | 51 | 34 |
| Zeng et al. 2019 | 2019 | Retrospective study | 43 | N | N | Hepatocellular carcinoma | 156 | 52 |
| Yang et al. 2019 | 2019 | Prospective study | 178 | 55.59±12.87 | 119 (66.9) | Colorectal cancer | 178 | 72 |
| Li et al. 2019 | 2019 | Retrospective study | 30 | 41.6±14.8 | 13 (43.3) | Papillary thyroid carcinoma | 99 | 70 |
| He et al. 2019 | 2019 | Prospective study | 51 | N | 16 (31.4) | Papillary thyroid carcinoma | 212 | 124 |
| Zhang et al. 2018 | 2018 | Prospective study | 193 | 47.6±10.1 | 0 (0.0) | Breast cancer | 337 | 76 |
| Foust et al. 2018 | 2018 | Retrospective study | 8 | N | N | Squamous cell carcinoma of the oropharynx | 29 | 13 |
| Zhao et al.2017 | 2017 | Retrospective study | 34 | 42.24±14.65 | 16 (47.1) | Papillary carcinoma and medullary carcinoma | 136 | 102 |
| Li et al. 2016 | 2016 | Retrospective study | 61 | 59.5±8.7 | 37 (60.7) | Lung cancer | 40 | 20 |
| Liu et al. 2015 | 2015 | Prospective study | 45 | 34.0±7.8 | 11 (24.4) | Papillary thyroid carcinoma | 175 | 63 |
| Liu et al. 2015 | 2015 | Prospective study | 55 | N | 39 (70.9) | Colorectal cancer | 152 | 60 |
| Kato et al. 2015 | 2015 | Retrospective study | 28 | N | N | Colorectal cancer | 81 | 35 |
The data of age are expressed as mean ± standard deviation. N, not reported; LN, lymph node; MLN, metastatic lymph node.
Table 2
| Reference | DECT brand | DECT type | kV1 | kV2 | Tube current | Slice thickness (mm) | Collimation | Rotation time (s) | Contrast | Dose | Flow rate | Arterial phase (s) | Venous phase (s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zou et al. 2021 | Siemens | Dual source | N | N | 600 mA | 1 | 64 mm ×0.6 mm | N | Iohexol | 1 mL/kg | 3 mL/s | 25 | 45 |
| Zhuo et al. 2021 | Siemens | Dual source | 90 | 150 | 250 mA, 125 mA | 0.75 | 2 mm ×192 mm ×0.6 mm | 0.5 | Iopromide | N | 4 mL/s | N | 50 |
| Wu et al. 2021 | GE | Single source fast switching kV | 80 | 140 | 260 mA | 1.25 | N | N | Iohexol 350 | 1.2 mL/kg | 3.1 mL/s | N | 50 |
| Qiu et al. 2021 | Canon | Single source fast switching kV | 80 | 135 | 112–187 mA | 0.5 | N | N | Ultravist 300 | 1.0 mL/kg | 3 mL/s | 40 | 70 |
| Sun et al. 2020 | Siemens | N | 90 | 150 | N | 1 | N | 0.25 | Iohexol | N | 23 mgI/kg/s | N | N |
| Zeng et al. 2019 | GE | Single source fast switching kV | 80 | 140 | 600 mA | 1.25 | N | 0.8 | Ioversol 320 | 1.0 mL/kg | 3.0 mL/s | 25 | 65 |
| Yang et al. 2019 | GE | Single source fast switching kV | 80 | 140 | 375 mA | 1.25 | N | N | Iohexol | 1.5 mL/kg | 4ml/s | N | N |
| Li et al. 2019 | GE | Single source fast switching kV | 80 | 140 | 260 mA | 5 | N | 0.7 | N | N | 3.0 mL/s | N | 45 |
| He et al. 2019 | Siemens | Dual source | 80 | 150 | 130 mA, 65 mA | 1.5 | 128 mm ×0.6 mm | 1 | Ioversol 370 | 85 mL | 3.0 mL/s | N | 30 |
| Zhang et al. 2018 | GE | N | N | N | 275 mA | 1.25 | N | N | Iohexol | 1.5 mL/kg | 4 mL/s | N | N |
| Foust et al. 2018 | Siemens | Dual source | 80 | 140 | 302 mA, 157 mA | 0.75 | 0.6 mm | 0.28 | N | 50 mL | 3 mL/s | N | 35 |
| Zhao et al.2017 | GE | Single source fast switching kV | 80 | 140 | 260 mA | 5 | N | 0.7 | N | 90 mL | 3.0 mL/s | N | 45 |
| Li et al. 2016 | GE | Single source fast switching kV | 80 | 140 | N | 1.25 | N | N | Iohexol | 1.2 mL/kg | 2.5mL/s | N | N |
| Liu et al. 2015 | GE | Single source fast switching kV | N | N | 550 mA | 1.25 | 0.625 mm | N | Iopamidol 300 | N | 4 mL/s | 25 | 50 |
| Liu et al. 2015 | GE | Single source fast switching kV | 80 | 140 | 600 mA | 1.25 | N | 0.6 | Ultravist | 1.5 mL/kg | 3 mL/s | N | N |
| Kato et al. 2015 | Siemens | N | N | N | N | N | N | N | N | N | N | 44 | 70 |
CT, computed tomography; DECT, dual-energy computed tomography; GE, GE Healthcare; kV, kilovoltage; N, not reported.
