
Age-related changes in lumbar bone mineral density measured using quantitative computed tomography in healthy female cynomolgus monkeys
Introduction
Osteoporosis is a systemic disorder characterized by a reduction in bone mass and deterioration of the bone tissue microarchitecture, leading to increased bone fragility and a higher risk of fracture (1). A meta-analysis showed that the global prevalence of osteoporosis based on World Health Organization criteria was 19.7%. The prevalence varied among countries, ranging from 4.1% in Netherlands to 52.0% in Turkey. In terms of gender, the prevalence of osteoporosis was 10.6% in males and 24.8% in females, while it was higher in postmenopausal women at about 27.4% (2). A multicenter study based on a Chinese population showed that the prevalence of osteoporosis in people >50 years of age was approximately 29.0% in women and 13.5% in men (3). An increasing number of people suffer from osteoporosis, and the occurrence of osteoporotic fractures poses a heavy economic burden on the healthcare system (4). Several species of animals are available for osteoporosis research, among which nonhuman primates such as cynomolgus monkeys are highly valuable. Female cynomolgus monkeys are similar to women with regard to the menstrual cycle, estrogen and progesterone secretion patterns, and reduced bone mass associated with natural menopause, making them an ideal model for studying osteoporosis (5). Ovariectomized cynomolgus monkeys are commonly used in studies related to osteoporosis, including investigations into the therapeutic effects of specific drugs on osteoporosis (6,7), which contributes greatly to the treatment and prevention of osteoporosis in humans.
Several methods are available for clinically diagnosing osteoporosis, including dual-energy X-ray absorptiometry (DXA), quantitative computed tomography (QCT), peripheral QCT (pQCT), quantitative ultrasound, and magnetic resonance imaging (8,9). In nonhuman primates, methods used for bone mineral density (BMD) measurements include DXA, pQCT, and micro-CT (6,7), among which DXA is the most commonly used. DXA can be used to measure the areal BMD (aBMD) of the whole body, lumbar spine, and radius using a region of interest (ROI) that includes both the trabecular and cortical bone (10). Whole-body aBMD of cynomolgus monkeys measured by DXA mainly reflects changes in the cortical bone and cannot reflect changes in the cancellous bone (11), and so this measure is not recommended for monitoring therapeutic efficacy. The International Society for Clinical Densitometry (ISCD) recommends measuring the BMD of the lumbar spine to monitor efficacy. The trabecular bone in the spine is approximately eight times more metabolically active than the cortical bone, and changes in lumbar trabecular BMD measured by QCT are greater than changes in aBMD measured by DXA; therefore, QCT is more sensitive for monitoring age- and treatment-related changes in the BMD (12). Several studies have shown that QCT has a higher detection rate than DXA in diagnosing osteoporosis because QCT measures the true volumetric bone mineral density (vBMD), which is not size dependent and unaffected by calcifications, osteophytes, and spinal deformity (13-16). During the natural growth of nonhuman primates, spinal osteoarthritis becomes more common in older monkeys and it may be more accurate to measure their lumbar vBMD using QCT (17).
Studies on the lumbar BMD of cynomolgus monkeys using pQCT have been reported, mainly measuring the in vitro vertebral BMD of monkeys in a single age group (18-20). Currently, pQCT is not routinely used in clinical practice, and the QCT lumbar vBMD measurement is widely used in clinical and scientific research. Previous study has shown that the aBMD and bone mineral content measured by DXA in female cynomolgus monkeys experience age-related changes (21). However, no studies have used QCT-based techniques to investigate age-related changes in the BMD in nonhuman primates. In addition, QCT is used to measure body components, which include abdominal adipose tissue and paravertebral muscles (22,23). Lumbar vBMD measured by QCT may be affected by body components in humans, which include visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), and lean mass (LM) (23-27). At present, there are no reports on the measurement of abdominal adipose tissue and LM using QCT in nonhuman primates. In addition, the relationship between lumbar vBMD and body composition in nonhuman primates is unclear. To ensure the accuracy of QCT measurements, the ISCD recommends establishing a new in vivo precision level for spine QCT operations (12). Several studies have reported the precision of pQCT for measuring BMD in nonhuman primates, but they measured peripheral bones, and some of these studies were performed in vitro (28-30). This study aimed to establish in vivo precision data of QCT to measure lumbar vBMD in female cynomolgus monkeys.
QCT is an accurate method for measuring vBMDs, and cynomolgus monkeys are ideal models for studying osteoporosis. Therefore, this study aimed to explore the value of QCT in healthy female cynomolgus monkeys. Our study was performed on female cynomolgus monkeys to investigate the following: (I) age-related changes in lumbar vBMD and in the relationship between lumbar vBMD and body composition, and (II) short-term in vivo precision of QCT measurements. We present the following article in accordance with the ARRIVE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-763/rc).
Methods
Animals
This study included 72 healthy female cynomolgus monkeys. All experimental animals were absent of severe organ diseases, and none of the animals showed evidence of clinical disorders. Animals with signs of disease were excluded. All female cynomolgus monkeys were specific pathogen-free animals in compliance with national laboratory animal standards. Animals were obtained from Guangdong Landau Biotechnology Co., Ltd., Guangzhou, China, which holds international accreditation from the Association for Assessment and Accreditation of Laboratory Animal Care. The animals were housed in groups of 10–15 monkeys, which consisted of one mature male, several mature females, and their progenies. The animal room was mainly illuminated by natural lighting, adjusted with incandescent lighting to achieve 12/12 h light/dark cycles. The animals were fed fruits, a semi-purified diet containing ~1.0% calcium, ~0.7% phosphorus, and drinking water ad libitum. The sitting height was recorded as the trunk length, which was approximately the distance from the crown to the base of the pubic bone. Body mass index (BMI) was calculated as the weight (kg) divided by the square of the trunk length (m2) (31). Experiments were performed under a project license (No. LDIACUC2018-0004) granted by the Laboratory Animal Ethics Committee of Guangdong Landau Biotechnology Co., Ltd., in compliance with international Association for Assessment and Accreditation of Laboratory Animal Care guidelines for the care and use of animals. Our protocol was not registered. Based on the progression of sexual maturation and development, healthy female cynomolgus monkeys were stratified into different age groups in our study: a juvenile group (≤4 years old), young group (5–10 years old), middle-aged group (11–19 years old), and older group (≥20 years old) (32).
