
Age-related changes in Scheimpflug corneal densitometry and their correlations with corneal topographic measurements in a healthy Chinese population
Introduction
Clear corneas are essential to good visual quality (1). Clinically, corneal infections, trauma, surgery, and congenital abnormalities may affect corneal transparency (2). The commonly used grading of corneal opacity in clinical practice is based on slit-lamp findings and is classified as corneal pannus, clouding, or leukoplakia (3,4). However, assessing the degree of corneal haze by slit-lamp examination remains a gross and subjective procedure, meaning that minor increases in corneal density may not be macroscopically discernible.
The objective measurements of corneal densitometry (CD) values include the Oculus Pentacam (Oculus Optikgerate GmbH, Wetzlar, Germany), anterior segment optical coherence tomography (AS-OCT), and corneal confocal microscopy (5-8). The Oculus Pentacam, which is designed according to the Scheimpflug principle, comprehensively analyzes the cornea and its CD by acquiring anterior segment images. CD measurement by Pentacam has been used to study different corneal diseases and surgeries (9-14). Corneal transparency is impaired in vernal keratoconjunctivitis even when the corneal epithelium is not involved (3). CD is significantly increased in keratoconic eyes and is positively correlated with severity (12,13). In one study, CD values measured by the Scheimpflug imaging system in patients with myopia were not changed significantly during the 1-year follow-up period after implantation of an implantable collamer lens V4c (14).
A previous study reported no correlation between CD and corneal parameters, including keratometry, corneal thickness, and spherical equivalent for individuals between 20 and 51 years of age (15). However, the relationship between CD and corneal parameters, including spherical aberration, has not been assessed in healthy juvenile and older individuals. This study sought to investigate age-related changes in Scheimpflug CD values in healthy Chinese individuals aged 5 to 90 years of age, as well as the correlations with age, sex, and corneal topographic parameters, such as keratometry, corneal thickness, and spherical aberration. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-486/rc).
Methods
Participants
This study design was a retrospective observational study. Eligible participants who underwent Oculus Pentacam eye health examination at Shenzhen Eye Hospital from April 1, 2018, to April 30, 2021, were recruited for the study, and both eyes were included in the observation when possible. There were no sex restrictions. Owing to reduced cooperation during eye examinations in children under 5 years of age, participants aged 5 to 90 years were included in this study. They were divided into 9 groups according to age (5–9, 10–19, 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, and 80–90 years). Individuals were excluded if they had any of the following: corneal diseases (such as infection, keratoconus, congenital corneal abnormalities, and corneal scars), ocular diseases requiring long-term medication or recent history of ocular medication (such as dry eye, glaucoma, and allergic conjunctivitis), previous ocular trauma or surgical history, and systemic diseases that might have affected the eye (such as diabetes, connective tissue diseases, and other autoimmune diseases). Written informed consent was obtained from the participants or their legal guardians prior to data collection. Ethical approval for this study was obtained from the Ethics Committee of Shenzhen Eye Hospital. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Measurement technique
The measurements were carried out by the same experienced doctor using the same Pentacam anterior segment analyzer (Oculus Optikgerate GmbH, Wetzlar, Germany) with software version 1.26r28. Examinations were performed in a darkroom environment, with the participant seated, the lower jaw placed on a rest, and with fixation of the target in both eyes. In all, 25 Scheimpflug images of the cornea with different meridians were automatically acquired using a three-dimensional scanning mode. For images with data gaps, lid closure or blinking error, the images were retaken until a high-quality image was obtained with the oll korrect (OK) quality specification (QS) value for analysis. CD and cornea-related parameters, including central corneal thickness (CCT), thinnest corneal thickness (TCT), flat-axis keratometry (K1), steep-axis keratometry (K2), and spherical aberration, were all obtained by Pentacam measurements. CD ranges from 0 to 100 gray-scale units (GSU), with 0 representing complete clearing and 100 representing complete opacification of the cornea. CD was subdivided into 4 concentric radial regions based on corneal diameter, including the corneal apex of diameter 0–2, 2–6, 6–10, and 10–12 mm. The cornea was divided into 3 layers according to depth for the evaluation of CD: the anterior 120 µm, the center, and the posterior 60 µm layer.
