
Early cerebral hemodynamic changes following unilateral carotid artery stenting in patients with different degrees of carotid stenosis
Introduction
In recent years, carotid artery stenting (CAS) has become an effective alternative treatment to carotid endarterectomy (CEA) for carotid artery stenosis (1-3). The postprocedural complications related to hemodynamic changes, such as cerebral hyperperfusion syndrome (CHS) or hemodynamic instability (HI), often occur in the first few hours following CAS (4-7). In particular, the incidence of CHS after CAS is 3.1–6.8%, and it often leads to disabling stroke (8). A recent meta-analysis recommended further investigations into cerebral hemodynamic monitoring to prevent CHS following CAS (8). However, few studies have focused on cerebral hemodynamics in the early postprocedural stage (within 6 hours). A preliminary study of our research group proved that transcranial color-code Doppler (TCCD) or transcranial Doppler (TCD) could be effective for routine clinical monitoring of cerebral hemodynamic changes immediately after CAS (9). Our present study further assessed the immediate variation in cerebral hemodynamics following unilateral CAS in patients with different degrees of carotid stenosis. We aimed to investigate the quantitative relationship between the degree of carotid stenosis and cerebral hemodynamic changes after CAS. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-511/rc).
Methods
Participants
Between January 2013 and January 2019, all patients who underwent CAS and TCCD monitoring at the Department of Interventional Radiology and Vascular Surgery, Peking University First Hospital, were enrolled in our study. The exclusion criteria were simultaneous bilateral carotid stenting, contralateral internal carotid artery (ICA) total occlusion, and moderate to severe contralateral carotid artery stenosis. The remaining patients were retrospectively enrolled in the study. Data collection was conducted in 2020. The degree of carotid stenosis was evaluated by angiography and graded according to the North American symptomatic carotid endarterectomy trial (NASCET) criteria. The diagnostic criteria of near occlusion (NO) were as follows: (I) delayed cranial arrival of ICA contrast compared with the external carotid artery (ECA); (II) intracranial collaterals seen as cross-filling of contralateral vessels or ipsilateral contrast dilution; (III) obvious diameter reduction of ICA compared with opposite ICA; or (IV) ICA diameter reduction compared with ipsilateral ECA, in accordance with Fox et al. (10). Patients meeting at least 2 of the 4 criteria were included in the NO group. Based on the degree of carotid artery stenosis, the 104 patients were divided into 3 groups. Patients with 70–89% stenosis were included in the severe stenosis (SS) group, and those who met the diagnostic criteria of NO were included in the NO group. The remaining patients with ≥90% carotid artery stenosis but angiographic exclusion of NO were included in the extreme stenosis (ES) group (Figure 1).
Ethics approval
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol for this study was approved by the Ethics Committee of Peking University First Hospital. All data were retrieved from the Peking University First Hospital, Department of Interventional Radiology and Vascular Surgery after obtaining written informed consent from all participants.
CAS protocol
CAS was performed in symptomatic or asymptomatic patients with ≥70% stenosis as per the NASCET criteria. The operation indication was confirmed by 2 or more attending physicians. Written informed consent was obtained from all patients who underwent CAS. Patients received antiplatelet premedication (100 mg aspirin and 75 mg clopidogrel) at least 72 hours before CAS. All patients received intensive lipid-lowering treatment. A transfemoral approach with local anesthesia by 2% lidocaine was used in all cases. All patients received distal embolic protection. We routinely performed predilation with a 4.0- to 5.0-mm balloon catheter (Boston Scientific, Natick, MA, USA) and selected the appropriate stent device (Precise RX, Cordis Endovascular; Acculink, Abbott Vascular; and Carotid Wallstent; Boston Scientific) according to the anatomic location and the diameter of the artery on the surgeon’s discretion. Postdilation was not performed unless the residual stenosis was more than 30%. A completion angiogram of the carotid artery and distal cerebral vasculature was obtained after stent deployment. Follow-up was completed in the outpatient department 1 and 6 months after the procedure.