QCT examination
All animals underwent whole-body CT scans using a PET/CT scanner (GE Discovery Elite 690, USA) at the PET CT/MRI center of the First Affiliated Hospital of Jinan University. For quality control purposes, we calibrate the machine with a phantom once a month. Asynchronous QCT was performed according to the following parameters: tube voltage 120 kV; tube current 300 mA; slice thickness 1.25 mm; slice interval 1.0 mm; and field of view 500 mm. Asynchronous QCT calibrates data in Hounsfield units by using phantom data originally obtained from CT scans for measuring vBMD. The installation of the QCT Pro Asynchronous Calibration Module makes it possible to measure vBMD directly from CT images without simultaneously using the calibration phantom during the subject scan (33). All monkeys were starved for 8–12 h and weighed prior to scanning. Before examination, the animals were anesthetized by a specialized veterinarian using ketamine hydrochloride (5–10 mg/kg, intramuscularly) and 3% pentobarbital sodium (0.5–1 mL/kg, intravenously). A few cynomolgus monkeys may vomit during anesthesia. In order to avoid causing asphyxia or death, the vital signs of the animals need to be monitored every 10 minutes to ensure that they can successfully complete the QCT scan. If the animal’s vital signs were not stable after anesthesia, the QCT scan was not performed. The animals were placed in a supine position on the scanning bed, with the spine parallel to the long axis of the examination bed. All CT images were sent to the QCT workstation and analyzed using the three-dimensional spine function and tissue composition module in the application (QCT Pro Version 6.1, Mindways Software).
QCT measurements of lumbar vBMD
QCT measurements of the lumbar vBMD were performed on the L2-4 vertebrae. Most cynomolgus monkeys have seven lumbar vertebrae, whereas some have six. In this study, we calculated the vertebral sequences from bottom to top, meaning that the bottom vertebra was defined as L7. On the axial and sagittal images, the ROIs were placed in the central trabecular bone of the vertebral bodies, parallel to the endplates of the upper and lower margins of the vertebral bodies, avoiding the inclusion of the cortical bone. The area of each ROI was approximately 20–30 mm2. If any vertebra from among L2-4 vertebrae showed deformation or an uneven increase in bone density, three adjacent vertebrae from among L1-7 vertebrae were selected for vBMD measurements. To reduce the error in manually drawing the ROI, the vBMD of each vertebra was measured three times by the same well-trained investigator, and the average of the three measurements was chosen as the final vBMD of the vertebra. The vBMD of each cynomolgus monkey was the average vBMD of L2-4 or of the three adjacent vertebrae.
QCT measurements of body components
Abdominal adipose tissue was measured at the L4-5 intervertebral disc level. The QCT Pro software was used to automatically color the adipose tissue based on the CT value. Anatomical positions in cynomolgus monkeys differ from those in humans. The selected slice may include the limbs, which required manual adjustment of the closed spline to remove non-abdominal tissues, after which the data were recorded as the total adipose tissue (TAT). The closed spline was adjusted to the external edge of the abdominal muscles to distinguish VAT from SAT, and the data within the closed spline were recorded as the VAT. SAT values were obtained by subtracting the VAT from the TAT.
The fascial boundaries of the LM of the paravertebral muscles (including the bilateral psoas major and erector spinae muscles) were manually traced and segmented from the image at the L3 mid-plane level. Voxels with density values in the muscle tissue range were defined as the LM (23). Some animals had low abdominal adipose tissue levels, and the contours of the paravertebral muscles could not be clearly displayed and were therefore outlined based on the CT images.
Precision of QCT measurements
The ISCD recommends a degree of freedom of 30 for precision studies, so the scanning methods include scanning 4 times each of 10 individuals, or 3 times each of 15 individuals, or twice each of 30 individuals (34,35). We selected the last scanning method in order to reduce the radiation exposure of female cynomolgus monkeys. Older female cynomolgus monkeys in the group aged ≥20 years old may experience osteoporosis and are not suitable for precision study (36). Due to the differences in lumbar vBMD in female cynomolgus monkeys of different ages, we used stratified random sampling method to randomly select 10 animals among those aged ≤10 years and 20 animals among those aged 11–19 years, adding up to a total of 30 animals for precision study. Two animals in the middle-aged group were excluded from the precision study due to an uneven increase in bone density of any lumbar vertebra on QCT images. Therefore, we randomly selected two more animals among those aged 11–19 years. Finally, there were 30 female cynomolgus monkeys included in the short-term in vivo precision study, consisting of seven from the juvenile group (1–4 years old), three from the young group (5–10 years old), and 20 from the middle-aged group (11–19 years old). All intact animals were scanned twice under the same conditions within 10 min with repositioning before the second scan.
A well-trained investigator measured the vBMD of the seven vertebrae in L1-7. The vBMD of each vertebra was recorded separately, and the average vBMD of the combination of two and three adjacent lumbar vertebrae was calculated. TAT, VAT, and SAT levels were measured at each intervertebral disc level from L1-2 to L6-7 in each cynomolgus monkey. The LM of the paravertebral muscles was measured in the mid-plane of each vertebra from L2 to L6. All the QCT measurements above were performed by the same investigator on the same day. In order to control the potential measurement bias from non-blinding, the investigator performed the QCT measurements strictly following the pre-defined criteria, as mentioned in the QCT measurements of lumbar vBMD and QCT measurements of body components above. The QCT images of the two scans were measured and recorded separately. The investigator measured the QCT images of the 1st scan first and recorded the results, while measuring the images of the 2nd scan, the investigator was not allowed to read the results of the first recording.
The root-mean-square standard deviation (RMS-SD) and root-mean-square coefficient of variation (RMS-CV%) are generally used to express short-term precision:
In Eqs. [1] and [2], m is the number of animals, SD is the standard deviation, and CV% is the percent coefficient of variation.
Once the precision of the measurement at a site is determined, the least significant change (LSC) representing the real biological change at that site can be calculated. Generally, an 80% confidence interval is adequate for clinical purposes, even though a 95% confidence interval is ideal. The formula for the LSC is as follows:
where Z' is the statistical confidence of the desired level obtained from tables or mathematical statistical texts, Pr is the precision value (RMS-SD or RMS-CV%), n1 is the number of measurements at baseline, and n2 is the number of measurements at follow-up. In general, one measurement is performed for both baseline and follow-up (34).
Statistical analysis
All data were analyzed using the SPSS software (version 26.0) and R software (version 4.2.1). For normally distributed variables, values are presented as the mean ± standard deviation (SD), and for non-normally distributed variables, values are presented as the median and interquartile range. For comparisons between groups, one-way ANOVA was used for normally distributed variables, and the Kruskal–Wallis H test was used for non-normally distributed variables. By evaluating and comparing different models such as linear, logarithmic, quadratic, cubic, compound, power, growth, and exponential, we found that the cubic regression model was the best for describing age-related changes in vBMD and body components. The mean value of peak vBMD and accumulated bone loss rates (ABLR) were calculated. ABLR was computed using the following formula: (mean vBMD – mean peak vBMD)/mean peak vBMD × 100%. The correlations between vBMD and body components were analyzed using Spearman rank correlation. We found strong correlations between some of the variables and performed collinearity diagnostics, which revealed the existence of multicollinearity. Finally, we used ridge regression instead of multiple linear regression in order to make the regression coefficients more stable. Ridge regression analysis was applied to evaluate the degree of contribution of each factor to vBMD using vBMD as the dependent variable, whereas age, LM, VAT, and SAT were independent variables, adjusted for BMI. Differences were considered statistically significant at P<0.05. The precision of the vBMD and body components was expressed using RMS-SD or RMS-CV%, and the LSCs with 80% and 95% confidence intervals were calculated, respectively, as shown in the above formula.