Statistical analysis
Statistical analyses were performed using SPSS 19 statistical software (IBM Corp., Armonk, NY, USA). All data sets were tested for normality using the Kolmogorov-Smirnov test. The statistical description of parameters meeting the normal distribution is expressed as the mean ± standard deviation (SD), and the statistical description of parameters not meeting the normal distribution is expressed as P50 (P25, P75). Correlation coefficients were assessed using a Spearman correlation analysis. The Kruskal-Wallis test was used to compare multiple groups of measurement data not conforming to a normal distribution, and a Mann-Whitney test was used to compare two independent samples of data not conforming to a normal distribution. Statistical significance was set to P<0.05. Sample size calculations were performed using an online power and sample size calculator (http://powerandsamplesize.com/Calculators). Based on previous studies (16,17), and using α=0.05 and power =0.8, at least 172 participants were needed for the study to be sufficiently powered.
Results
Comparison of corneal topography parameters by age and sex
A total of 347 eyes from 181 healthy Chinese participants (43.6% men, 56.4% women) were included. Table 1 shows the significant differences in corneal topography parameters (including K1, CCT, TCT, and corneal spherical aberration) among age groups (all P values <0.05), but there was no significant difference in K2 among age groups (χ2=13.193; P=0.105). K1, K2, CCT, TCT, and corneal spherical aberration were not significantly different between men and women, nor between the right and left eyes (all P values >0.05).
Table 1
| Age groups (number/eyes) | Sex (M/F) | Age (y) | K1 (D) | K2 (D) | CCT (μm) | TCT (μm) | Spherical aberration |
|---|---|---|---|---|---|---|---|
| 5–9 (16/31) | 7/9 | 6.00 (5.00, 9.00) | 42.49±1.42 | 44.08±2.00 | 530.72±70.75 | 494.83±52.07 | 0.21±0.09 |
| 10–19 (13/26) | 6/7 | 11.00 (10.00, 12.00) | 42.89±1.25 | 43.93±1.31 | 528.37±58.53 | 497.50 (357.00, 538.25) | 0.19±0.07 |
| 20–29 (38/76) | 16/22 | 24.00 (22.00, 26.00) | 42.27±1.56 | 43.5±1.64 | 526.00 (504.00, 552.00) | 522.00 (494.00, 547.00) | 0.20 (0.15, 0.25) |
| 30–39 (17/33) | 6/11 | 32.00 (30.00, 35.00) | 43.30 (42.80, 43.60) | 44.13±1.07 | 520.63±41.86 | 512.59±43.69 | 0.21±0.08 |
| 40–49 (23/44) | 11/12 | 45.00 (42.25, 47.00) | 43.50 (42.53, 43.90) | 44.40±1.48 | 527.50 (504.75, 540.00) | 517.00 (495.75, 533.50) | 0.26±0.09 |
| 50–59 (13/25) | 4/9 | 53.00 (52.00, 55.00) | 43.75±1.78 | 44.54±2.03 | 551.59±35.38 | 543.86±35.64 | 0.32 (0.23, 0.35) |
| 60–69 (32/61) | 16/16 | 67.00 (63.50, 68.50) | 43.20 (42.50, 44.80) | 44.00 (42.90, 45.40) | 523.98±26.88 | 515.45±26.35 | 0.33 (0.25, 0.42) |
| 70–79 (20/35) | 11/9 | 74.00 (72.00, 78.00) | 43.75±1.54 | 44.76±1.79 | 518.93±33.06 | 509.34±34.43 | 0.34 (0.27, 0.47) |
| 80–89 (9/16) | 2/7 | 83.00 (82.00, 86.00) | 42.38±1.59 | 43.79±1.45 | 502.00 (496.75, 607.50) | 498.00 (486.75, 569.50) | 0.32±0.26 |
| χ2 | N/A | N/A | 30.598 | 13.193 | 16.644 | 21.161 | 55.784 |
| P | N/A | N/A | <0.001 | 0.105 | 0.034 | 0.007 | <0.001 |
Normally distributed parameter values are expressed as mean ± SD, and nonnormally distributed parameter values are expressed as P50 (P25, P75). All comparisons were made using Kruskal-Wallis test. P<0.05 was considered statistically significant. M, male; F, female; y, years; K1, flat-axis keratometry; K2, steep-axis keratometry; CCT, central corneal thickness; TCT, thinnest corneal thickness; N/A, not applicable; SD, standard deviation.