Methodological quality
We used the QUADAS-2 tool to evaluate the quality of primary diagnostic accuracy studies, and Figure 2 summarizes the overall risk of bias and applicability concerns for this research.

For the diagnostic experiments to be evaluated (whether or not predetermined thresholds were used in the original studies), we judged the risk of bias for diagnostic experiments to be evaluated (QUADAS-2, domain 2) to be high in all studies. We assessed the risk of bias by determining whether there was a time interval between the diagnostic experiment and the gold standard (QUADAS-2, domain 4). In 11 of the 16 studies included, the time interval between the DECT examination and the pathological gold standard was not clearly stated, and, therefore, the risk of bias was considered to be unclear for these 11 articles. The evaluation of clinical applicability included the selection of cases, the implementation and interpretation of the experiments, and the evaluation of the applicability of the gold standard.
Results of data synthesis
Value of each parameter of DECT in the diagnosis of metastatic lymph nodes
Due to the small amount of studies on some parameters, the diagnostic performance of only 15 indicators was analyzed. The data on the TPs, FPs, TNs, FNs, sensitivities, and specificities of each parameter, as well as main characteristics, are shown in Appendix 3. Each parameter included in the analysis had varying degrees of heterogeneity (available online: https://cdn.amegroups.cn/static/public/qims-22-527-1.pdf). The effect sizes of the diagnostic sensitivity, specificity, positive likelihood ratio, NLR, diagnostic ratio, and area under the SROC curve (AUC) are summarized for each parameter of DECT in Table 3.
Table 3
| Parameter | Sensitivity | Specificity | PLR | NLR | DOR | AUC | Spearman correlation | P value |
|---|---|---|---|---|---|---|---|---|
| IC in AP | 0.77 (0.70–0.83) | 0.78 (0.70–0.84) | 3.42 (2.32–5.04) | 0.3 (0.22–0.43) | 12.01 (5.73–25.16) | 0.84 | –0.429 | 0.397 |
| NIC in AP | 0.78 (0.69–0.86) | 0.79 (0.66–0.88) | 3.49 (2.19–5.57) | 0.29 (0.19–0.46) | 13.74 (5.48–34.45) | 0.85 | –0.042 | 0.907 |
| Slope in AP | 0.74 (0.65–0.82) | 0.85 (0.72–0.93) | 4.12 (2.50–6.77) | 0.32 (0.22–0.47) | 13.82 (6.46–25.96) | 0.85 | −1.017 | 0.819 |
| IC in VP | 0.80 (0.73–0.86) | 0.84 (0.79–0.88) | 4.58 (3.52–5.98) | 0.23 (0.15–0.36) | 21.89 (11.90–40.28) | 0.86 | −0.429 | 0.289 |
| NIC in VP | 0.83 (0.74–0.89) | 0.78 (0.74–0.82) | 3.54 (2.93–4.26) | 0.24 (0.18–0.34) | 15.85 (10.21–24.63) | 0.85 | 0.098 | 0.762 |
| Slope in VP | 0.75 (0.66–0.83) | 0.87 (0.79–0.92) | 5.26 (3.59–7.72) | 0.29 (0.22–0.39) | 20.75 (11.63–37.04) | 0.88 | 0.345 | 0.308 |
| IC in AP + NIC in AP | 0.95 (0.78–0.99) | 0.66 (0.44–0.82) | 2.59 (1.76–3.81) | 0.11 (0.02–0.51) | 21.00 (11.69–37.70) | 0.88 | 0.7 | 0.188 |