Results
Characteristics of lumbar vBMD and body composition
A total of 72 healthy female cynomolgus monkeys, with an age range of 1–25 years and a weight range of 2.0–9.1 kg, were included in this study and divided into four groups according to age (Table 1). There were statistically significant differences in the average lumbar vBMD between the juvenile and young groups, young and older groups, and middle-aged and older groups (P<0.05). The differences in BMI, LM, TAT, VAT, and SAT were statistically significant (P<0.05) between the juvenile and young groups, juvenile and middle-aged groups, and juvenile and older groups. In addition, the LM of the older group was significantly different from that of the young group (P<0.05), and the SAT of the older group was significantly different from that of the middle-age group (P<0.05).
Table 1
| Variables | Juvenile (≤4 years) | Young (5–10 years) | Middle-aged (11–19 years) | Older (≥20 years) |
|---|---|---|---|---|
| Number of animals | 16 | 19 | 24 | 13 |
| Age (years) | 1.5 (1.0–4.0) | 9.0 (7.0–10.0) | 14.0 (13.0–15.0) | 22.0 (21.0–24.0) |
| BMI (kg/m2) | 22.14±6.75 | 34.41±9.08a | 33.17±6.23a | 30.18±8.31a |
| Average vBMD (mg/cm3) | 382.17±31.02 | 433.08±51.44a | 402.93±47.53 | 337.14±61.94bc |
| LM (g) | 1.21±0.26 | 1.72±0.26a | 1.65±0.28a | 1.50±0.23ab |
| TAT (g) | 0.85 (0.80–1.35) | 9.50 (4.10–11.70)a | 10.10 (5.98–11.90)a | 8.50 (4.95–9.90)a |
| VAT (g) | 0.80 (0.60–1.05) | 6.60 (3.20–8.50)a | 7.05 (4.55–8.10)a | 6.80 (4.00–7.90)a |
| SAT (g) | 0.20 (0.10–0.30) | 2.20 (1.50–3.20)a | 3.10 (1.45–3.50)a | 1.50 (0.65–2.15)ac |
Values are presented as the mean ± standard deviation (SD) for normally distributed variables and as the median and interquartile range (IQR) for non-normally distributed variables. a, P<0.05 versus juvenile group; b, P<0.05 versus young group; c, P<0.05 versus middle-aged group. BMI, body mass index; vBMD, volumetric bone mineral density; LM, lean mass; TAT, total adipose tissue; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue.
Age-related changes in the vBMD and body composition
Figures 1,2 demonstrate the changes in the average lumbar vBMD and body components associated with age in healthy female cynomolgus monkeys. The average vBMD of the lumbar spine tended to increase with age until the age of 10 years, reaching a peak bone mass at approximately 10 years, with bone mass plateauing between 8 and 12 years of age. After the age of 13 years, the average lumbar vBMD showed a decreasing trend with age (Figure 1). From the scatter plot, a few female cynomolgus monkeys had relatively high vBMD after reaching peak bone mass, which may be due to the individual differences, so we mainly described the result of the fitted curve of 72 monkeys above.
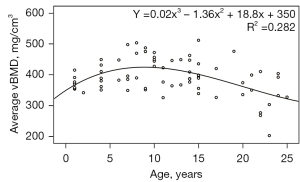
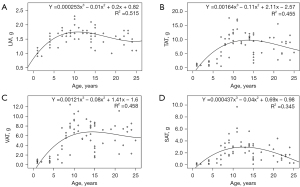
The LM of the paravertebral muscles increased with age until the age of 10 years, decreased mildly with age from 11 to 15 years, and decreased more rapidly with age after 15 years (Figure 2A). With regard to abdominal adipose tissue, TAT, VAT, and SAT levels showed a rapid increase before the age of 10 years, stabilized around the ages of 11–15 years, and showed a decreasing trend after the age of 15 years (Figure 2B-2D).
ABLR in female cynomolgus monkeys
As shown in Figure 1, the bone mass of female cynomolgus monkeys was at a high level between approximately 8 and 12 years of age, and the mean value of their lumbar vBMD was calculated. After reaching the peak bone mass, the lumbar vBMD of female cynomolgus monkeys tended to decrease with age. Female cynomolgus monkeys were divided into premenopausal (13–19 years old) and postmenopausal groups (20–25 years old) according to the age of menopause, and their ABLRs were calculated separately, as shown in Table 2.
Table 2
| Age (years) | Number of animals | vBMD (mg/cm3) | ABLR (%) |
|---|---|---|---|
| 8–12 | 18 | 428.05±53.75 | – |
| 13–19 | 19 | 406.95±47.89 | –4.9 |
| 20–25 | 13 | 337.14±61.94 | –21.2 |
vBMD, volumetric bone mineral density, presented as mean ± standard deviation; ABLR, accumulated bone loss rate; ABLR = (mean vBMD – mean peak vBMD)/mean peak vBMD × 100%.
Spearman correlation analysis of the average lumbar vBMD and body components
According to the age at peak bone mass, the female cynomolgus monkeys were divided into a pre-peak bone mass group and a post-peak bone mass group with the age of 10 years as the cut-off. In the group aged ≤10 years, the average lumbar vBMD was positively correlated with age, BMI, LM, VAT, and SAT. In the group aged >10 years, the average lumbar vBMD was positively correlated with BMI and SAT, while it was negatively correlated with age (Table 3).
Table 3
| Variables | Pre-peak bone mass group (≤10 years; n=35) | Post-peak bone mass group (>10 years; n=37) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| vBMD | VAT | SAT | LM | BMI | vBMD | VAT | SAT | LM | BMI | ||
| VAT | 0.508** | 0.317 | |||||||||
| SAT | 0.617** | 0.929** | 0.362* | 0.657** | |||||||
| LM | 0.519** | 0.674** | 0.589** | −0.053 | 0.193 | 0.248 | |||||
| BMI | 0.412* | 0.821** | 0.800** | 0.590** | 0.377* | 0.691** | 0.562** | 0.231 | |||
| Age | 0.534** | 0.826** | 0.781** | 0.813** | 0.756** | −0.345* | −0.218 | −0.611** | −0.405* | −0.379* | |
*P<0.05; **P<0.01. vBMD, volumetric bone mineral density; BMI, body mass index; LM, lean mass; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue.
