
Learning curve in ultrasound-guided vacuum-assisted excision of breast lesions for surgeons and ultrasound physicians
Introduction
Ultrasound-guided vacuum-assisted excision (VAE) of breast lesions has been an acceptable alternative to open resection of benign breast lesions over the years (1-4). Moreover, an 8-gauge or 11-gauge VAE needle retrieves a larger volume of specimen, thus allowing a more accurate diagnosis of breast lesions, including atypical ductal hyperplasia or ductal carcinoma in situ, compared to a typical 14-gauge core needle biopsy (5-7). Given the important role that VAE plays in the diagnosis and resection of breast masses, acquisition of this skill requires appropriate training, as well as the accumulation of time and cases, like stereotactic breast core biopsy or deep learning of breast masses detection and diagnose (8-10). The varying experience of surgeons and ultrasound physicians, as well as their collaboration with physicians, could affect the operation time and efficiency. This can be divided into the exploration and proficiency stages based on a time axis; therefore, there may be a learning curve. The accurate evaluation and summary of this learning process is of great significance in the guidance of future teams to carry out this kind of procedure (11,12). In the past, a simple grouping method was used to evaluate the learning process, which has the advantage of low data requirements, but the disadvantage is inaccurate calculation of turning points (11,13). Cumulative sum analysis (CUSUM) can be used to draw the learning curve, simulate the equation, and accurately calculate the turning point of learning from the exploration stage to the mastery stage (12,14,15). However, there are few studies on the different experience combinations of collaborative physicians. In China, most VAE operations are performed with the cooperation of a surgeon and an ultrasound physician. The ultrasound physician is responsible for positioning and guidance, and the surgeon is responsible for removing the lesions. Therefore, we aimed to use CUSUM to retrospectively analyze the learning curve of a combination of skilled surgeons and novice ultrasound physicians without VAE experience, as well as the combination of novice surgeons and skilled ultrasound physicians. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-573/rc).
Methods
Patients
This retrospective study was divided into two time periods: 49 consecutive patients from June 2018 to May 2019 completed by skilled surgeons and novice ultrasound physicians (ultrasound physician group, U group); and 30 consecutive patients from June 2020 to May 2021 completed by skilled ultrasound physicians and novice surgeons (surgeon group, S group). That’s all the consecutive cases in the first year that the novice physician start VAE. All patients underwent ultrasound breast examination. Inclusion criteria were patients with breast masses less than 3 cm in long diameter who wished to undergo VAE. The exclusion criteria were patients with obvious bleeding tendency, or other conditions that could not tolerate VAE procedures. Lesions proven to be malignant by biopsy were also excluded. The average age of patients in the U group was 41.92±12.71 years, and the average maximum diameter of masses was 1.34±0.50 cm; the average age of patients in the S group was 33.33±9.79 years, and the average maximum diameter of masses was 1.52±0.47 cm. The masses were classified into class 3 and 4A by the Breast Imaging Reporting and Data System (BI-RADS). Part of masses classified as BI-RADS 4A underwent ultrasound-guided biopsy before VAE and were confirmed to be benign lesions, while masses of BI-RADS 3 did not undergo preoperative biopsy. All patients underwent ultrasound-guided VAE at Peking University Third Hospital. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The requirement for ethical approval was waived for this retrospective analysis as the study carried out under normal education and training, based on the collection of previous data, presented no more than minimal risk to participants and no commercial interest is involved in the project. Postoperative pathology included hyperplasia, fibroadenoma, hamartoma, breast adenosis, tubular adenoma, intraductal papilloma, and invasive breast cancer. Another 548 patients who received ultrasound-guided VAE performed by a combination of skilled surgeons and skilled ultrasound physicians at Peking University Third Hospital during the same period (from June 2018 to May 2021) were selected as the reference group (R group).
Ultrasound physicians and surgeons without experience were defined as those who had never carried out ultrasound-guided VAE, while skilled ultrasound physicians and skilled surgeons were defined as those who had independently carried out ultrasound-guided VAE in more than 60 cases, as per Park et al. (11) and Esgueva et al. (16). All operating physicians were above the level of attending physicians with independent medical qualifications.