| NIC in AP + slope in AP | 0.94 (0.86–0.98) | 0.74 (0.52–0.88) | 3.52 (1.99–6.24) | 0.10 (0.04–0.27) | 38.86 (9.00–167.66) | 0.94 | −0.371 | 0.468 |
| NIC in AP + NIC in VP | 0.95 (0.91–0.98) | 0.60 (0.49–0.70) | 2.4 (1.84–3.13) | 0.09 (0.05–0.18) | 29.54 (12.78–68.27) | 0.9 | 0 | 1 |
| NIC in AP + slope in VP | 0.93 (0.88–0.97) | 0.73 (0.57–0.84) | 3.39 (2.22–5.18) | 0.10 (0.05–0.21) | 37.02 (13.05–105.02) | 0.93 | −0.086 | 0.872 |
| Slope in AP + NIC in VP | 0.95 (0.89–0.97) | 0.66 (0.56–0.75) | 2.75 (2.05–3.68) | 0.09 (0.04–0.20) | 31.89 (13.69–74.24) | 0.88 | 0.029 | 0.957 |
| Slope in AP + slope in VP | 0.92 (0.88–0.95) | 0.74 (0.61–0.83) | 3.25 (2.40–4.41) | 0.12 (0.07–0.19) | 32.00 (15.04–68.09) | 0.93 | 0.036 | 0.939 |
| IC in VP + NIC in VP | 0.97 (0.92–0.99) | 0.69 (0.62–0.75) | 2.97 (2.42–3.65) | 0.05 (0.02–0.15) | 53.29 (20.16–140.83) | 0.82 | −0.072 | 0.878 |
| Slope in VP + slope in VP | 0.96 (0.89–0.99) | 0.68 (0.61–0.74) | 2.85 (2.17–3.75) | 0.07 (0.03–0.20) | 41.03 (14.83–113.54) | 0.77 | −0.486 | 0.329 |
| NIC in VP + slope in VP | 0.95 (0.90–0.97) | 0.68 (0.62–0.74) | 2.94 (2.48–3.48) | 0.10 (0.06–0.17) | 31.23 (18.39–53.04) | 0.85 | 0.433 | 0.244 |
PLR, positive likelihood ratio; NLR, negative likelihood ratio; DOR, diagnostic odds ratio; AUC, area under curve; IC, iodine concentration; AP, arterial phase; NIC, normalized iodine concentration; VP, venous phase.
As can be seen from the summary in Table 3, of the 15 parameters, 4 had relatively large AUC values of 90% or more. These were normalized iodine concentration (NIC) in the arterial phase combined with the slope in the arterial phase, NIC in the arterial phase combined with NIC in the venous phase, NIC in the arterial phase combined with the slope in the venous phase, and the slope in the arterial phase combined with the slope in the venous phase, and their corresponding AUC values were 0.94, 0.90, 0.93, and 0.93, respectively. The sensitivity and specificity of NIC in the arterial phase combined with the slope in the arterial phase were 94% (95% CI: 86–98%) and 74% (95% CI: 52–88%) (Figure 3), respectively, with a large amount of heterogeneity in sensitivity (I2=88.96%) and specificity (I2=97.36%). The combined positive likelihood ratio (PLR) was 3.39 (95% CI: 2.22–5.18) (Figure 4A), the combined NLR was 0.10 (95% CI: 0.04–0.27) (Figure 4B), and the combined diagnostic odds ratio (DOR) was 38.86 (95% CI: 9.00–167.66) (Figure 4C). Among all the parameters, the AUC of NIC in the arterial phase combined with the slope in the arterial phase was the largest, with a value of 0.94 (Figure 4D).


Threshold effect
The results are shown in Table 3; the SROC curve lacked a shoulder-arm structure, suggesting that no threshold effect existed. After excluding the effect of threshold effects, we also considered the heterogeneity caused by non-threshold effects.