Ridge regression analysis of average lumbar vBMD and body components
Table 4 shows the results of the ridge regression analysis with the average lumbar vBMD as the dependent variable and age, LM, VAT, and SAT as independent variables, adjusted for BMI. In the group aged ≤10 years, age and SAT had a positive contribution to the average lumbar vBMD, and in the group aged >10 years, only age showed an independent negative influence on vBMD. The LM and VAT were not significantly correlated with the average lumbar vBMD in the ridge regression analysis in either group.
Table 4
| Variables | Average vBMD (≤10 years; n=35) | Average vBMD (>10 years; n=37) | |||||
|---|---|---|---|---|---|---|---|
| Standardized β | t | P value | Standardized β | t | P value | ||
| Age | 0.229 | 2.205 | 0.028 | −0.218 | 2.869 | 0.004 | |
| LM | 0.209 | 1.902 | 0.057 | −0.093 | 1.165 | 0.244 | |
| VAT | −0.142 | 1.479 | 0.139 | 0.055 | 0.768 | 0.442 | |
| SAT | 0.251 | 2.537 | 0.011 | 0.056 | 0.760 | 0.447 | |
Ridge parameter was 0.312 for the group aged ≤10 years and 0.841 for the group age >10 years, which were chosen automatically by the R software. vBMD, volumetric bone mineral density; BMI, body mass index; LM, lean mass; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue.
Short-term in vivo precision of QCT measurements of lumbar vBMD
The RMS-CV% and RMS-SD of lumbar vBMD for repeated QCT measurements of a single vertebra and of the combination of 2 and 3 adjacent vertebrae ranged from 0.47% to 1.60% and 1.91 to 6.31 mg/cm3, respectively. In addition, the LSCs with 80% and 95% confidence intervals for the above measurement sites are provided in Table 5.
Table 5
| Lumbar vertebrae | Average vBMD (mg/cm3) | RMS-CV% (%) | RMS-SD (mg/cm3) | LSC80 | LSC95 | |||
|---|---|---|---|---|---|---|---|---|
| RMS-CV% (%) | RMS-SD (mg/cm3) | RMS-CV% (%) | RMS-SD (mg/cm3) | |||||
| L1 | 418.38±41.18 | 0.75 | 3.11 | 1.35 | 5.64 | 2.06 | 8.63 | |
| L2 | 411.30±45.17 | 0.72 | 2.97 | 1.31 | 5.37 | 2.00 | 8.22 | |
| L3 | 402.40±44.88 | 1.09 | 4.24 | 1.97 | 7.68 | 3.01 | 11.75 | |
| L4 | 393.71±44.40 | 1.08 | 4.21 | 1.95 | 7.61 | 2.99 | 11.65 | |
| L5 | 390.41±48.82 | 1.60 | 6.31 | 2.89 | 11.42 | 4.25 | 17.48 | |
| L6 | 391.12±49.48 | 1.51 | 5.91 | 2.73 | 10.69 | 4.18 | 16.36 | |
| L7 | 395.70±48.48 | 0.94 | 3.64 | 1.71 | 6.59 | 2.62 | 10.08 | |
| L1-2 | 414.84±42.72 | 0.50 | 2.08 | 0.90 | 3.76 | 1.38 | 5.75 | |
| L2-3 | 406.85±44.52 | 0.61 | 2.39 | 1.10 | 4.33 | 1.68 | 6.62 | |
| L3-4 | 398.06±44.22 | 0.80 | 3.09 | 1.45 | 5.59 | 2.21 | 8.56 | |
| L4-5 | 392.06±46.08 | 0.93 | 3.72 | 1.68 | 6.73 | 2.58 | 10.29 | |
| L5-6 | 390.77±48.86 | 1.15 | 4.53 | 2.09 | 8.20 | 3.19 | 12.55 | |
| L6-7 | 393.41±48.04 | 0.94 | 3.77 | 1.70 | 6.82 | 2.60 | 10.44 | |
| L1-3 | 410.69±43.03 | 0.47 | 1.91 | 0.84 | 3.45 | 1.29 | 5.28 | |
| L2-4 | 402.47±44.11 | 0.58 | 2.26 | 1.05 | 4.09 | 1.60 | 6.26 | |
| L3-5 | 395.50±45.34 | 0.82 | 3.23 | 1.48 | 5.84 | 2.27 | 8.92 | |
| L4-6 | 391.74±46.85 | 0.84 | 3.32 | 1.52 | 6.01 | 2.32 | 9.19 | |
| L5-7 | 392.41±47.72 | 0.85 | 3.38 | 1.53 | 6.11 | 2.34 | 9.36 | |
QCT, quantitative computed tomography; vBMD, volumetric bone mineral density, presented as mean ± standard deviation; RMS-CV%, root-mean-square of coefficient of variation; RMS-SD, root-mean-square of standard deviation; LSC80, least significant changes at 80% confidential intervals; LSC95, least significant changes at 95% confidential intervals.
Short-term in vivo precision of QCT measurements of body components
Table 6 shows the RMS-CV% (RMS-SD) for the repeated QCT measurements of LM of the paravertebral muscles, TAT, VAT, and SAT at different levels, as well as the corresponding LSCs at 80% and 95% confidence intervals.
Table 6
| Anatomic slices | RMS-CV% (%) | RMS-SD (g) | LSC80 | LSC95 | |||
|---|---|---|---|---|---|---|---|
| RMS-CV% (%) | RMS-SD (g) | RMS-CV% (%) | RMS-SD (g) | ||||
| L2–LM | 5.40 | 0.08 | 9.77 | 0.15 | 14.95 | 0.23 | |
| L3–LM | 2.47 | 0.04 | 4.47 | 0.07 | 6.84 | 0.11 | |
| L4–LM | 2.78 | 0.05 | 5.03 | 0.09 | 7.70 | 0.13 | |
| L5–LM | 3.90 | 0.07 | 7.07 | 0.12 | 10.81 | 0.18 | |
| L6–LM | 4.21 | 0.07 | 7.62 | 0.13 | 11.66 | 0.20 | |
| L1/2 | |||||||
| TAT | 6.31 | 0.17 | 11.43 | 0.31 | 17.49 | 0.47 | |
| VAT | 7.18 | 0.18 | 13.00 | 0.32 | 19.89 | 0.50 | |
| SAT | 2.94 | 0.05 | 5.32 | 0.09 | 14.73 | 0.14 | |
| L2/3 | |||||||
| TAT | 5.22 | 0.09 | 9.81 | 0.16 | 14.46 | 0.25 | |
| VAT | 6.75 | 0.17 | 12.22 | 0.31 | 18.70 | 0.47 | |
| SAT | 16.78 | 0.13 | 30.37 | 0.24 | 46.48 | 0.37 | |
| L3/4 | |||||||
| TAT | 3.81 | 0.09 | 6.90 | 0.16 | 10.57 | 0.25 | |
| VAT | 6.11 | 0.14 | 11.07 | 0.26 | 16.93 | 0.40 | |
| SAT | 10.54 | 0.09 | 19.08 | 0.16 | 29.20 | 0.24 | |
| L4/5 | |||||||
| TAT | 3.18 | 0.13 | 5.76 | 0.23 | 8.82 | 0.36 | |
| VAT | 3.86 | 0.14 | 6.99 | 0.26 | 10.69 | 0.40 | |
| SAT | 8.71 | 0.10 | 15.76 | 0.19 | 24.12 | 0.29 | |
| L5/6 | |||||||
| TAT | 3.44 | 0.10 | 6.23 | 0.18 | 9.53 | 0.28 | |
| VAT | 3.45 | 0.11 | 6.24 | 0.20 | 9.55 | 0.30 | |
| SAT | 6.58 | 0.07 | 11.92 | 0.12 | 18.24 | 0.19 | |
| L6/7 | |||||||
| TAT | 4.98 | 0.10 | 9.02 | 0.18 | 13.81 | 0.28 | |
| VAT | 7.67 | 0.09 | 13.88 | 0.16 | 21.24 | 0.25 | |
| SAT | 8.22 | 0.07 | 14.89 | 0.13 | 22.78 | 0.20 | |
QCT, quantitative computed tomography; LM, lean mass; TAT, total adipose tissue; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; RMS-CV%, root-mean-square of coefficient of variation; RMS-SD, root-mean-square of standard deviation; LSC80, least significant changes at 80% confidential intervals; LSC95, least significant changes at 95% confidential intervals.
