
Coexistence of parathyroid adenoma, autonomous functioning thyroid nodule, and papillary thyroid carcinoma: a case description
Introduction
Parathyroid adenoma, autonomous functioning thyroid nodule (AFTN), and papillary thyroid carcinoma (PTC) are 3 common endocrine disorders. However, patients with coexisting parathyroid adenoma, AFTN, and PTC are extremely rare (1). Due to the anatomically close relationship of the thyroid and parathyroid, imaging manifestations of lesions in the thyroid and parathyroid are identical or similar, which increases the difficulty of diagnosis and the likelihood of misdiagnosis and missed diagnosis (2). This report describes the case of a 53-year-old woman with a 20-year history of thyroid nodules and was preoperatively diagnosed with concomitant AFTN and parathyroid adenoma. In addition, an unexpected PTC was revealed intraoperatively. The experience of this case stresses the significance of examination methods and the timely identification of parathyroid adenoma in patients with coexistent thyroid nodules, including benign thyroid nodules and thyroid cancer.
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A 53-year-old woman was admitted to our hospital in April 2022 with a 20-year history of thyroid nodules and 6-month history of mild dyspnea and dysphagia. The patient also had progressive weakness and pain in both lower extremities in the preceding month. She had previously been diagnosed with nodular goiter by ultrasound. As the nodules had increased in size and quantity, and the above symptoms were aggravated, the patient agreed to undertake recommended radiofrequency ablation, which was the reason for her hospitalization. Her family history was unremarkable. Physical examination revealed a severe enlargement of the thyroid, especially in the left lobe. Multiple and moderately tough nodules were palpable. Her cardiological, pulmonary, abdominal, and neurological evaluations were normal.
Thyroid function showed the following results: thyroid-stimulating hormone (TSH) <0.005 mU/L (normal range 0.27–4.20 mU/L); free triiodothyronine (FT3), 8.32 pmol/L (normal range 3.1–6.8 pmol/L); free thyroxine (FT4), 23.5 pmol/L (normal range 12.0–22.0 pmol/L); and thyroglobulin (TG), thyroid peroxidase antibody (TPOAb), and thyroglobulin antibody (TGAb) within normal limits. Parathyroid hormone (PTH) was increased significantly at 1,028 ng/L (normal range 15–65 ng/L), and blood calcium was 3.32 mmol/L (normal range 2.11–2.52 mmol/L), while serum phosphate was decreased at 0.72 mmol/L (0.85–1.51 mmol/L). Liver and kidney functions were normal.
The initial diagnosis was multiple nodular goiter Thyroid Imaging Reporting and Data System (TI-RADS) 3 according to ultrasound. Given the significant increase of PTH and irregularities in serum calcium and phosphorus, technetium-99m-methoxy-isobutyl-isonitrile (99mTc-MIBI) scintigraphy was performed to localize parathyroid lesions. The results showed normal accumulation of radioactivity in the early phase, with radioactivity aggregated in the lower right lobe and the upper left lobe, especially in the mass of the upper left lobe, which was large and irregular. The radiotracer partially subsided in the delayed phase, while the concentration of MIBI in the lower right lobe and the upper left lobe was still clear (Figure 1A,1B). Single-photon emission computed tomography-computed tomography (SPECT-CT) fusion imaging showed elevated activity in the heterogeneous masses, with calcification and low-density foci in the lower right lobe and upper left lobe. The size of the largest lesion was 5.5 cm. CT also revealed a heterogeneous nodule in the lower left lobe without concentration of activity (Figure 1C-1E). In addition, given the multiple nodules and abnormalities in thyroid function, pertechnetate (99mTcO4−) scintigraphy was undertaken the next day to preliminarily evaluate the nature of the nodules. 99mTcO4− planar imaging (Figure 1F) and SPECT-CT fusion imaging (Figure 1G-1I) revealed an enhancement of 99mTcO4− absorption in the nodule of the lower right lobe, with no obvious radioactivity in the whole left lobe or the upper right lobe. In summary, the nodule of the lower right lobe could absorb both MIBI and 99mTcO4−, the mass of the upper left lobe concentrated MIBI only, and the nodule of the lower left lobe failed to take in any kind of radiotracer.