All patients signed an informed consent form before the procedure. Immediate intraoperative ultrasound showed complete resection of the breast target masses based on imaging. The operative time was recorded from the surgeon punctured the needle until the target mass completely removed. All specimens obtained were divided into the center of the masses and the periphery of the masses. The central part was the target mass, and the surrounding part was normal breast tissue, which was considered to have a clear margin. Large amounts of local bleeding were removed through vacuum-assisted suction or extrusion. There were no serious complications at the two-week follow-up.
Surgical procedure and equipment
Ultrasound-guided VAE was performed by the ultrasound physician and a surgeon using a Philips iU 22 (Royal Dutch Philips Electronics Ltd., Amsterdam, The Netherlands) with a liner probe of L12-5 MHz. Preoperative ultrasound was used to determine the target breast masses and the direction, expressed as a quadrant, and defined as follows. Left breast: 12–3 o’clock (excluding 12 o’clock) was the upper outer quadrant; 3–6 o’clock (excluding 3 o’clock) was the lower outer quadrant; 6–9 o’clock (excluding 6 o’clock) was the lower inner quadrant; 9–12 o’clock (excluding 9 o’clock) was the upper inner quadrant; right breast: 12–3 o’clock (excluding 12 o’clock) was the upper inner quadrant; 3–6 o’clock (excluding 3 o’clock) was the lower inner quadrant; 6–9 o’clock (excluding 6 o’clock) was the lower outer quadrant; 9–12 o’clock (excluding 9 o’clock) was the upper outer quadrant. The distance from the nipple, size, and depth of the masses were confirmed and recorded again through ultrasound during the operation, and then marked.
The procedures were performed under local anesthesia. The surgeon performed routine disinfection, towel spreading, and local anesthesia. Subsequently, ultrasound-guided resection of the mass was performed through the collaboration of two physicians. Timing began from the insertion of the biopsy needle until the tumor was completely resected. In this procedure, we placed the needle just posterior to the target and continued to excise until the entire target was removed. The direction of resection was adjusted often under ultrasound guidance. Intraoperative ultrasound was used to determine whether the mass was completely resected. If there was bleeding that affected judgment, the biopsy needle was temporarily pulled out, and a pressure scan was used to determine whether it was local bleeding or residual mass. If the mass was not completely removed, the needle was reinserted and the excision continued. A Mammotome VAE and resection system (Ethicon Endo-Surgery Inc., New Brunswick, NJ, USA), together with a matching 8-gauge biopsy needle, was used.
Research method
Calculation of operative time CUSUM
All patients in the U and S groups were separately numbered according to the operation sequence. The CUSUM of operative time of the first patient was the difference between the operative time of the first patient and the average operative time of all patients. The CUSUM of operative time of the Nth patient was the sum of the difference between the cumulative sum of the operative time of the previous patient, the operative time of the Nth patient, and the average operative time of all patients, and the cumulative sum of the operative time of the last patient was zero. The calculation formula is as follows:
where xirepresents the actual operation time of each patient, and u represents the average operation time of the same group of patients. N represents the order of patients.
Drawing of the learning curve and calculation of the turning point
The cumulative number of cases as the abscissa and the cumulative sum of the operation time as the ordinate were taken, and IBM SPSS software (Version 25.0, IBM Corp., Armonk, NY, USA) was used to calculate the simulation equation. The learning curve was drawn, and P<0.05 was considered as a successful fit of the equation. R2 was used to determine how good the fit was, and the turning point of the learning curve was calculated.
Influencing factors between the exploration and proficiency stages
Taking the turning point as the boundary, patients in the U and S groups were divided into an exploration stage and proficiency stage (U-E stage and U-P stage; and S-E stage and S-P stage, respectively). We then analyzed the data to determine if there was a statistically significant difference in patient age, nodule location, quadrant, BI-RADS classification, distance from the nipple, long diameter, short diameter, depth from the epidermis, and operation time between the stages. This was done to demonstrate whether there was a learning curve and whether the turning point was appropriate.
Influencing factors with reference group
The U group and S group were compared with the R group in terms of patient age, nodule location, quadrant, BI-RADS classification, distance from the nipple, long diameter, short diameter, depth from the epidermis, and operation time. We subsequently analyzed whether there were other factors that affected the learning curve.