TCCD
Middle cerebral artery (MCA) peak systolic velocity (PSV) and pulsatility index (PI) were measured using TCCD equipment (LOGIQ e, GE Healthcare, Chicago, IL, USA) fitted with a 2.0-MHz sector array transducer or a 2-MHz probe connected to TCD equipment (Companion III TC2021, EME, Uberlingen, Germany). All TCCD or TCD examinations were performed by a single physician to avoid bias induced by the variation of the insonation angle. Each patient received only one of either TCD or TCCD before and after the procedure. In fact, only 16 patients (before June 2014) underwent TCD. Doppler angle correction was used for TCCD measurements when necessary. The ipsilateral and contralateral MCA were insonated at a depth of 45–60 mm through the temporal window. PSV and PI were recorded before and 1 and 3 hours after CAS. We recorded the maximum value of PSV in a respiratory cycle. Post-CAS hyperperfusion was defined as MCA-PSV exceeding twice the pre-CAS value measured with TCCD (11,12).
Blood pressure (BP) control
BP was monitored, and a standard cuff was maintained throughout the periprocedural period of CAS. Systolic BP (SBP) was controlled below 160 mmHg before predilation. After predilation and stent deployment, SBP was preliminarily managed in the range of 90–150 mmHg. In cases with potential hyperperfusion or hypoperfusion indicated by postoperative TCCD examination, BP was further adjusted with intravenous administration of urapidil or nicardipine. The dosage of urapidil or nicardipine was adjusted until the BP was controlled within the target range. Hemodynamic depression (HD) was defined as periprocedural hypotension (BP <90/60 mmHg) or bradycardia (heart rate <50 beats/minute). Persistent HD was defined as continuous HD status over 1 hour. Dopamine or/and atropine were administered intravenously to patients with HD. CHS was characterized by ipsilateral headache, hypertension, seizures, focal neurological deficit, nausea, or vomiting.
Statistical analysis
Statistical analyses were performed using SPSS software version 23.0 (IBM Corp., Armonk, NY, USA). Continuous variables are presented as mean ± standard deviation (SD). Categorical variables are presented as percentages. In each group, changes in BP, PSV, and PI (before CAS and at 1 and 3 hours following CAS) were evaluated using a paired t-test. The Chi-squared and Fisher exact tests were used to evaluate the differences in categorical variables between the groups. The Kolmogorov-Smirnov test was conducted to determine whether continuous variables conformed to the normal distribution. Data conforming to the normal distribution are expressed as mean ± SD. Analysis of variance (ANOVA) was used to analyze differences in the continuous variables between groups. Data not conforming to the normal distribution are expressed as median [interquartile range (IQR)]. Kruskal-Wallis test was used to analyze differences in the variables between groups. A P value <0.05 was considered statistically significant.
Results
Between January 2013 and January 2019, 203 patients underwent CAS. Of these patients, 45 failed to be monitored using TCCD or TCD because of a poor temporal window, MCA stenosis/occlusion, or other reasons. Of the remaining 158 patients, 54 were excluded due to simultaneous bilateral carotid stenting (18 patients), contralateral ICA total occlusion (17 patients), or moderate to severe contralateral carotid artery stenosis (19 patients). The remaining 104 patients were retrospectively enrolled in the present study. The mean ± SD age of the 104 patients was 67±9 years. Most of the patients were symptomatic (65 patients, 63%), male (84 patients, 81%), and had a history of hypertension (75 patients, 72%), hyperlipidemia (84 patients, 81%), and smoking (62 patients, 60%). In the total cohort, 33 (32%) patients had diabetes. There were 50 patients in the SS group, 34 in the ES group, and 20 in the NO group. Except for the low proportion of diabetic patients in the ES group, there was no statistical difference in the demographic data among the 3 groups (Table 1).