Discussion
Female nonhuman primates experience menarche at approximately 2.5–3 years of age, with regular menstrual cycles at around 4 years of age, and experience natural menopause at approximately 20 years of age (32). Accordingly, we divided them into juvenile, young, middle-aged and older groups. The time of epiphyseal closure of different skeletons varies in cynomolgus monkeys, with their long bones closing at about 5–6.5 years of age (37), while the epiphyses of the spine close at least 15 years of age (38). There has still existed confusion as to which skeletal epiphyseal closure should be used as a marker of bone maturity. X-ray radiography was commonly used to determine the closure of the epiphyses (37), while we only performed QCT in this study. There were differences in the determination of epiphyseal closure by different devices, leading to differences in the determination of skeletal maturity age. Therefore, we grouped monkeys according to their reproductive age rather than their skeletal maturity age. Our study showed that the young group had the highest average vBMD of the lumbar spine, followed by the middle-aged and juvenile groups, and the older group had the lowest average vBMD. There were some differences in lumbar vBMD between the different age groups in our study, consistent with the process of growth and physiological changes in healthy female cynomolgus monkeys. Our study showed that female cynomolgus monkeys in the postmenopausal group had higher ABLR than the premenopausal group, suggesting that postmenopausal animals lose bone mass more rapidly. Colman et al. [1999] also showed that the aBMD of the lumbar spine measured using DXA was higher in adult premenopausal female rhesus monkeys than in growing and postmenopausal animals (17).
In the ridge regression analysis, our study revealed that age contributed positively to the average lumbar vBMD in the group aged ≤10 years, while it contributed negatively to vBMD in the group aged >10 years. Our study showed that the age at peak bone mass in healthy female cynomolgus monkeys was approximately 10 years. Studies based on DXA have reported an age at peak bone mass of 9 years in female cynomolgus monkeys (21,39), and Champ et al. [1996] reported an age at peak bone mass of 11 years in female rhesus monkeys (10). The results from our study were similar to those reported in the above studies. Hence, we recommend using cynomolgus monkeys aged no less than 10 years for osteoporosis-related studies based on QCT. The average lumbar vBMD of female cynomolgus monkeys decreased significantly after 13 years of age in our study. As reported by Jayo [1994], skeletally mature monkeys showed a decreasing trend in bone mass with age after reaching peak bone mass (21), which was consistent with the results of the present study. However, after reaching peak bone mass, some studies based on DXA have shown that the bone mass of the lumbar spine in nonhuman primates remains relatively stable with age (10,39), which may be due to the increased susceptibility of older monkeys to osteoarthritis, leading to overestimation of the bone mass of the lumbar spine (17). Since DXA measures aBMD, it may not always reflect the true bone strength of animals or patients with osteochondrosis or osteoarthritis. The vBMD of the trabecular bone measured by QCT reflects more accurately bone mass and is less likely to be affected by osteoarthritis (12). In our study, lumbar vBMD showed a decreasing trend with age after reaching peak bone mass, which was consistent with the findings in female humans (23). However, nonhuman primates are distinguished from humans by their diverse positional modes, including tripedal walking, quadrupedal walking and so on (40). Different postural or locomotor habits may affect the skeleton development of monkeys and thus indirectly affect lumbar vBMD. The impact of these factors should be taken into account when using monkeys as animal models. Currently, there is a lack of QCT-based diagnostic criteria for osteoporosis in female cynomolgus monkeys. Our study calculated the mean peak vBMD and ABLRs in female cynomolgus monkeys, and the data provided in this study may be used to determine whether the osteoporosis model in cynomolgus monkey was successfully established and to provide reference information for screening suitable animal models.
In our study, the TAT, VAT, and SAT of female cynomolgus monkeys increased with age before the age of 10 years, while after the age of 15 years, they showed a decreasing trend. Ng et al. [2013] reported that in women, total body fat measured by DXA, VAT and STA measured by QCT increased with age until approximately 70 years, after which it decreased (41), showing a similar trend to our study in female cynomolgus monkeys. LM increased in female cynomolgus monkeys with age until the age of 10 years and decreased rapidly with age after the age of 15 years. Colman et al. [2005] showed that whole-body LM measured by DXA reached a peak at approximately 14–15 years of age, after which it declined significantly with age in female rhesus monkeys (42). Although there were some differences between the whole-body LM measured by DXA and LM of paravertebral muscles measured by QCT, they exhibited similar characteristics regarding the changes associated with age. Our study describes age-related characteristics of the LM of paravertebral muscles, which may provide valuable information for sarcopenia-related studies.
The relationship between lumbar vBMD and abdominal adipose tissue remains controversial. Our study revealed that SAT had a positive effect on the average lumbar vBMD in the group aged ≤10 years, adjusted for BMI. Zhang et al. [2019] also showed a positive contribution of SAT to lumbar vBMD in women (23). Leptin is produced by SAT and affects bone metabolism and promotes bone formation (43). SAT may exert mechanical stress on the bone and therefore has a positive effect on BMD (26). However, some studies have shown no correlation between the SAT and the average lumbar vBMD in women (24-26). The reasons for this discrepancy may be due to differences in the sample size or the different groupings of the studies. Our study demonstrated that VAT was positively associated with the average lumbar vBMD in the Spearman correlation analysis, whereas it had no effect on vBMD in the ridge regression analysis in the group aged ≤10 years adjusted for BMI. This may be due to factors such as the BMI, which may confound the effect of VAT on the vBMD. Similarly, previous human studies showed that VAT was not correlated with lumbar vBMD (23,25). However, studies have shown that VAT has a negative effect on lumbar vBMD in women, and that increased VAT may be detrimental to the maintenance of bone mass (24,26). This may be due to the secretion of inflammatory mediators and adipokines by VAT, which affects the health of bones (44). These results suggest that the effects of VAT on vBMD may be complicated, and some differences may exist between humans and nonhuman primates. The correlation between adipose tissue and lumbar vBMD in nonhuman primates reported in this study needs to be further confirmed.