Since there was no threshold effect in this study, the presence of heterogeneity was deemed not to be related to the threshold effect. Therefore, this study used a random effects model to combine effect sizes and analyze the sources of heterogeneity.
Sensitivity analysis
The above effect size synthesis and data analysis found that the NIC in the arterial phase combined with slope in the arterial phase had the highest AUC. We focused on the sensitivity analysis of this combination. As shown in Figure 5, regardless of which study was excluded, the final combined result was not significantly affected, indicating that each study had little influence on the general results. As such, the results of this study were stable. We also examined sensitivity analysis results for other parameter combinations, which are shown in https://cdn.amegroups.cn/static/public/qims-22-527-2.pdf, and these results were all stable.

Investigation of heterogeneity
Sources of clinical variability were explored by meta-regression. Based on clinical practice, we then formally evaluated the effects of the following variables on sensitivity and specificity: DECT manufacturers (Siemens vs. General Electric Company), blood supply (abundant vs. insufficient), contrast flow rate (>3 vs. ≤3 mL/s), and study design (prospective vs. retrospective). The results of the meta-regression for each parameter are shown in Table 4. For the NIC in the arterial phase combined with the slope in the arterial phase, meta-regression analysis showed a significant effect of experimental design on the heterogeneity of sensitivity.
Table 4
| Parameter | Diagnostic indicators | DECT brand | Blood supply | Contrast flow rate | Study design |
|---|---|---|---|---|---|
| IC in AP | Sensitivity | − | − | + | − |
| Specificity | − | − | − | − | |
| NIC in AP | Sensitivity | − | − | − | + |
| Specificity | − | − | − | − | |
| Slope in AP | Sensitivity | − | − | − | + |
| Specificity | − | − | − | − | |
| IC in VP | Sensitivity | − | + | − | − |
| Specificity | + | + | + | − | |
| NIC in VP | Sensitivity | − | − | − | + |
| Specificity | − | − | − | − | |
| Slope in VP | Sensitivity | − | − | − | + |
| Specificity | − | − | − | − | |
| IC in AP + NIC in AP | Sensitivity | + | − | − | − |
| Specificity | − | − | − | − | |
| NIC in AP + slope in AP | Sensitivity | − | − | − | + |
| Specificity | − | − | − | − | |
| NIC in AP + NIC in VP | Sensitivity | − | + | − | + |
| Specificity | − | − | − | − | |
| NIC in AP + slope in VP | Sensitivity | − | − | − | + |
| Specificity | − | − | − | − | |
| Slope in AP + NIC in VP | Sensitivity | − | − | − | + |
| Specificity | − | − | − | − | |
| Slope in AP + slope in VP | Sensitivity | + | − | − | + |
| Specificity | − | − | − | − | |
| IC in VP + NIC in VP | Sensitivity | − | − | − | − |
| Specificity | + | + | + | − | |
| Slope in VP + slope in VP | Sensitivity | − | − | + | − |
| Specificity | − | − | − | − | |
| NIC in VP + slope in VP | Sensitivity | − | − | − | + |
| Specificity | − | − | − | + |
“+”: significant effect on the heterogeneity; “−”: nonsignificant effect on the heterogeneity. IC, iodine concentration; AP, arterial phase; NIC, normalized iodine concentration; VP, venous phase; DECT, dual-energy computed tomography.
Publication bias
The Deeks test with Stata software was used to examine publication bias; P>0.05 indicated that there was no substantial publication bias in the included papers. Figure 6 shows the publication bias test for NIC in the arterial phase combined with the slope in the arterial phase. The publication bias tests for the remaining parameters are shown in https://cdn.amegroups.cn/static/public/qims-22-527-3.pdf.