Table 1
| Variables | Total, n [%] | SS group, n [%] | ES group, n [%] | NO group, n [%] | c2 | P* |
|---|---|---|---|---|---|---|
| Number | 104 | 50 | 34 | 20 | ||
| Male | 84 [81] | 43 [86] | 25 [74] | 16 [80] | 2.036 | 0.36 |
| Age ≥70 years | 40 [38] | 16 [32] | 18 [53] | 6 [30] | 4.499 | 0.11 |
| Hypertension | 75 [72] | 38 [76] | 24 [71] | 13 [65] | 0.918 | 0.63 |
| Diabetes mellitus | 33 [32] | 20 [40] | 5 [15] | 8 [40] | 6.759 | 0.034 |
| Smoker | 62 [60] | 27 [54] | 22 [65] | 13 [65] | 1.262 | 0.53 |
| Symptomatic | 65 [63] | 30 [60] | 21 [62] | 14 [70] | 0.621 | 0.73 |
| Hyperlipidemia | 84 [81] | 44 [88] | 25 [74] | 15 [75] | 3.259 | 0.20 |
| Stent | ||||||
| Precise RX | 74 [71] | 35 [70] | 26 [76] | 13 [65] | 0.870 | 0.65 |
| Wallstent | 12 [12] | 8 [16] | 8 [24] | 1 [5] | 2.058 | 0.36 |
| Acculink | 18 [17] | 7 [14] | 5 [15] | 6 [30] | 2.794 | 0.25 |
| Simultaneous VA or SA stenting | 18 [17] | 7 [14] | 7 [21] | 4 [20] | 0.739 | 0.69 |
| Outcome | ||||||
| Transient or permanent HD | 30 [29] | 12 [24] | 12 [35] | 6 [30] | 1.274 | 0.53 |
| Transient HD | 19 [18] | 9 [18] | 6 [18] | 4 [20] | 0.057 | 0.98 |
| Permanent HD | 11 [11] | 3 [6] | 6 [18] | 2 [10] | 2.911 | 0.23 |
| Headache | 1 [1] | 0 | 0 | 1 [5] | 4.241 | 0.12 |
| Minor stroke | 2 [2] | 1 [2] | 1 [3] | 0 | 0.581 | 0.74 |
| Intracranial hemorrhage | 0 | 0 | 0 | 0 | – | – |
*, P<0.05 was considered statistically significant. SS, severe stenosis (70–89%); ES, extreme stenosis (≥90%); NO, near occlusion; HD, hemodynamic depression; VA, vertebral artery; SA, subclavian artery.
Complication rates following CAS were similar among the groups. None of the 104 patients experienced intracranial hemorrhage, myocardial infarction, or renal failure, and there were no deaths or cases with disabling strokes during the 30-day follow-up after CAS. Minor stroke after the CAS procedure occurred in 1 patient in the SS group and 1 patient in the ES group. There were 30 (29%) cases of transient (19/104, 18%) or permanent HD (11/104, 11%) after CAS. Of these, 10 patients with permanent HD received intravenous dopamine treatment, and the symptoms were relieved within 48 hours.
Ipsilateral MCA-PSV increased significantly at 1 hour after CAS in all 104 patients (81.3±23.0 to 98.5±28.6 cm/s; 23.6%; P<0.001). Similar changes in MCA-PSV were observed at 3 hours after CAS (81.3±23.0 to 97.5±27.3 cm/s; 22.4%; P<0.001). There was no significant difference between the PSV values at 1 and 3 hours after CAS. Subgroup analyses were performed according to symptoms, sex, smoking status, history of hypertension, hyperlipidemia, or diabetes. The increase in the magnitude of ipsilateral MCA-PSV showed no significance among the subgroups.
Hemodynamic data of the groups with different degrees of carotid stenosis are shown in Table 2. The baseline BP (P=0.47) and pre-CAS ipsilateral MCA-PSV (P=0.16) were comparable among the 3 groups. Mean BP in the 3 groups decreased following CAS, while ipsilateral MCA-PSV increased. Ipsilateral PI of the MCA also increased in the 3 groups at 1 and 3 hours following CAS. At 3 hours after CAS, ipsilateral MCA PI further increased significantly compared with that at 1 hour after CAS in the SS group (1 vs. 3 hours: 0.94±0.23 vs. 0.98±0.23 cm/s; P=0.001) and ES group (1 vs. 3 hours: 0.99±0.20 vs. 1.04±0.18 cm/s; P=0.001). In the NO group, at 3 hours after CAS, BP (1 vs. 3 hours: 129±20 vs. 123±18; P=0.04) and ipsilateral MCA-PSV (1 vs. 3 hours: 109±29 vs. 104±31; P=0.04) decreased significantly compared with those at 1 hour after CAS. BP and ipsilateral MCA-PSV showed no significant change from 1 to 3 hours following CAS in the SS group and ES group. In the ES group, the 3-hour contralateral PI increased significantly compared with that at 1 hour after CAS (P=0.02). On the contralateral side, there was no other significant increase in MCA-PSV and PI at 1 or 3 hours following CAS.