A study has shown that LM contributes to BMD and that an increase in LM is beneficial for maintaining bone mass (27). Muscles may produce osteocyte viability factors that exert a protective effect on osteocytes. The mechanical view implies that a decrease in muscle function leads to a reduction in skeletal load and consequently to a reduction in bone mass (45). Wagner et al. [2018] also showed that men with low LM may have accelerated bone deterioration, whereas those with higher LM and muscle strength had less deterioration of the bone microarchitecture (46). In female cynomolgus monkeys, our study showed no contribution of LM of the paravertebral muscles to the average lumbar vBMD in ridge regression analysis. This might be due to the fact that the postural or locomotor habits of primates are different from those of humans, and therefore the effect of LM on vBMD in primates may not be consistent with that of humans. Very few studies have reported similar issues, and more research is needed to elucidate these issues further.
This is the first study to report the short-term in vivo precision of QCT measurements of the lumbar vBMD and body composition in female cynomolgus monkeys. Short-term precision reflects the reproducibility of the measurement technique and allows for the calculation of the LSC, which can be used for efficacy assessment and estimation of the follow-up interval. Precision study is statistically required to have a degree of freedom of no less than 30 (34), and the ISCD recommends that new QCT techniques establish in vivo precision levels (12). Previous studies using QCT-based techniques to analyze the precision of BMD in nonhuman primates did not meet the above requirements (28-30), whereas our study met the relevant requirements and the results were reliable. For the short-term in vivo precision of our study, the RMS-CV% of single lumbar vBMD measured by QCT ranged from 0.72% to 1.60%, among which the precision of the L2 vertebra was the best. The RMS-CV% of the vBMD for the two-and three-lumbar combinations ranged from 0.50% to 1.15% and 0.47% to 0.85%, respectively. The precision of the average vBMD of the three adjacent lumbar combinations was relatively better than that of the two adjacent lumbar combinations, with the best combination of precision being for the L1-3 vertebrae. We selected the L2-4 vertebrae for vBMD measurements, as these sites also showed good precision. Wang et al. [2017] used asynchronous QCT in the European Spine Phantom to determine the RMS-CV%, which ranged from 0.2% to 0.7% between two scans, and the RMS-CV%, which ranged from 2.2% to 2.6% between observers (47). The precision of QCT in measuring human spinal trabecular BMD has been reported to be 1.3–2.4% (12). Another study reported that the precision error of QCT measurements of spinal trabecular bone was 2–4% (48). Bligh et al. [2009] showed that the precision of helical multidetector-row QCT ranged from 1.4% to 3.6% (49). Regarding the precision of lumbar vBMD in female cynomolgus monkeys, the RMS-CV% of all measurements in our study was <2%, which is generally good and roughly consistent with that reported above. Therefore, QCT can be used for measuring lumbar vBMD in monkeys and accurately reflects their bone mass. In studies based on pQCT in nonhuman primates, Hotchkiss [1999] reported a precision <3% for the measurement of lumbar BMD in cynomolgus monkeys (28).
The present study showed that the RMS-CV% of the LM of paravertebral muscles measured by QCT in cynomolgus monkeys ranged between 2.47% and 5.40%, with the best precision in the L3 mid-plane, which was also the level analyzed in this study. The precision of LM measured in nonhuman primates using QCT has not been reported, and the precision results of our study may provide reference information for future related studies. This study showed that the RMS-CV% was <6.4% for TAT and <7.7% for VAT, and greater variability in the precision for SAT, ranging from 2.94% to 16.78%. The precision at the L4-5 intervertebral disc level was comparatively superior among the measurement levels; therefore, we selected this level to measure abdominal adipose tissue. Baum et al. [2012] showed that QCT had a reproducibility error of 0.12% for measuring SAT levels and 3.74% for measuring VAT levels in humans (50). This discrepancy may arise due to differences in measurement software and the fact that the above study measured the volume of abdominal adipose tissue, whereas our study measured the mass of abdominal adipose tissue. Contrarily, the small size of cynomolgus monkeys, the low levels of abdominal adipose tissue in some animals, and the very few animals in which SAT could not be measured may also account for the large variation in precision. In addition, since SAT levels in cynomolgus monkeys are significantly lower than those of TAT and VAT, it is more significantly influenced by respiratory movements, and the error of measurement is greater, resulting in a greater error of precision.
Our study had the following limitations. First, although the present study included female cynomolgus monkeys of different ages ranging from 1 to 25 years, the number of animals was unevenly distributed at each year of age, and the results may have been more conclusive if the number of animals was ≥5 per year of age. Second, due to differences in measurement techniques, our study could only analyze the correlation between lumbar vBMD and body components at the individual level based on QCT, and we could not confirm whether there was a correlation between lumbar vBMD and whole-body fat mass or between lumbar vBMD and whole-body LM. Third, long-term precision is crucial for efficacy monitoring and follow-up; however, due to limited experimental conditions, long-term precision studies were not performed in this study, and relevant data could not be obtained.
Conclusions
In conclusion, age-related changes in lumbar vBMD in healthy female cynomolgus monkeys based on QCT were similar to those in humans. Our study also described the age-related characteristics of body components in healthy female cynomolgus monkeys. Age and SAT levels are factors that may influence the lumbar vBMD in female cynomolgus monkeys. QCT showed good in vivo precision for measuring the vBMD of the lumbar spine in female cynomolgus monkeys.
Acknowledgments
We thank Guangdong Landau Biotechnology Co. Ltd. for providing female cynomolgus monkeys for this study and their veterinarians for their full cooperation during the study to ensure the smooth running of the experiments.