Linear correlation analysis
We used operation time as the dependent variable, Y; and patient age, nodule location, quadrant, BI-RADS classification, distance from the nipple, long diameter, short diameter, and depth from the epidermis as independent variables X1–8, respectively. Linear regression was then used to analyze the relationship between the dependent variable and the independent variable in the three groups, respectively, to find other factors that may affect the learning curve.
Statistical analysis
Measurement data are expressed as , and enumeration data as the number of cases. The curve estimation analysis was used for the learning curve fitting equation; the one-way analysis of variance (ANOVA) test was used for the comparison between the measurement data groups; χ2 or Fisher’s exact test was used for the comparison between the enumeration data groups. The relationship between the dependent variable and the independent variable was analyzed using linear regression. IBM SPSS software version 25.0 was used for data analysis. The difference was statistically significant at the test level of P<0.05.
Results
Patients and groups
There were 627 patients underwent ultrasound-guided VAE (49 in the U group, 30 in the U group and 548 in the R group; Figure 1) were included in this study.
Learning curve and turning point
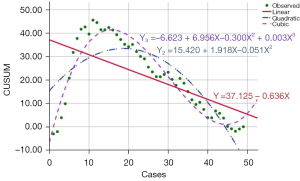
The fit R2 of the quadratic fitting equation of the learning curve of the U group was 0.684 (P<0.05), the degree of fit was good, and the curve turning point was the 19th case. The R2 of the cubic fitting equation was 0.910 (P<0.05), the degree of fit was also relatively good, and the turning point of the curve was the 15th case, as shown in Figure 2.
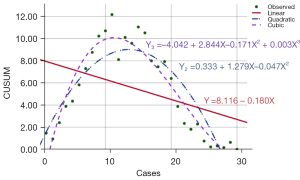
The R2 of the quadratic fitting equation of the learning curve of the S group was 0.786 (P<0.05), and the turning point of the curve was the 14th case. The R2 of the cubic fitting equation was 0.903 (P<0.05), and the turning point of the curve was the 11th case. The degrees of fit of all the curves were good, as shown in Figure 3.
Influencing factors between exploration and proficiency stages
The U group and the S group were divided into the exploratory and proficiency stages, with the 19th case and the 14th case as the boundary, respectively. The operative time required in the proficiency stage was significantly shorter than that in the exploration stage in both groups (P=0.012), and the difference in the mean operative time between the two stages was statistically significant (P=0.003). The actual operation time of the 19th patient was 3 minutes in the S group, while the actual time of the 14th patient was 2 minutes in the U group.
In the U group, 78.9% (15/19) of the masses were BI-RADS 4a in the exploration stage, while the masses in the proficiency stage were mainly BI-RADS 3, and the difference was statistically significant (P=0.004). The average age of the patients in the exploratory stage in the S group was higher than that of the patients in the proficient stage, and the difference was statistically significant (P=0.025). There were no statistically significant differences in other indicators between the two stages in the two groups, as shown in Table 1.