Discussion
Metastatic lymph nodes are the key to predicting the prognosis of cancer, and early detection of metastatic lymph nodes can help with the staging and treatment of cancer (38). DECT provides more quantitative parameters than does traditional CT, while also providing quantitative indicators, especially IC (39). This study focused on the influence of various parameters provided by an iodine map and the slope of the energy spectrum curve in the diagnosis of metastatic lymph nodes. IC and NIC can reflect the difference in iodine content between benign and malignant lymph nodes, and, thus, indirectly reflect the blood supply. Additionally, NIC avoids the effect of individual differences, and previous studies have found higher NIC values in metastatic lymph nodes than in benign lymph nodes. This is probably because tumor cells release a large number of regulatory factors before metastasis occurs, thus, stimulating the proliferation of blood vessels and lymphatic vessels in the lymph nodes which results in a widening of the microvasculature and an increase in blood flow (40). Both metastatic and no-metastatic lymph nodes follow a descending spectrum curve patterns, but the curve pattern of the metastatic nodes is much steeper, suggesting that the slope of the spectral Hounsfeld unit curve [λHU = (CT value (40 keV) − CT value (100 keV)]/(100−40). “CT value (40 keV)” and “CT value (100 keV)” are the CT attenuation measurements at 40 and 100 keV, respectively] could reflect the different statuses of lymph nodes (metastatic or non-metastatic) (37).
A total of 16 publications were included in this study comprising 2,577 lymph nodes, with disease types including thyroid cancer, lung cancer, colorectal cancer, oropharyngeal squamous cell carcinoma, breast cancer, esophageal cancer, and liver cancer,. Of the 15 indicators included in the analysis, 4 of the combined ones had a good diagnostic effect with an AUC greater than or equal to 0.90 (NIC in the arterial phase combined with slope in the arterial phase, NIC in the arterial phase combined with NIC in the venous phase, NIC in the arterial phase combined with slope in the venous phase, and slope in the arterial phase combined with slope in the venous phase). The combination of the two parameters of NIC in the arterial phase and the slope in the arterial phase not only increases the sensitivity but also has a high specificity. For this combination, the diagnostic energy efficiency analysis included 6 studies involving 1,274 lymph nodes. The sensitivity was 94%, the specificity was 74%, the AUC value of the included studies was 0.94, and the Q* value was 0.93, suggesting that the NIC in the arterial phase combined with the slope in the arterial phase has good diagnostic value for the diagnosis of metastatic lymph nodes. Moreover, to a certain extent, it avoids the missed diagnosis of metastatic lymph nodes.
Diagnostic studies are usually more heterogeneous than are other types of studies because of differences in case selection, gold standard settings, and experimental procedures. Based on sensitivity analysis and clinical practice, we then formally evaluated the effect of the following variables on sensitivity and specificity: DECT brand (Siemens vs. General Electric Company); blood supply (abundant vs. insufficient), contrast flow rate (>3 vs. ≤3 mL/s), and study design (prospective vs. retrospective). For the NIC in the arterial phase combined with the slope in the arterial phase, meta-regression analysis showed a significant effect of the experimental design on the heterogeneity of sensitivity (P<0.00). These 4 variables also affected the sensitivity and specificity of the remaining partial parameters. In addition, the heterogeneity in this study may also be due to the different sizes of lymph nodes, the different blood supplies to lymph nodes during different periods, the different types of cancer, the different settings of machine parameters, and the different types and doses of contrast agents. In the studies by Li et al. (16), Zhao et al. (33) and Kato et al. (37), the contrast agents used were not described in the text, and the patients included in the study by Zhang et al. (31) were all female, which might have contributed to the large heterogeneity observed in these studies.
This meta-analysis had some limitations. While a very extensive literature search was conducted for this study, variables such as different types of cancer, lymph node sizes, machine characteristics, and DECT technologies might have affected diagnostic accuracy. However, this review did not evaluate the combined role of these variables.
Conclusions
With the development and refinement of various imaging techniques, DECT has great clinical significance and application prospects in detecting metastatic and benign lymph nodes. To make the best use of DECT in this respect, it is helpful to combine the two parameters of NIC in the arterial phase and slope in the arterial phase. To further investigate and validate the reliability of the results of this analysis, we need to design prospective cohort studies of high quality, with large sample sizes, homogeneous study populations, homogeneous control populations, and homogeneous detection methods and combine morphological features to model the best combination of parameters to provide clinical guidance for the differential diagnosis of benign and metastatic lymph nodes. In addition, DECT parameters with the best diagnostic performance for each tumor type and the best DECT techniques should also be sought to be able to provide individual guidance for the differentiation of benign and malignant lymph nodes in each patient with cancer, and this should be the focus of further clinical research.