Funding: This study was supported by the National Natural Science Foundation of China (No. 81871383).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-763/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-763/coif). All authors reported that this work was supported by the National Natural Science Foundation of China (No. 81871383). Yuefeng Li is the head of Research and Development Department in Guangdong Landau Biotechnology Co. Ltd. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. LDIACUC2018-0004) granted by the Laboratory Animal Ethics Committee of Guangdong Landau Biotechnology Co., Ltd., in compliance with international Association for Assessment and Accreditation of Laboratory Animal Care guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Consensus development conference. diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 1993;94:646-50. [Crossref] [PubMed]
- Xiao PL, Cui AY, Hsu CJ, Peng R, Jiang N, Xu XH, Ma YG, Liu D, Lu HD. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: a systematic review and meta-analysis. Osteoporos Int 2022;33:2137-53. [Crossref] [PubMed]
- Cheng X, Zhao K, Zha X, Du X, Li Y, Chen S, et al. Opportunistic Screening Using Low-Dose CT and the Prevalence of Osteoporosis in China: A Nationwide, Multicenter Study. J Bone Miner Res 2021;36:427-35. [Crossref] [PubMed]
- Si L, Winzenberg TM, Jiang Q, Chen M, Palmer AJ. Projection of osteoporosis-related fractures and costs in China: 2010-2050. Osteoporos Int 2015;26:1929-37. [Crossref] [PubMed]
- Smith SY, Jolette J, Turner CH. Skeletal health: primate model of postmenopausal osteoporosis. Am J Primatol 2009;71:752-65. [Crossref] [PubMed]
- Doyle N, Varela A, Haile S, Guldberg R, Kostenuik PJ, Ominsky MS, Smith SY, Hattersley G. Abaloparatide, a novel PTH receptor agonist, increased bone mass and strength in ovariectomized cynomolgus monkeys by increasing bone formation without increasing bone resorption. Osteoporos Int 2018;29:685-97. [Crossref] [PubMed]
- Fujihara R, Mashiba T, Yoshitake S, Komatsubara S, Iwata K, Takao-Kawabata R, Yamamoto T. Weekly teriparatide treatment increases vertebral body strength by improving cortical shell architecture in ovariectomized cynomolgus monkeys. Bone 2019;121:80-8. [Crossref] [PubMed]
- Pezzuti IL, Kakehasi AM, Filgueiras MT, de Guimarães JA, de Lacerda IAC, Silva IN. Imaging methods for bone mass evaluation during childhood and adolescence: an update. J Pediatr Endocrinol Metab 2017;30:485-97. [Crossref] [PubMed]
- Cheng X, Yuan H, Cheng J, Weng X, Xu H, Gao J, Huang M, Wáng YXJ, Wu Y, Xu W, Liu L, Liu H, Huang C, Jin Z, Tian W. Chinese expert consensus on the diagnosis of osteoporosis by imaging and bone mineral density. Quant Imaging Med Surg 2020;10:2066-77. [Crossref] [PubMed]
- Champ JE, Binkley N, Havighurst T, Colman RJ, Kemnitz JW, Roecker EB. The effect of advancing age on bone mineral content of female rhesus monkeys. Bone 1996;19:485-92. [Crossref] [PubMed]
- Jerome CP, Johnson CS, Lees CJ. Effect of treatment for 3 months with human parathyroid hormone 1-34 peptide in ovariectomized cynomolgus monkeys (Macaca fascicularis). Bone 1995;17:415S-20S. [Crossref] [PubMed]
- Engelke K, Adams JE, Armbrecht G, Augat P, Bogado CE, Bouxsein ML, Felsenberg D, Ito M, Prevrhal S, Hans DB, Lewiecki EM. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: the 2007 ISCD Official Positions. J Clin Densitom 2008;11:123-62. [Crossref] [PubMed]
- Mao SS, Li D, Syed YS, Gao Y, Luo Y, Flores F, Child J, Cervantes M, Kalantar-Zadeh K, Budoff MJ. Thoracic Quantitative Computed Tomography (QCT) Can Sensitively Monitor Bone Mineral Metabolism: Comparison of Thoracic QCT vs Lumbar QCT and Dual-energy X-ray Absorptiometry in Detection of Age-relative Change in Bone Mineral Density. Acad Radiol 2017;24:1582-7. [Crossref] [PubMed]
- Yuan Y, Zhang P, Tian W, Deng X, Yue R, Ge X, Li X. Application of bone turnover markers and DXA and QCT in an elderly Chinese male population. Ann Palliat Med 2021;10:6351-8. [Crossref] [PubMed]
- Li N, Li XM, Xu L, Sun WJ, Cheng XG, Tian W. Comparison of QCT and DXA: Osteoporosis Detection Rates in Postmenopausal Women. Int J Endocrinol 2013;2013:895474. [Crossref] [PubMed]
- Adams JE, Engelke K, Zemel BS, Ward KA. Quantitative computer tomography in children and adolescents: the 2013 ISCD Pediatric Official Positions. J Clin Densitom 2014;17:258-74. [Crossref] [PubMed]
- Colman RJ, Kemnitz JW, Lane MA, Abbott DH, Binkley N. Skeletal effects of aging and menopausal status in female rhesus macaques. J Clin Endocrinol Metab 1999;84:4144-8. [Crossref] [PubMed]
- Boyce RW, Varela A, Chouinard L, Bussiere JL, Chellman GJ, Ominsky MS, Pyrah IT. Infant cynomolgus monkeys exposed to denosumab in utero exhibit an osteoclast-poor osteopetrotic-like skeletal phenotype at birth and in the early postnatal period. Bone 2014;64:314-25. [Crossref] [PubMed]
- Yamada H, Ochi Y, Mori H, Nishikawa S, Hashimoto Y, Tanaka M, Deacon S, Kawabata K. Cortical bone mineral density is increased by the cathepsin K inhibitor ONO-5334, which leads to a robust increase in bone strength: results from a 16-month study in ovariectomised cynomolgus monkeys. J Bone Miner Metab 2019;37:636-47. [Crossref] [PubMed]
- Sato M, Westmore M, Clendenon J, Smith S, Hannum B, Zeng GQ, Brommage R, Turner CH. Three-dimensional modeling of the effects of parathyroid hormone on bone distribution in lumbar vertebrae of ovariectomized cynomolgus macaques. Osteoporos Int 2000;11:871-80. [Crossref] [PubMed]
- Jayo MJ, Jerome CP, Lees CJ, Rankin SE, Weaver DS. Bone mass in female cynomolgus macaques: a cross-sectional and longitudinal study by age. Calcif Tissue Int 1994;54:231-6. [Crossref] [PubMed]
- Peng X, Li X, Xu Z, Wang L, Cai W, Yang S, Liao W, Cheng X. Age-related fatty infiltration of lumbar paraspinal muscles: a normative reference database study in 516 Chinese females. Quant Imaging Med Surg 2020;10:1590-601. [Crossref] [PubMed]