Table 2
| Group | Parameter | Pre-CAS | 1 hour post-CAS | P* | 3 hours post-CAS | P* | P1–3h |
|---|---|---|---|---|---|---|---|
| SS group | BP (mmHg) | 143±17 | 116±12 | <0.001 | 116±12 | <0.001 | 0.53 |
| iMCA PSV (cm/s) | 84±21 | 93±27 | <0.001 | 94±26 | <0.001 | 0.87 | |
| iMCA PI | 0.86±0.15 | 0.94±0.23 | 0.001 | 0.98±0.23 | <0.001 | 0.001 | |
| cMCA PSV (cm/s) | 82±21 | 82±21 | 0.81 | 83±22 | 0.90 | 0.62 | |
| cMCA PI | 0.90±0.14 | 0.93±0.19 | 0.16 | 0.93±0.19 | 0.11 | 0.50 | |
| ES group | BP (mmHg) | 143±16 | 119±16 | <0.001 | 118±15 | <0.001 | 0.76 |
| iMCA PSV (cm/s) | 83±24 | 100±29 | <0.001 | 100±27 | <0.001 | 0.52 | |
| iMCA PI | 0.85±0.16 | 0.99±0.20 | <0.001 | 1.04±0.18 | <0.001 | 0.001 | |
| cMCA PSV (cm/s) | 96±24 | 101±29 | 0.04 | 99±25 | 0.25 | 0.17 | |
| cMCA PI | 0.99±0.19 | 0.99±0.24 | 0.91 | 1.03±0.22 | 0.14 | 0.02 | |
| NO group | BP (mmHg) | 148±13 | 129±20 | <0.001 | 123±18 | <0.001 | 0.04 |
| iMCA PSV (cm/s) | 73±24 | 109±29 | <0.001 | 104±31 | <0.001 | 0.04 | |
| iMCA PI | 0.72±0.20 | 0.93±0.28 | <0.001 | 0.91±0.30 | 0.001 | 0.35 | |
| cMCA PSV (cm/s) | 96±31 | 97±29 | 0.51 | 98±30 | 0.28 | 0.67 | |
| cMCA PI | 0.91±0.24 | 0.91±0.31 | 0.89 | 0.91±0.31 | 0.97 | 0.77 |
Data are presented as mean ± standard deviation. *, P<0.05 was considered statistically significant. P1–3h: P values between 1 and 3 hours after CAS. SS, severe stenosis (70–89%); ES, extreme stenosis (≥90%); NO, near occlusion; BP, blood pressure; ICA, internal carotid artery; iMCA, ipsilateral middle cerebral artery; cMCA, contralateral middle cerebral artery; PSV, peak systolic velocity; PI, pulsatility index; CAS, carotid artery stenting; VA, vertebral artery; SA, subclavian artery.
The magnitude of increase in the ipsilateral MCA-PSV increased with the severity of carotid stenosis, and the difference was significant (Table 3, Figure 2). In the SS group, the increase in ipsilateral MCA-PSV was 8.1% (IQR, 1.4–20.1%) at 1 hour following CAS and 7.5% (IQR, 3.4–20.0%) at 3 hours following CAS. In the ES group, the increase in ipsilateral MCA-PSV was 20.8% (IQR, 5.3–33.1%) at 1 hour and 21.1% (IQR, 9.4–32.9%) at 3 hours following CAS. In the NO group, the increase in ipsilateral MCA-PSV was 45.8% (IQR, 24.3–73.1%) at 1 hour and 44.5% (IQR, 19.1–71.8%) at 3 hours after CAS. However, the decrease in BP was not significantly different among the 3 groups.