Acknowledgments
Funding: This study received financial support from the Science and Technology Department of Jilin Province (No. 20220203151SF).
Footnote
Reporting Checklist: The authors have completed the PRISMA-DTA reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-527/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-527/coif). All authors reported that this work was supported by the Science and Technology Department of Jilin Province (No. 20220203151SF). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Gillot L, Baudin L, Rouaud L, Kridelka F, Noël A. The pre-metastatic niche in lymph nodes: formation and characteristics. Cell Mol Life Sci 2021;78:5987-6002. [Crossref] [PubMed]
- Nguyen AT, Luu M, Lu DJ, Hamid O, Mallen-St Clair J, Faries MB, Gharavi NM, Ho AS, Zumsteg ZS. Quantitative metastatic lymph node burden and survival in Merkel cell carcinoma. J Am Acad Dermatol 2021;84:312-20. [Crossref] [PubMed]
- Kato H, Kanematsu M, Kato Z, Teramoto T, Mizuta K, Aoki M, Makita H, Kato K. Necrotic cervical nodes: usefulness of diffusion-weighted MR imaging in the differentiation of suppurative lymphadenitis from malignancy. Eur J Radiol 2013;82:e28-35. [Crossref] [PubMed]
- Li J, Fang M, Wang R, Dong D, Tian J, Liang P, Liu J, Gao J. Diagnostic accuracy of dual-energy CT-based nomograms to predict lymph node metastasis in gastric cancer. Eur Radiol 2018;28:5241-9. [Crossref] [PubMed]
- Okada K, Matsuda M, Tsuda T, Kido T, Murata A, Nishiyama H, Nishiyama K, Yamasawa H, Kamei Y, Kurata M, Fukushima M, Kitazawa R, Mochizuki T. Dual-energy computed tomography for evaluation of breast cancer: value of virtual monoenergetic images reconstructed with a noise-reduced monoenergetic reconstruction algorithm. Jpn J Radiol 2020;38:154-64. [Crossref] [PubMed]
- Ji GW, Zhang YD, Zhang H, Zhu FP, Wang K, Xia YX, Zhang YD, Jiang WJ, Li XC, Wang XH. Biliary Tract Cancer at CT: A Radiomics-based Model to Predict Lymph Node Metastasis and Survival Outcomes. Radiology 2019;290:90-8. [Crossref] [PubMed]
- Xie T, Li Y, He G, Zhang Z, Shi Q, Cheng G. The influence of liver fat deposition on the quantification of the liver-iron fraction using fast-kilovolt-peak switching dual-energy CT imaging and material decomposition technique: an in vitro experimental study. Quant Imaging Med Surg 2019;9:654-61. [Crossref] [PubMed]
- Grillet F, Busse-Coté A, Calame P, Behr J, Delabrousse E, Aubry S. COVID-19 pneumonia: microvascular disease revealed on pulmonary dual-energy computed tomography angiography. Quant Imaging Med Surg 2020;10:1852-62. [Crossref] [PubMed]
- Weidman EK, Plodkowski AJ, Halpenny DF, Hayes SA, Perez-Johnston R, Zheng J, Moskowitz C, Ginsberg MS, Dual-Energy CT. Angiography for Detection of Pulmonary Emboli: Incremental Benefit of Iodine Maps. Radiology 2018;289:546-53. [Crossref] [PubMed]
- Booz C, Nöske J, Martin SS, Albrecht MH, Yel I, Lenga L, Gruber-Rouh T, Eichler K, D'Angelo T, Vogl TJ, Wichmann JL. Virtual Noncalcium Dual-Energy CT: Detection of Lumbar Disk Herniation in Comparison with Standard Gray-scale CT. Radiology 2019;290:446-55. [Crossref] [PubMed]
- Zhang W, Zhao S, Pan H, Zhao Y, Zhao X. An iterative reconstruction method based on monochromatic images for dual energy CT. Med Phys 2021;48:6437-52. [Crossref] [PubMed]
- Hoover KB, Starks AO, Robila V, Riddle DL. Quantitative contrast enhanced dual energy CT to predict avascular necrosis: a feasibility study of proximal humerus fractures. BMC Med Imaging 2021;21:191. [Crossref] [PubMed]