- Zhang X, Hua T, Zhu J, Peng K, Yang J, Kang S, Xu T, Hu J, Tang G. Body compositions differently contribute to BMD in different age and gender: a pilot study by QCT. Arch Osteoporos 2019;14:31. [Crossref] [PubMed]
- Zhang L, Ruan X, Ju R, Qin S, Wang B, Dou Z, Xu X, Mueck AO. Lumbar bone mineral density measured by quantitative computed tomography (QCT): association with abdominal adipose tissue in different menopausal periods of Chinese women. Gynecol Endocrinol 2021;37:264-8. [Crossref] [PubMed]
- Zhang W, Ma X, Xue P, Gao Y, Wu X, Zhao J, Wang Y, Li S. Associations between fat distribution and volumetric bone mineral density in Chinese adults. Endocrine 2014;47:862-8. [Crossref] [PubMed]
- Wang L, Wang W, Xu L, Cheng X, Ma Y, Liu D, Guo Z, Su Y, Wang Q. Relation of visceral and subcutaneous adipose tissue to bone mineral density in chinese women. Int J Endocrinol 2013;2013:378632. [Crossref] [PubMed]
- Garvey ME, Shi L, Gona PN, Troped PJ, Camhi SM. Age, Sex, and Race/Ethnicity Associations between Fat Mass and Lean Mass with Bone Mineral Density: NHANES Data. Int J Environ Res Public Health 2021; [Crossref] [PubMed]
- Hotchkiss CE. Use of peripheral quantitative computed tomography for densitometry of the femoral neck and spine in cynomolgus monkeys (Macaca fascicularis). Bone 1999;24:101-7. [Crossref] [PubMed]
- Jayakar RY, Cabal A, Szumiloski J, Sardesai S, Phillips EA, Laib A, Scott BB, Pickarski M, Duong LT, Winkelmann CT, McCracken PJ, Hargreaves R, Hangartner TN, Williams DS. Evaluation of high-resolution peripheral quantitative computed tomography, finite element analysis and biomechanical testing in a pre-clinical model of osteoporosis: a study with odanacatib treatment in the ovariectomized adult rhesus monkey. Bone 2012;50:1379-88. [Crossref] [PubMed]
- Hermsen SA, Larsson S, Arima A, Muneoka A, Ihara T, Sumida H, Fukusato T, Kubota S, Yasuda M, Lind PM. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) affects bone tissue in rhesus monkeys. Toxicology 2008;253:147-52. [Crossref] [PubMed]
- Kavanagh K, Fairbanks LA, Bailey JN, Jorgensen MJ, Wilson M, Zhang L, Rudel LL, Wagner JD. Characterization and heritability of obesity and associated risk factors in vervet monkeys. Obesity (Silver Spring) 2007;15:1666-74. [Crossref] [PubMed]
- Vidal JD. The Impact of Age on the Female Reproductive System. Toxicol Pathol 2017;45:206-15. [Crossref] [PubMed]
- Brown JK, Timm W, Bodeen G, Chason A, Perry M, Vernacchia F, DeJournett R. Asynchronously Calibrated Quantitative Bone Densitometry. J Clin Densitom 2017;20:216-25. [Crossref] [PubMed]
- Bonnick SL, Johnston CC Jr, Kleerekoper M, Lindsay R, Miller P, Sherwood L, Siris E. Importance of precision in bone density measurements. J Clin Densitom 2001;4:105-10. [Crossref] [PubMed]
- Baim S, Wilson CR, Lewiecki EM, Luckey MM, Downs RW Jr, Lentle BC. Precision assessment and radiation safety for dual-energy X-ray absorptiometry: position paper of the International Society for Clinical Densitometry. J Clin Densitom 2005;8:371-8. [Crossref] [PubMed]
- Glüer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int 1995;5:262-70. [Crossref] [PubMed]
- Fukuda S, Cho F, Honjo S. Bone growth aud development of secondary ossification centers of extremities in the cynomolgus monkey (Macaca fascicularis). Jikken Dobutsu 1978;27:387-97. [Crossref] [PubMed]
- Iwata M, Yamamoto W, Shimomoto T, Okada Y, Oosawa S, Miura D, Hara Y. Persistence of vertebral growth plate cartilage in aged cynomolgus monkeys. J Toxicol Pathol 2018;31:151-4. [Crossref] [PubMed]
- Chen Y, Shimizu M, Sato K, Koto M, Tsunemi K, Yoshida T, Yoshikawa Y. Effects of aging on bone mineral content and bone biomarkers in female cynomolgus monkeys. Exp Anim 2000;49:163-70. [Crossref] [PubMed]
- Hunt KD, Cant JGH, Gebo DL, Rose MD, Walker SE, Youlatos D. Standardized descriptions of primate locomotor and postural modes. Primates 1996;37:363-87. [Crossref]
- Ng AC, Melton LJ 3rd, Atkinson EJ, Achenbach SJ, Holets MF, Peterson JM, Khosla S, Drake MT. Relationship of adiposity to bone volumetric density and microstructure in men and women across the adult lifespan. Bone 2013;55:119-25. [Crossref] [PubMed]
- Colman RJ, McKiernan SH, Aiken JM, Weindruch R. Muscle mass loss in Rhesus monkeys: age of onset. Exp Gerontol 2005;40:573-81. [Crossref] [PubMed]
- Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, Hamnvik OP, Koniaris A. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab 2011;301:E567-84. [Crossref] [PubMed]
- Liu CT, Broe KE, Zhou Y, Boyd SK, Cupples LA, Hannan MT, Lim E, McLean RR, Samelson EJ, Bouxsein ML, Kiel DP. Visceral Adipose Tissue Is Associated With Bone Microarchitecture in the Framingham Osteoporosis Study. J Bone Miner Res 2017;32:143-50. [Crossref] [PubMed]
- Brotto M, Bonewald L. Bone and muscle: Interactions beyond mechanical. Bone 2015;80:109-14. [Crossref] [PubMed]
- Wagner P, Chapurlat R, Ecochard R, Szulc P. Low Muscle Strength and Mass Is Associated With the Accelerated Decline of Bone Microarchitecture at the Distal Radius in Older Men: the Prospective STRAMBO Study. J Bone Miner Res 2018;33:1630-40. [Crossref] [PubMed]
- Wang L, Su Y, Wang Q, Duanmu Y, Yang M, Yi C, Cheng X. Validation of asynchronous quantitative bone densitometry of the spine: Accuracy, short-term reproducibility, and a comparison with conventional quantitative computed tomography. Sci Rep 2017;7:6284. [Crossref] [PubMed]
- Genant HK, Engelke K, Fuerst T, Glüer CC, Grampp S, Harris ST, Jergas M, Lang T, Lu Y, Majumdar S, Mathur A, Takada M. Noninvasive assessment of bone mineral and structure: state of the art. J Bone Miner Res 1996;11:707-30. [Crossref] [PubMed]
- Bligh M, Bidaut L, White RA, Murphy WA Jr, Stevens DM, Cody DD. Helical multidetector row quantitative computed tomography (QCT) precision. Acad Radiol 2009;16:150-9. [Crossref] [PubMed]
- Baum T, Yap SP, Karampinos DC, Nardo L, Kuo D, Burghardt AJ, Masharani UB, Schwartz AV, Li X, Link TM. Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? J Magn Reson Imaging 2012;35:117-24. [Crossref] [PubMed]