Table 3
| Variables | Increase rate | SS group | ES group | NO group | P | P1–2 | P1–3 | P2–3 | P* |
|---|---|---|---|---|---|---|---|---|---|
| 1 hour post-CAS | BP, % | −18.6±9.8 | −16.2±13.1 | −12.5±11.8 | 0.13 | – | – | – | <0.001 |
| iMCA PSV, % | 8.1 (1.4–20.1) | 20.8 (5.3–33.1) | 45.8 (24.3–73.1) | <0.001 | 0.045 | <0.001 | 0.006 | ||
| 3 hours post-CAS | BP, % | −20.0 (−25.0 to −11.7) | −20.7 (−25.5 to −6.8) | −15.0 (−22.4 to −6.8) | 0.64 | – | – | – | |
| iMCA PSV, % | 7.5 (3.4–20.0) | 21.1 (9.4–32.9) | 44.5 (19.1–71.8) | <0.001 | 0.04 | <0.001 | 0.04 | ||
| Patients with hyperperfusion | – | 0 | 0 | 4 (20%) | – | – | – | – | – |
Data of increase rate of BP at 1 hour are presented as mean ± standard deviation. Data of increase rate of BP at 3 hours and data of increase rate of MCA-PSV are presented as median (interquartile range). P<0.05 was considered statistically significant. P: P value among the 3 groups; P1–2: P values between SS group and ES group; P1–3: P values between SS group and NO group; P2–3: P values between ES group and NO group; P*: P values between NO group and the other two groups. SS, severe stenosis (70−89%); ES, extreme stenosis (≥90%); NO, near occlusion; CAS, carotid artery stenting; BP, blood pressure; iMCA, ipsilateral middle cerebral artery; PSV, peak systolic velocity.
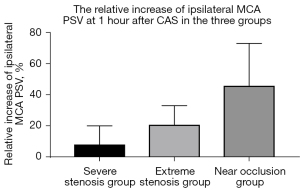
Hyperperfusion was detected on TCCD in 4 patients (4/104, 3.8%), and all 4 cases belonged to the NO group (4/20, 20.0%, P<0.001). Of these 4 patients, 3 received intravenous nicardipine treatment and were asymptomatic throughout the periprocedural period. One patient experienced a headache during intravenous administration of dopamine, and the symptom was relieved when the BP was decreased to 85/50 mmHg by reducing the dose of dopamine.
Discussion
The present study investigated the changes in cerebral hemodynamics in the early stage following CAS in patients with different degrees of carotid stenosis. Patients with unstable cerebral hemodynamics showed a higher risk of post-CAS complications, including CHS and HD. Ogasawara et al. reviewed the data of 4,494 patients and demonstrated that CHS usually occurred in the first 24 hours after CAS (13). A recent meta-analysis by Huibers et al. indicated that the risk of CHS after CAS was 4.6% (8). They also suggested that the clinical importance of cerebral hemodynamic monitoring should be elaborated in future studies. However, there has been limited research on this subject. Our team has conducted a series of studies on early cerebral hemodynamic after CAS (14,15). The present study comprises the largest sample size to date for the evaluation of early cerebral hemodynamic changes following CAS.
Cerebral blood flow can be measured by computed tomography angiography (CTA), CT perfusion (CTP), and magnetic resonance imaging (MRI) (16), but these are not bedside examinations. Our previous study concluded that TCCD and TCD were feasible for monitoring early cerebral hemodynamic changes following CAS (9). The MCA blood flow velocity will increase due to the recanalization of carotid artery (9). We also focused on changes in patients with NO in comparison with corresponding results from cases of SS without NO (15). In the latter research, ipsilateral MCA-PSV in patients with NO increased significantly compared to that in patients with SS stenosis but without NO. However, because of the limited number of patients without NO, we found no significant differences in the changes in PSV with ES stenosis or SS stenosis. We further increased the sample size and noted that the results were statistically significant. The study is the first to confirm the disparate cerebral hemodynamic changes following CAS in patients with varying degrees of initial carotid stenosis.
Our study innovatively quantified the variation in periprocedural cerebral blood flow velocity in patients with varying degrees of carotid stenosis. In the SS group, the increase in ipsilateral MCA-PSV was about 8% at 1 and 3 hours following CAS. In the ES group, the increase in ipsilateral MCA-PSV was approximately 20% at 1 and 3 hours after CAS. Considering that cerebral hyperperfusion is defined as the increase in cerebral blood flow exceeding 100% of the preprocedural value (11), we speculated that the change in the cerebral blood flow in the 2 groups was unlikely to result in post-CAS CHS. The postprocedural PI increased significantly in ipsilateral MCA in all 3 groups, which could be explained by the vasoconstriction of the resistance arterioles (15,17,18).
In recent years, TCD and TCCD have been used for the early detection of CHS following CAS as well as CEA (19,20). In the NO group, cerebral hyperperfusion was observed in 4 patients (4/20, 20.0%) following CAS using TCCD measurement, the rate of which was significantly higher than that in the other 2 groups. With hemodynamic surveillance using TCCD measurement, we maintained the BP to a relatively lower level, and the symptoms of the patients were relieved in the early stage before the diagnosis of CHS. In fact, due to the TCCD-based BP monitoring, none of the 158 patients experienced severe CHS (8). Hence, immediate TCD or TCCD measurement following CAS should be recommended in routine clinical practice in patients at a high risk of CHS, such as those with NO stenosis.