- Albrecht MH, Vogl TJ, Martin SS, Nance JW, Duguay TM, Wichmann JL, De Cecco CN, Varga-Szemes A, van Assen M, Tesche C, Schoepf UJ. Review of Clinical Applications for Virtual Monoenergetic Dual-Energy CT. Radiology 2019;293:260-71. [Crossref] [PubMed]
- Chen S, Zhang J, Quan X, Xie Y, Deng X, Zhang Y, Shi S, Liang Z. Diagnostic accuracy of dual-energy computed tomography to differentiate intracerebral hemorrhage from contrast extravasation after endovascular thrombectomy for acute ischemic stroke: systematic review and meta-analysis. Eur Radiol 2022;32:432-41. [Crossref] [PubMed]
- Li L, Cheng SN, Zhao YF, Wang XY, Luo DH, Wang Y. Diagnostic accuracy of single-source dual-energy computed tomography and ultrasonography for detection of lateral cervical lymph node metastases of papillary thyroid carcinoma. J Thorac Dis 2019;11:5032-41. [Crossref] [PubMed]
- Cao Y, Zhang J, Bao H, Zhang G, Yan X, Wang Z, Ren J, Chai Y, Zhao Z, Zhou J. Development of a Nomogram Combining Clinical Risk Factors and Dual-Energy Spectral CT Parameters for the Preoperative Prediction of Lymph Node Metastasis in Patients With Colorectal Cancer. Front Oncol 2021;11:689176. [Crossref] [PubMed]
- McCollough CH, Boedeker K, Cody D, Duan X, Flohr T, Halliburton SS, Hsieh J, Layman RR, Pelc NJ. Principles and applications of multienergy CT: Report of AAPM Task Group 291. Med Phys 2020;47:e881-912. [Crossref] [PubMed]
- Otrakji A, Digumarthy SR, Lo Gullo R, Flores EJ, Shepard JA, Kalra MK, Dual-Energy CT. Spectrum of Thoracic Abnormalities. Radiographics 2016;36:38-52. [Crossref] [PubMed]
- Singh R, Sharma A, McDermott S, Homayounieh F, Rastogi S, Flores EJ, Shepard JAO, Gilman MD, Digumarthy SR. Comparison of image quality and radiation doses between rapid kV-switching and dual-source DECT techniques in the chest. Eur J Radiol 2019;119:108639. [Crossref] [PubMed]
- McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018;319:388-96. [Crossref] [PubMed]
- Wade R, Corbett M, Eastwood A. Quality assessment of comparative diagnostic accuracy studies: our experience using a modified version of the QUADAS-2 tool. Res Synth Methods 2013;4:280-6. [Crossref] [PubMed]
- Zhuo S, Sun J, Chang J, Liu L, Li S. Dual-source dual-energy thin-section CT combined with small field of view technique for small lymph node in thyroid cancer: a retrospective diagnostic study. Gland Surg 2021;10:1347-58. [Crossref] [PubMed]
- Zou Y, Zheng M, Qi Z, Guo Y, Ji X, Huang L, Gong Y, Lu X, Ma G, Xia S. Dual-energy computed tomography could reliably differentiate metastatic from non-metastatic lymph nodes of less than 0.5 cm in patients with papillary thyroid carcinoma. Quant Imaging Med Surg 2021;11:1354-67. [Crossref] [PubMed]
- Yang Z, Zhang X, Fang M, Li G, Duan X, Mao J, Shen J. Preoperative Diagnosis of Regional Lymph Node Metastasis of Colorectal Cancer With Quantitative Parameters From Dual-Energy CT. AJR Am J Roentgenol 2019;213:W17-25. [Crossref] [PubMed]
- Sun X, Niwa T, Ozawa S, Endo J, Hashimoto J. Detecting lymph node metastasis of esophageal cancer on dual-energy computed tomography. Acta Radiol 2022;63:3-10. [Crossref] [PubMed]
- Zeng YR, Yang QH, Liu QY, Min J, Li HG, Liu ZF, Li JX. Dual energy computed tomography for detection of metastatic lymph nodes in patients with hepatocellular carcinoma. World J Gastroenterol 2019;25:1986-96. [Crossref] [PubMed]