Table 1
| Influencing factor | U group | S group | |||||
|---|---|---|---|---|---|---|---|
| U-E stage | U-P stage | P | S-E stage | S-P stage | P | ||
| Age (years), mean ± SD | 45.68±12.30 | 39.53±12.57 | 0.099 | 36.14±11.39 | 30.88±7.68 | 0.025 | |
| Nodule location, n (%) | |||||||
| R breast | 5 (26.3) | 14 (46.7) | 0.154 | 6 (42.9) | 8 (50.0) | 0.730 | |
| L breast | 14 (73.7) | 16 (53.3) | 8 (57.1) | 8 (50.0) | |||
| Quadrant, n (%) | |||||||
| Upper outer | 9 (47.4) | 20 (66.7) | 0.560 | 9 (64.3) | 11 (68.8) | 0.924 | |
| Lower outer | 2 (10.5) | 2 (6.7) | 2 (14.3) | 1 (6.3) | |||
| Upper inner | 5 (26.3) | 4 (13.3) | 3 (21.4) | 3 (18.8) | |||
| Lower inner | 3 (15.8) | 4 (13.3) | 0 (0.0) | 1 (6.3) | |||
| BI-RADS, n (%) | |||||||
| 3 | 4 (21.1) | 19 (63.3) | 0.004 | 9 (64.3) | 11 (68.8) | 1.000 | |
| 4A | 15 (78.9) | 11 (36.7) | 5 (35.7) | 5 (31.2) | |||
| Distance from nipple (cm), mean ± SD | 2.28±1.55 | 2.44±1.96 | 0.766 | 2.51±1.24 | 2.09±0.52 | 0.083 | |
| Long diameter (cm), mean ± SD | 1.23±0.53 | 1.41±0.49 | 0.226 | 1.60±0.54 | 1.44±0.42 | 0.239 | |
| Short diameter (cm), mean ± SD | 0.69±0.21 | 0.69±0.30 | 0.986 | 0.75±0.23 | 0.87±0.19 | 0.541 | |
| Depth (cm), mean ± SD | 0.90±0.27 | 0.81±0.25 | 0.227 | 0.72±0.37 | 0.41±0.10 | 0.944 | |
| Operation time (min), mean ± SD | 5.89±4.58 | 2.87±1.94 | 0.012 | 4.21±1.05 | 2.94±1.12 | 0.003 | |
U, surgeries completed by skilled surgeons and novice ultrasound physicians; S, surgeries completed by skilled ultrasound physicians and novice surgeons; E, exploration; P, proficiency; SD, standard deviation; BI-RADS, Breast Imaging Reporting and Data System.
Influencing factors with reference group
The mean operation time of the U group was 4.04±3.52 min, while the operation time of the R group was 3.03±2.02 min, and there was a significant difference between the two groups (P=0.002). No other parameters were significantly different between these two groups. The long diameter of lesions in the S group was slightly larger than that in the R group, and there was a statistically significant difference (P=0.026), but the difference in operation time was not statistically significant (P=0.219). The mean age of patients in the U group was higher than that in the S group (P=0.031), and the masses were deeper than those in the S group (P=0.023). The mean operating time was 3.53±1.25 min in the S group, lower than the U group (P=0.001). The specific data are shown in Table 2.
Table 2
| Influencing factor | R group | U group | S group | P | ||
|---|---|---|---|---|---|---|
| R vs. U | R vs. S | U vs. S | ||||
| Age (years), mean ± SD | 39.33±12.92 | 41.92±12.71 | 33.33±9.79 | 0.179 | 0.053 | 0.031 |
| Nodule location, n (%) | ||||||
| R breast | 278 (50.7) | 19 (38.8) | 14 (46.7) | 0.109 | 0.710 | 0.490 |
| L breast | 270 (49.3) | 30 (61.2) | 16 (53.3) | |||
| Quadrant, n (%) | ||||||
| Upper outer | 249 (45.4) | 29 (59.2) | 20 (66.7) | 0.132 | 0.187† | 0.496 |
| Lower outer | 88 (16.1) | 4 (8.1) | 3 (10.0) | |||
| Upper inner | 152 (27.7) | 9 (18.4) | 6 (20.0) | |||
| Lower inner | 59 (10.8) | 7 (14.3) | 1 (3.3) | |||
| BI-RADS, n (%) | ||||||
| 3 | 291 (53.1) | 23 (46.9) | 20 (66.7) | 0.408 | 0.207 | 0.088 |
| 4A | 257 (46.9) | 26 (53.1) | 10 (33.3) | |||
| Distance from nipple (cm), mean ± SD | 2.46±1.50 | 2.38±1.80 | 3.03±1.76 | 0.779 | 0.980 | 0.367 |
| Long diameter (cm), mean ± SD | 1.38±0.59 | 1.34±0.50 | 1.52±0.47 | 0.605 | 0.026 | 0.159 |
| Short diameter (cm), mean ± SD | 0.75±0.32 | 0.69±0.26 | 0.81±0.22 | 0.252 | 0.097 | 0.466 |
| Depth (cm), mean ± SD | 0.78±0.35 | 0.84±0.26 | 0.70±0.38 | 0.231 | 0.497 | 0.023 |
| Operation time (min), mean ± SD | 3.03±2.02 | 4.04±3.52 | 3.53±1.25 | 0.002 | 0.219 | 0.001 |
†, Fisher’s exact test. R, vacuum-assisted breast excision performed by a combination of skilled surgeons and skilled ultrasound physicians (reference); U, surgeries completed by skilled surgeons and novice ultrasound physicians; S, surgeries completed by skilled ultrasound physicians and novice surgeons; E, exploration; P, proficiency; SD, standard deviation; BI-RADS, Breast Imaging Reporting and Data System.