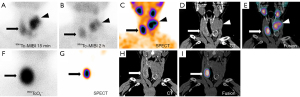
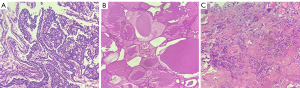
Giant parathyroid tumor with AFTN was suspected based on the imaging findings and the laboratory findings. Therefore, after oral administration of propylthiouracil (PTU) to control thyroid function, we elected to proceed with thyroid and parathyroid surgery after obtaining informed consent. Intraoperative exploration showed that there was diffuse enlargement of bilateral thyroid lobes, with the largest nodule located in the left lobe and measuring 7 cm in diameter. In addition, there was a moderately tough nodule in the lower left lobe which invaded the thyroid capsule and adhered to the anterior wall of the trachea. Multiple enlarged lymph nodes were found in the left central cervical region. Therefore, a total thyroidectomy + left central cervical lymph node dissection + left upper parathyroid adenoma resection was finally selected. The pathology report confirmed that the lesion in the left lobe was a giant parathyroid adenoma (Figure 2A) with an unclear boundary and fibrous hyperplasia measuring about 5.5×4.0×3.0 cm while the nodule in the lower right lobe was a nodular goiter with cystic degeneration and calcification (Figure 2B). Unexpectedly, there was a PTC (Figure 2C) in the lower left lobe (1 cm in diameter), with immunohistochemical (IHC) staining positive for CK19, HBME-1, galectin-3, CD56, BRAF V600E, and Ki-67 (positivity rate about 1%). Metastasis was found in the left central cervical lymph nodes (4/6). Gene detection revealed the BRAF V600E mutation level was 29.52%. In brief, the final diagnosis was parathyroid adenoma with AFTN and PTC.
The patient recovered well after surgery. Her PTH level reached 21.50 ng/L a day after the operation and decreased to 14.00 ng/L on the third postoperative day. Her serum calcium concentration reached 2.24 mmol/L a day after the operation and decreased to 2.07 mmol/L on the third postoperative day. She received levothyroxine, calcitriol, and calcium supplementation daily after surgery. Four weeks after the operation, examination of thyroid function showed FT3 and FT4 were normal, with slightly low TSH. Her PTH level decreased to 54.30 pmol/L, and serum calcium concentration reached 2.12 mmol/L. Four months after the surgery, the patient had no discomfort. Serum thyroxin and calcium levels remained normal.
Discussion
Thyroid disorders occur in about 15–75% of patients with parathyroid diseases (2,3). While hyperparathyroidism (HPT) and concomitant medullary thyroid cancer (MTC) are well described in multiple endocrine neoplasia type 2A (MEN2A) syndrome, no obvious genetic link between PTC and HPT has been demonstrated yet (1). One study reported that the link may be related to a BRAF V600E mutation (4). Other studies (5-7) have found that there may be a rare germline variation in cyclin-dependent kinase inhibitor 1B (CDKN1B) gene. Beebeejaun et al. (8) put forward the hypothesis that elevated PTH, decreased 1,25-dihydroxyvitamin D, and hypercalcemia may lead to high levels of angiogenic factors and promote tumor formation. However, there have been no definite conclusions to date, and a number of scholars believe further study of the relationship between the two disorders is needed (4,9,10). In this case, preoperative examination showed that PTH was significantly elevated, which strongly suggested abnormality of the parathyroid gland. Meanwhile, ultrasound failed to identify parathyroid lesion and thyroid cancer, likely because the parathyroid tumor was too large and had an unclear boundary with thyroid tissue. In addition, the malignant thyroid tumor was relatively occult and concealed by the parathyroid lesion. As a result, it was difficult to distinguish the tissue composition with ultrasound or CT.
Currently, the occurrence of AFTN is believed to be mainly caused by the mutation of thyrotropin receptors and guanylate-stimulating protein subunit α (11,12). 99mTcO4− scanning is an important method to distinguish AFTN and other nodules. Because of its hyperfunction and abundant mitochondria, AFTN can also accumulate MIBI, which can easily result in a false positive in the detection of parathyroid hyperplasia or adenoma. The combination of 99mTcO4− thyroid imaging and MIBI imaging is helpful to identifying this false positive. However, since the uptake of MIBI is closely associated with the extent of tumor malignancy, as an inert tumor, PTC generally presents little or even no uptake of MIBI. PTC in 99mTcO4− imaging is also visualized as a “cold nodule.” This could explain why the PTC of the lower left lobe in this case showed no uptake of either MIBI or 99mTcO4−. The identification of thyroid cancer may need to be combined with other malignant signs, such as surrounding tissue invasion and lymph node metastasis, among others. Finally, the diagnosis of PTC requires intraoperative exploration and pathological confirmation.