We excluded patients with simultaneous bilateral carotid stenting, total occlusion of the contralateral ICA, and moderate to severe contralateral carotid artery stenosis. The reason for these exclusion criteria was that the above situations could influence the cerebral hemodynamics after carotid artery recanalization (8,9,14). However, there was no definite conclusion as to whether age, sex, smoking status, symptoms, history of hypertension, diabetes, or hyperlipidemia was a potential confounding factor for changes in cerebral hemodynamics. In the present research, changes in the cerebral hemodynamics showed no significant difference in the subgroup analysis when patients were categorized according to the above factors.
The present research did not study parameters such as intracranial pressure or cerebrovascular reactivity. Measurement of MCA-PSV and PI can facilitate TCD or TCCD examination to ensure that the data of all patients are collected in a timely manner. Some medications, including statins, vasopressors, and antihypertensive drugs, might result in changes in cerebral circulation (21). The potential effect of these medications on cerebral hemodynamics after CAS will be further investigated in the future. An imbalanced sex ratio was a limitation in the present research due to the higher incidence of poor temporal window in female patients.
Conclusions
In patients with unilateral severe carotid stenosis, ipsilateral MCA-PSV and PI increased significantly at 1 and 3 hours following CAS. The increase in ipsilateral MCA-PSV was higher in patients with a higher degree of original stenosis. NO was an independent risk factor of hyperperfusion after unilateral CAS. TCD or TCCD measurement immediately after CAS is recommended in patients at a high risk of hemodynamic complications.
Acknowledgments
Funding: This work was funded by the National Program on Key Research Project (No. 2017YFC0109105).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-511/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-511/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol for this study was approved by the Ethics Committee of Peking University First Hospital, and informed consent was obtained from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ederle J, Bonati LH, Dobson J, Featherstone RL, Gaines PA, Beard JD, Venables GS, Markus HS, Clifton A, Sandercock P, Brown MM. Endovascular treatment with angioplasty or stenting versus endarterectomy in patients with carotid artery stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long-term follow-up of a randomised trial. Lancet Neurol 2009;8:898-907. [Crossref] [PubMed]
- Kim LK, Yang DC, Swaminathan RV, Minutello RM, Okin PM, Lee MK, Sun X, Wong SC, McCormick DJ, Bergman G, Allareddy V, Singh H, Feldman DN. Comparison of trends and outcomes of carotid artery stenting and endarterectomy in the United States, 2001 to 2010. Circ Cardiovasc Interv 2014;7:692-700. [Crossref] [PubMed]
- Mott M, Koroshetz W, Wright CB. CREST-2: Identifying the Best Method of Stroke Prevention for Carotid Artery Stenosis: National Institute of Neurological Disorders and Stroke Organizational Update. Stroke 2017;48:e130-1. [Crossref] [PubMed]
- Huibers A, Calvet D, Kennedy F, Czuriga-Kovács KR, Featherstone RL, Moll FL, Brown MM, Richards T, de Borst GJ. Mechanism of Procedural Stroke Following Carotid Endarterectomy or Carotid Artery Stenting Within the International Carotid Stenting Study (ICSS) Randomised Trial. Eur J Vasc Endovasc Surg 2015;50:281-8. [Crossref] [PubMed]
- Gupta R, Abou-Chebl A, Bajzer CT, Schumacher HC, Yadav JS. Rate, predictors, and consequences of hemodynamic depression after carotid artery stenting. J Am Coll Cardiol 2006;47:1538-43. [Crossref] [PubMed]