Linear correlation analysis
The operation time (Y) in the R group was negatively correlated with patient age (X1), and positively correlated with nodule long diameter (X3), nodule short diameter (X4), and distance from the epidermis (X5). The correlation equation was Y = 1.771 − 0.022 × X1 + 0.862 × X3 + 1.278 × X4 + 0.571 × X5 [correlation coefficient r =0.453, P=0.000 (P<0.05)]. Operation time (Y) was not correlated with other indicators in the R group. The operation time (Y) in the U group was positively correlated with nodule thickness (X4). The correlation equation was Y = 0.142 + 5.619 × X4 [correlation coefficient r=0.421, P=0.003 (P<0.05)]. The operation time (Y) with no correlation was found for other indicators in the U group. The operation time (Y) in the S group was positively correlated with the nodule long diameter (X3); the correlation equation was Y = 1.688 + 1.216 × X3 [correlation coefficient r=0.461, P=0.010 (P<0.05)]. The operation time (Y) with no correlation was found for other indicators in the S group.
Post-procedural histopathology results
The pathological results of eight patients in the R group obtained by VAE were malignant, and the pathological types were five cases of intraductal carcinoma, one case of medullary carcinoma and two cases of unspecified invasive breast cancer. The pathological result of one patient in the U group was malignant, and the pathological type was medullary carcinoma. The pathological results of all patients in the S group were benign. Malignant patients underwent radical surgery and follow-up treatment.
Discussion
The best learning curve of this study group was the quadratic fitting equation, and the turning points were the 19th case in the U group and the 14th case in the S group. Total operation times of the proficiency stage were significantly shorter than those of the exploration stage in the U and S group (5.89±4.58 vs. 2.87±1.94 min and 4.21±1.05 vs. 2.94±1.12 min, respectively). Patient age, long diameter, short diameter, and depth of masses related to the operation time.
Recently, the detection rate of breast masses in women has been increasing year on year. With the advancement of diagnosis and treatment technology, patient expectations of breast-conserving treatment, and the desire to reduce the pain of treatment, the treatment of breast masses has gradually changed from simple open surgery to microscopic surgery (17). As a new surgical method, ultrasound-guided VAE has been used in clinical settings because of its advantages of providing more tissue information for pathological diagnosis, less complications, and good cosmetic effect (18,19).
The results of our study show that there is a learning curve, not only for the combination of novice ultrasound physicians and skilled surgeons, but also for novice surgeons and skilled ultrasound physicians in ultrasound-guided VAE procedures. In the ultrasound physician group, the R2 of the cubic equation was better than that of the quadratic equation (0.684 vs. 0.910), and the calculated turning points were the 15th and 19th case, respectively. In the S group, the R2 of the cubic equation was also better than that of the quadratic equation (0.786 vs. 0.903), with the turning points at the 11th case and the 14th case, respectively. Compared to other complicated surgeries, such as breast plastic surgery which needs about 45−100 cases to reach a plateau (20), the turning points of VAE have been significantly reduced, which reflects that ultrasound-guided VAE is easy to be mastered by new doctors. Since the turning points of the different equations are approximate, given the clinical safety, our study shows that it is more appropriate to use the 19th case for the ultrasound physician and the 14th case for the surgeon as a training reference. Our results are similar to those of Park et al., who considered the 20th case as an appropriate turning point (11). The study by Salazar et al. demonstrated that 11 lesions were required to acquire the necessary skills to perform complete excision in more than 80% of patients at the end of ultrasound-guided VAE using CUSUM (12). These differences may be because their study was performed by radiologists with two years of experience in breast interventions, without cooperation between ultrasound physicians and surgeons as seen in our study. In addition, their study mainly used a 10-gauge needle, while our study used a thicker 8-gauge needle, with no postoperative residual masses after ultrasound-guided VAE. Alternatively, Kim et al. believed that the initial size is the only variable that correlates significantly with recurrence (21). The average maximum diameter in our study was 1.38–1.52 cm smaller than that of 1.685 cm in the study by Salazar et al. (12).