With the popularization of routine laboratory tests and thyroid ultrasound examination, the clinical diagnosis of parathyroid adenoma is gradually increasing. Some patients with parathyroid adenoma are first diagnosed with ultrasound as having thyroid nodules. In order to avoid misdiagnosis and missed diagnosis, clinicians should improve their understanding of thyroid and parathyroid diseases, especially the coexistence of both disorders. Moreover, long-term and close multidisciplinary cooperation, including nuclear medicine, radiology, ultrasound, and surgery, should be established. In addition, for patients with thyroid nodules of unknown origin, attention should be given to the laboratory examination of thyroid function, serum calcium, phosphorus, and PTH.
Acknowledgments
All the authors appreciate the collaboration of the patient and her family.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-847/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- He Y, Liu S, Guo H, Shi B. Incidental finding of papillary thyroid carcinoma with BRAFV600E mutation in a patient with coexistent primary hyperparathyroidism and Graves' hyperthyroidism. BMJ Case Rep 2014;2014:bcr2013203436. [Crossref] [PubMed]
- Alajaimi A, Altooq N, Chandran N, Alderazi Y. Synchronous Parathyroid Carcinoma and Noninvasive Follicular Thyroid Neoplasm With Papillary-Like Nuclear Features. Cureus 2022;14:e24006. [Crossref] [PubMed]
- Pickard AL, Gridley G, Mellemkjae L, Johansen C, Kofoed-Enevoldsen A, Cantor KP, Brinton LA. Hyperparathyroidism and subsequent cancer risk in Denmark. Cancer 2002;95:1611-7. [Crossref] [PubMed]
- Mahmoodzadeh H, Harirchi I, Hassan Esfehani M, Alibakhshi A. Papillary thyroid carcinoma associated with parathyroid adenoma. Acta Med Iran 2012;50:353-4. [PubMed]
- Bugalho MJ, Domingues R. Uncommon association of cerebral meningioma, parathyroid adenoma and papillary thyroid carcinoma in a patient harbouring a rare germline variant in the CDKN1B gene. BMJ Case Rep 2016;2016:bcr2015213934. [Crossref] [PubMed]
- Costa-Guda J, Marinoni I, Molatore S, Pellegata NS, Arnold A. Somatic mutation and germline sequence abnormalities in CDKN1B, encoding p27Kip1, in sporadic parathyroid adenomas. J Clin Endocrinol Metab 2011;96:E701-6. [Crossref] [PubMed]
- Tonelli F, Giudici F, Giusti F, Marini F, Cianferotti L, Nesi G, Brandi ML. A heterozygous frameshift mutation in exon 1 of CDKN1B gene in a patient affected by MEN4 syndrome. Eur J Endocrinol 2014;171:K7-K17. [Crossref] [PubMed]
- Beebeejaun M, Chinnasamy E, Wilson P, Sharma A, Beharry N, Bano G. Papillary carcinoma of the thyroid in patients with primary hyperparathyroidism: Is there a link? Med Hypotheses 2017;103:100-4. [Crossref] [PubMed]
- Zhang F, Pan X, Tong N, Lü Q. Coexistence of Graves’ disease and primary hyperparathyroidism: a case description. Quant Imaging Med Surg 2022;12:3014-9. [Crossref] [PubMed]
- Cinamon U, Levy D, Marom T. Is Primary hyperparathyroidism a Risk Factor for Papillary Thyroid Cancer? An Exemplar Study and Literature Review. Int Arch Otorhinolaryngol 2015;19:42-5. [PubMed]
- Tonacchera M, Chiovato L, Pinchera A, Agretti P, Fiore E, Cetani F, Rocchi R, Viacava P, Miccoli P, Vitti P. Hyperfunctioning thyroid nodules in toxic multinodular goiter share activating thyrotropin receptor mutations with solitary toxic adenoma. J Clin Endocrinol Metab 1998;83:492-8. [Crossref] [PubMed]
- Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN, Walter MA. 2016 American Thyroid Association Guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 2016;26:1343-421. [Crossref] [PubMed]