- Qiu L, Hu J, Weng Z, Liu S, Jiang G, Cai X. A prospective study of dual-energy computed tomography for differentiating metastatic and non-metastatic lymph nodes of colorectal cancer. Quant Imaging Med Surg 2021;11:3448-59. [Crossref] [PubMed]
- Wu YY, Wei C, Wang CB, Li NY, Zhang P, Dong JN. Preoperative Prediction of Cervical Nodal Metastasis in Papillary Thyroid Carcinoma: Value of Quantitative Dual-Energy CT Parameters and Qualitative Morphologic Features. AJR Am J Roentgenol 2021;216:1335-43. [Crossref] [PubMed]
- He M, Lin C, Yin L, Lin Y, Zhang S, Ma M. Value of Dual-Energy Computed Tomography for Diagnosing Cervical Lymph Node Metastasis in Patients With Papillary Thyroid Cancer. J Comput Assist Tomogr 2019;43:970-5. [Crossref] [PubMed]
- Zhang X, Zheng C, Yang Z, Cheng Z, Deng H, Chen M, Duan X, Mao J, Shen J. Axillary Sentinel Lymph Nodes in Breast Cancer: Quantitative Evaluation at Dual-Energy CT. Radiology 2018;289:337-46. [Crossref] [PubMed]
- Foust AM, Ali RM, Nguyen XV, Agrawal A, Prevedello LM, Bourekas EC, Boulter DJ. Dual-Energy CT-Derived Iodine Content and Spectral Attenuation Analysis of Metastatic Versus Nonmetastatic Lymph Nodes in Squamous Cell Carcinoma of the Oropharynx. Tomography 2018;4:66-71. [Crossref] [PubMed]
- Zhao Y, Li X, Li L, Wang X, Lin M, Zhao X, Luo D, Li J. Preliminary study on the diagnostic value of single-source dual-energy CT in diagnosing cervical lymph node metastasis of thyroid carcinoma. J Thorac Dis 2017;9:4758-66. [Crossref] [PubMed]
- Li X, Meng X, Ye Z. Iodine quantification to characterize primary lesions, metastatic and non-metastatic lymph nodes in lung cancers by dual energy computed tomography: An initial experience. Eur J Radiol 2016;85:1219-23. [Crossref] [PubMed]
- Liu H, Yan F, Pan Z, Lin X, Luo X, Shi C, Chen X, Wang B, Zhang H. Evaluation of dual energy spectral CT in differentiating metastatic from non-metastatic lymph nodes in rectal cancer: Initial experience. Eur J Radiol 2015;84:228-34. [Crossref] [PubMed]
- Liu X, Ouyang D, Li H, Zhang R, Lv Y, Yang A, Xie C. Papillary thyroid cancer: dual-energy spectral CT quantitative parameters for preoperative diagnosis of metastasis to the cervical lymph nodes. Radiology 2015;275:167-76. [Crossref] [PubMed]
- Kato T, Uehara K, Ishigaki S, Nihashi T, Arimoto A, Nakamura H, Kamiya T, Oshiro T, Ebata T, Nagino M. Clinical significance of dual-energy CT-derived iodine quantification in the diagnosis of metastatic LN in colorectal cancer. Eur J Surg Oncol 2015;41:1464-70. [Crossref] [PubMed]
- Choi Y, Bin-Manie M, Roh JL, Cho KJ, Lee YS, Choi SH, Nam SY, Kim SY. Metastatic lymph node burden predictive of survival in patients undergoing primary surgery for laryngeal and hypopharyngeal cancer. J Cancer Res Clin Oncol 2019;145:2565-72. [Crossref] [PubMed]
- Wu H, Dong S, Li X, Shi L, Shao D, Zhang Q, Chen M, Cao Y, Thant M, Huang X. Clinical utility of dual-energy CT used as an add-on to 18F FDG PET/CT in the preoperative staging of resectable NSCLC with suspected single osteolytic metastases. Lung Cancer 2020;140:80-6. [Crossref] [PubMed]
- Lee SY, Chao-Nan Q, Seng OA, Peiyi C, Bernice WH, Swe MS, Chii WJ, Jacqueline HS, Chee SK. Changes in specialized blood vessels in lymph nodes and their role in cancer metastasis. J Transl Med 2012;10:206. [Crossref] [PubMed]