In addition, this study further used the 19th case and the 14th case as the boundary to compare the indicators before and after the exploration stage and the proficiency stage in the U and S groups, respectively. It was found that there were statistically significant differences in the operation time and BI-RADS classification for the ultrasound physician, and patient age and operation time for the surgeon. Differences in other indicators were not statistically significant. Differences in operative time suggest the existence of a learning curve. The BI-RADS classification may only be an accidental factor in the U group. In the S group, the age of the patients in the proficient stage was lower than that in the exploratory stage, but in the subsequent multivariate analysis of the R group, we found that age and operation time were negatively correlated; that is, the older the patient, the shorter the operation time, and the younger the patient, the longer the operation time. This may be related to the fact that the breast glands in young patients are denser and thicker, and it is challenging to insert needles and remove them (11). Nakano et al. believe that sample collection depends on the hardness of the target lesion, and that suction time could potentially be increased to overcome this issue (22). Our results can also be explained by this, since glands in younger patients are generally thicker and denser. In the S group, the patients in the proficiency stage were younger, and it was predicted that the operation time should be longer, but the actual operation time was shorter. It also proves that it is appropriate to use the 14th case as the turning point and, in the proficiency stage, surgeons can master VAE skills and shorten the operation time.
The results of the comparative analysis between the U group and the R group showed that there was no significant difference in other indicators except for the operation time. On one hand, it shows that when these indicators are the same; the only factor that can cause the difference in operation time is the difference in the experience of the combined physicians. This result also proves that there is a learning curve in the combination of new ultrasound physicians and skilled clinicians to perform VAE. The comparison between the S group and the R group showed that there was no significant difference in the operation time. The surgeons involved in this study have more than five years of surgical experience. Although there is a learning process for the individual surgeon to master VAE, the overall operating time was not significantly longer compared to other surgeons skilled in VAE. This may be due to the manual dexterity of the individual surgeon and his background knowledge of surgical anatomy (23). On the other hand, it suggests that the speed-limiting factor of VAE under ultrasound guidance lies in the ultrasound physician, because many influencing factors may lead to prolonged operation time, such as the inability to identify masses after the administration of local anesthetics, or the ultrasound probe was not properly positioned (11,24). The proficiency of the ultrasound physician to guide is more important for the smooth progress of VAE.
Further analysis in this study showed that the operation time in the U group was positively correlated with the short diameter of the nodule, while positively correlated with the long diameter of the nodule in the S group, but not with other indicators. The operation time in the R group showed a negative correlation with patient age, and a positive correlation with the long diameter, short diameter, and depth of the nodule. The thickness and density of the breast glands may be the reason the operation time in the R group was negatively correlated with age. Moreover, in our study, a small incision was made in the skin with a surgical blade before the rotary cutter was inserted, which also avoided the influence of the skin being too tight and hard to be punctured. The operation time in the R group was positively correlated with the other three indicators. The reason may be that the larger and deeper the nodule, the deeper the needle insertion, the more rotational cuts, the more difficult the ultrasound guidance, and the longer the operation time. Other studies have also identified nodule volume and position as a relevant factor affecting the surgical procedure and residual (15,25). Although there was a linear correlation between the operation time and the above factors, the correlation coefficients were all lower than 0.5, showing a low correlation and only used as a reference factor affecting the operation time.