- Widecka-Ostrowska K, Modrzejewski A, Gorący J. Haemodynamic depression during carotid angioplasty and stenting. Pol J Radiol 2010;75:34-7. [PubMed]
- Abou-Chebl A, Yadav JS, Reginelli JP, Bajzer C, Bhatt D, Krieger DW. Intracranial hemorrhage and hyperperfusion syndrome following carotid artery stenting: risk factors, prevention, and treatment. J Am Coll Cardiol 2004;43:1596-601. [Crossref] [PubMed]
- Huibers AE, Westerink J, de Vries EE, Hoskam A, den Ruijter HM, Moll FL, de Borst GJ. Editor's Choice - Cerebral Hyperperfusion Syndrome After Carotid Artery Stenting: A Systematic Review and Meta-analysis. Eur J Vasc Endovasc Surg 2018;56:322-33. [Crossref] [PubMed]
- Yan Z, Yang M, Niu G, Zou Y. Analysis of Hemodynamic Changes in Early Stage after Carotid Stenting by Transcranial Doppler-A Preliminary Study. Ann Vasc Surg 2017;45:85-91. [Crossref] [PubMed]
- Fox AJ, Eliasziw M, Rothwell PM, Schmidt MH, Warlow CP, Barnett HJ. Identification, prognosis, and management of patients with carotid artery near occlusion. AJNR Am J Neuroradiol 2005;26:2086-94. [PubMed]
- Pennekamp CW, Moll FL, De Borst GJ. Role of transcranial Doppler in cerebral hyperperfusion syndrome. J Cardiovasc Surg (Torino) 2012;53:765-71. [PubMed]
- van Mook WN, Rennenberg RJ, Schurink GW, van Oostenbrugge RJ, Mess WH, Hofman PA, de Leeuw PW. Cerebral hyperperfusion syndrome. Lancet Neurol 2005;4:877-88. [Crossref] [PubMed]
- Ogasawara K, Sakai N, Kuroiwa T, Hosoda K, Iihara K, Toyoda K, Sakai C, Nagata I, Ogawa A. Intracranial hemorrhage associated with cerebral hyperperfusion syndrome following carotid endarterectomy and carotid artery stenting: retrospective review of 4494 patients. J Neurosurg 2007;107:1130-6. [Crossref] [PubMed]
- Yan Z, Yang M, Niu G, Zhang B, Tong X, Guo H, Zou Y. Hemodynamic Surveillance of Unilateral Carotid Artery Stenting in Patients With or Without Contralateral Carotid Occlusion by TCD/TCCD in the Early Stage Following Procedure. Front Neurol 2019;10:958. [Crossref] [PubMed]
- Yan Z, Yang M, Niu G, Zhang B, Tong X, Zou Y. Cerebral Hemodynamic Variations in the Early Stage after Carotid Artery Stenting in Patients with and without Near Occlusion. Ann Vasc Surg 2019;59:5-11. [Crossref] [PubMed]
- Wang J, Li R, Liu M, Nie Z, Jin L, Lu Z, Li Y. Impaired cerebral hemodynamics in late-onset depression: computed tomography angiography, computed tomography perfusion, and magnetic resonance imaging evaluation. Quant Imaging Med Surg 2020;10:1763-74. [Crossref] [PubMed]
- Sánchez-Arjona MB, Sanz-Fernández G, Franco-Macías E, Gil-Peralta A. Cerebral hemodynamic changes after carotid angioplasty and stenting. AJNR Am J Neuroradiol 2007;28:640-4. [PubMed]
- Nowacki P, Zywica A, Podbielski J, Kornacewicz-Jach Z, Drechsler H, Drechsler D. Middle cerebral artery flow after angioplasty and stenting of symptomatic internal carotid artery stenosis. Neurol Neurochir Pol 2009;43:9-15. [PubMed]
- Kablak-Ziembicka A, Przewlocki T, Pieniazek P, Musialek P, Tekieli L, Rosławiecka A, Motyl R, Zmudka K, Tracz W, Podolec P. Predictors of cerebral reperfusion injury after carotid stenting: the role of transcranial color-coded Doppler ultrasonography. J Endovasc Ther 2010;17:556-63. [Crossref] [PubMed]
- Fassaert LMM, Immink RV, van Vriesland DJ, de Vries JPM, Toorop RJ, Kappelle LJ, Westerink J, Tromp SC, de Borst GJ. Transcranial Doppler 24 Hours after Carotid Endarterectomy Accurately Identifies Patients Not at Risk of Cerebral Hyperperfusion Syndrome. Eur J Vasc Endovasc Surg 2019;58:320-7. [Crossref] [PubMed]
- Giannopoulos S, Katsanos AH, Tsivgoulis G, Marshall RS. Statins and cerebral hemodynamics. J Cereb Blood Flow Metab 2012;32:1973-6. [Crossref] [PubMed]