This study was a retrospective analysis, and there were some limitations. First, we did not conduct long-term follow-up for nodule recurrence, only immediate postoperative observation. Our institution strictly controls the inclusion criteria of VAE, and the diameter of the tumor in the included cases did not exceed 3 cm, which may be an important reason the complete resection rate of all patients could reach 100%. The learning curve indicator is only based on the operation time, not the existence of residual, which is different from some previous studies (15,25). Second, whether new ultrasound physicians and surgeons have other similar surgical experience before surgery, the length of preoperative training, the pathological type of masses, postoperative complications, and other factors have not been considered. Future studies will further explore the influencing factors of these aspects to conduct a comprehensive analysis of VAE guided by ultrasound. Third, to the best of our knowledge, this article is one of the few on learning curve considering collaboration among different physicians; however, the collaboration did not involve a combination of novice surgeons and novice ultrasound physicians, to which the turning point obtained in this study may not apply. Fourth, the collection of retrospective data is subject to information bias, including recalling bias and investigation bias.
Conclusions
There is a learning curve in ultrasound-guided vacuum-assisted breast lesion excision. For novice ultrasound physicians, the experience of performing VAE procedures in 19 patients is required to acquire stable skills. For surgeons, it takes 14 patients to reach a plateau. Through the accumulation of experience, it is feasible to safely perform ultrasound-guided VAE procedures. Patient age, long diameter, short diameter, and depth of masses may be factors affecting the operation time.
Acknowledgments
Thanks to Yutao Lei, Hongmei Zhao, and Ying Peng for their contributions in the case collection process. We would like to thank Editage (www.editage.cn) for English language editing.
Funding: This work was supported by Key clinical projects in Peking University Third Hospital (No. Y78480-02).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-573/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-573/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The requirement for ethical approval was waived for this retrospective analysis as the study carried out under normal education and training, based on the collection of previous data, presented no more than minimal risk to participants and no commercial interest is involved in the project. All patients provided written informed consent before VAE.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang WJ, Wang Q, Cai QP, Zhang JQ. Ultrasonographically guided vacuum-assisted excision for multiple breast masses: non-randomized comparison with conventional open excision. J Surg Oncol 2009;100:675-80. [Crossref] [PubMed]
- Maxwell AJ. Ultrasound-guided vacuum-assisted excision of breast papillomas: review of 6-years experience. Clin Radiol 2009;64:801-6. [Crossref] [PubMed]
- Wang ZL, Li JL, Su L, Zhang YF, Tang J. An evaluation of a 10-gauge vacuum-assisted system for ultrasound-guided excision of clinically benign breast lesions. Breast 2009;18:192-6. [Crossref] [PubMed]
- Mathew J, Crawford DJ, Lwin M, Barwick C, Gash A. Ultrasound-guided, vacuum-assisted excision in the diagnosis and treatment of clinically benign breast lesions. Ann R Coll Surg Engl 2007;89:494-6. [Crossref] [PubMed]
- Sohn V, Arthurs Z, Herbert G, Keylock J, Perry J, Eckert M, Fellabaum D, Smith D, Brown T. Atypical ductal hyperplasia: improved accuracy with the 11-gauge vacuum-assisted versus the 14-gauge core biopsy needle. Ann Surg Oncol 2007;14:2497-501. [Crossref] [PubMed]
- Cho N, Moon WK, Cha JH, Kim SM, Kim SJ, Lee SH, Chung HK, Cho KS, Park IA, Noh DY. Sonographically guided core biopsy of the breast: comparison of 14-gauge automated gun and 11-gauge directional vacuum-assisted biopsy methods. Korean J Radiol 2005;6:102-9. [Crossref] [PubMed]
- Londero V, Zuiani C, Linda A, Battigelli L, Brondani G, Bazzocchi M. Borderline breast lesions: comparison of malignancy underestimation rates with 14-gauge core needle biopsy versus 11-gauge vacuum-assisted device. Eur Radiol 2011;21:1200-6. [Crossref] [PubMed]
- Koskela A, Berg M, Sudah M, Malinen A, Kärjä V, Mustonen P, Kataja V, Soimakallio S, Vanninen R. Learning curve for add-on stereotactic core needle breast biopsy. Acta Radiol 2006;47:454-60. [Crossref] [PubMed]
- Niu S, Huang J, Li J, Liu X, Wang D, Wang Y, Shen H, Qi M, Xiao Y, Guan M, Li D, Liu F, Wang X, Xiong Y, Gao S, Wang X, Yu P, Zhu J. Differential diagnosis between small breast phyllodes tumors and fibroadenomas using artificial intelligence and ultrasound data. Quant Imaging Med Surg 2021;11:2052-61. [Crossref] [PubMed]
- Gao Y, Liu B, Zhu Y, Chen L, Tan M, Xiao X, Yu G, Guo Y. Detection and recognition of ultrasound breast nodules based on semi-supervised deep learning: a powerful alternative strategy. Quant Imaging Med Surg 2021;11:2265-78. [Crossref] [PubMed]
- Park HS, Jeon CW. Learning curve for breast mass excision using a vacuum-assisted biopsy system. Minim Invasive Ther Allied Technol 2014;23:235-40. [Crossref] [PubMed]
- Salazar JP, Miranda I, de Torres J, Rus MN, Espinosa-Bravo M, Esgueva A, Salvador R, Rubio IT. Percutaneous ultrasound-guided vacuum-assisted excision of benign breast lesions: A learning curve to assess outcomes. Br J Radiol 2019;92:20180626. [Crossref] [PubMed]
- Zografos GC, Zagouri F, Sergentanis TN. Vacuum-assisted breast biopsy: an easy-to-learn procedure? Am J Surg 2008;196:798. [Crossref] [PubMed]
- Novoa NM, Varela G. Monitoring surgical quality: the cumulative sum (CUSUM) approach. Mediastinum 2020;4:4. [Crossref] [PubMed]
- Ko EY, Bae YA, Kim MJ, Lee KS, Lee Y, Kim LS. Factors affecting the efficacy of ultrasound-guided vacuum-assisted percutaneous excision for removal of benign breast lesions. J Ultrasound Med 2008;27:65-73. [Crossref] [PubMed]
- Esgueva A, Rodríguez-Revuelto R, Espinosa-Bravo M, Salazar JP, Rubio IT. Learning curves in intraoperative ultrasound guided surgery in breast cancer based on complete breast cancer excision and no need for second surgeries. Eur J Surg Oncol 2019;45:578-83. [Crossref] [PubMed]
- Sebag P, Tourasse C, Rouyer N, Lebas P, Dénier JF, Michenet P. Value of vacuum assisted biopsies under sonography guidance: results from a multicentric study of 650 lesions. J Radiol 2006;87:29-34. [Crossref] [PubMed]
- Bromberg SE, Moraes PRAF, Ades F. Prime incision: A minimally invasive approach to breast cancer surgical treatment-A 2 cohort retrospective comparison with conventional breast conserving surgery. PLoS One 2018;13:e0191056. [Crossref] [PubMed]
- Yu YH, Liang C, Yuan XZ. Diagnostic value of vacuum-assisted breast biopsy for breast carcinoma: a meta-analysis and systematic review. Breast Cancer Res Treat 2010;120:469-79. [Crossref] [PubMed]
- Tapking C, Kowalewski KF, Hundeshagen G, Kneser U, Hirche C. A Systematic Review of Learning Curves in Plastic and Reconstructive Surgery Procedures. Ann Plast Surg 2020;85:324-31. [Crossref] [PubMed]
- Kim MJ, Park BW, Kim SI, Youk JH, Kwak JY, Moon HJ, Kim EK. Long-term follow-up results for ultrasound-guided vacuum-assisted removal of benign palpable breast mass. Am J Surg 2010;199:1-7. [Crossref] [PubMed]
- Nakano S, Imawari Y, Mibu A, Otsuka M, Oinuma T. Differentiating vacuum-assisted breast biopsy from core needle biopsy: Is it necessary? Br J Radiol 2018;91:20180250. [Crossref] [PubMed]
- Subramonian K, Muir G. The 'learning curve' in surgery: what is it, how do we measure it and can we influence it? BJU Int 2004;93:1173-4. [Crossref] [PubMed]
- Bhatt AA, Whaley DH, Lee CU. Ultrasound-Guided Breast Biopsies: Basic and New Techniques. J Ultrasound Med 2021;40:1427-43. [Crossref] [PubMed]
- Wang ZL, Liu G, Huang Y, Wan WB, Li JL. Percutaneous excisional biopsy of clinically benign breast lesions with vacuum-assisted system: comparison of three devices. Eur J Radiol 2012;81:725-30. [Crossref] [PubMed]