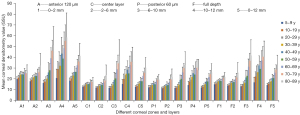
Comparison of CD in different corneal layers and regions by age and sex
Figure 1 represents CD images of 20, 50, and 80 years old. Meanwhile, Table 2 and Figure 2 represent the CD values of different corneal layers and regions from individuals 5–90 years of age. There were significant differences in CD among all age groups when divided into anterior 120 µm, central, and posterior 60 µm layers, or when analyzed at full depth (all P values <0.05). The highest CD value was identified in the anterior 120 µm corneal layer, and the lowest was seen in the posterior 60 µm corneal layer in all age groups (all P values <0.05). Among the 4 annular regions of corneal diameter, the lowest CD values were 6–10 mm at 5–29 years, 2–6 mm at 30–69 years, and 0–2 mm at 70–89 years, while the highest CD values were 10–12 mm at 5–79 years and 6–10 mm at 80–90 years (all corresponding to values of P<0.05). No significant difference was observed in CD between the right and left eyes in different corneal layers and regions (all P values >0.05). CD values of 10–12 mm in the anterior 120 µm corneal layer were significantly lower in men than in women (Z=−2.353; P=0.019). There were no significant differences in CD between men and women at 0–2, 2–6, and 6–10 mm across all corneal layers (all P values >0.05).

Table 2
| Cornea layer | Zones (mm) | 5–9 y group | 10–19 y group | 20–29 y group | 30–39 y group | 40–49 y group | 50–59 y group | 60–69 y group | 70–79 y group | 80–89 y group | χ2 | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anterior 120 μm | 0–2 | 19.7 (18.8, 21.2) | 21.2 (20.2, 22.6) | 23.1 (21.8, 25.0) | 23.4 (22.3, 25.3) | 24.1 (22.9, 25.1) | 24.3 (22.5, 25.6) | 25.1 (23.0, 27.0) | 24.9 (23.1, 27.1) | 27.1 (25.0, 30.6) | 84.299 | <0.001 |
| 2–6 | 17.6 (17.0, 19.3) | 19.3 (18.1, 20.2) | 20.7 (19.5, 22.5) | 21.2 (20.2, 23.1) | 22.3 (21.3, 24.5) | 22.8 (20.8, 24.0) | 25.0 (22.4, 27.1) | 26.1 (23.2, 28.6) | 30.5 (27.2, 35.4) | 146.175 | <0.001 | |
| 6–10 | 15.9 (14.3, 18.1) | 16.3 (16.0, 18.8) | 18.9 (17.2, 21.6) | 20.1 (18.0, 22.4) | 24.1 (21.1, 28.0) | 26.9 (24.8, 32.9) | 39.5 (31.3, 45.7) | 45.5 (37.3, 52.1) | 53.5 (36.7, 65.9) | 231.193 | <0.001 | |
| 10–12 | 19.0 (15.1, 26.3) | 27.1 (23.0, 36.1) | 33.3 (29.0, 41.0) | 30.4 (25.6, 37.1) | 39.7 (32.3, 49.3) | 35.9 (29.2, 46.5) | 45.5 (36.3, 58.8) | 54.5 (47.9, 65.1) | 63.5 (44.7, 78.0) | 100.345 | <0.001 | |
| 0–12 | 17.9 (16.0, 19.6) | 20.3 (19.0, 21.7) | 22.8 (21.3, 24.8) | 23.0 (21.2, 25.0) | 26.0 (23.6, 27.6) | 26.0 (24.7, 28.1) | 33.3 (28.4, 36.1) | 35.9 (31.3, 39.2) | 40.1 (37.4, 50.3) | 200.439 | <0.001 | |
| Center layer | 0–2 | 12.7 (12.5, 13.3) | 13.2 (12.8.13.8) | 14.9 (14.1, 15.4) | 15.0 (13.9, 16.2) | 15.4 (14.6, 16.1) | 14.9 (14.5, 16.3) | 16.5 (15.3, 17.9) | 16.9 (15.9, 18.3) | 17.7 (17.0, 19.5) | 147.188 | <0.001 |
| 2–6 | 11.8 (11.3, 12.0) | 12.1 (11.7, 12.3) | 13.4 (12.7, 14.1) | 13.6 (12.9, 14.6) | 14.5 (13.8, 15.4) | 14.0 (13.2, 15.7) | 16.5 (15.0, 18.0) | 17.8 (16.4, 19.0) | 21.3 (18.6, 25.3) | 200.407 | <0.001 | |
| 6–10 | 11.4 (11.0, 12.3) | 11.7 (11.3, 12.5) | 13.5 (12.2, 14.9) | 14.0 (12.8, 16.0) | 17.4 (15.6, 19.5) | 19.7 (18.5, 21.5) | 28.9 (24.1, 34.9) | 33.6 (29.7, 371.) | 41.1 (28.5, 57.1) | 244.020 | <0.001 | |
| 10–12 | 15.0 (12.6, 18.8) | 19.6 (17.1, 21.5) | 22.8 (19.3, 26.2) | 23.3 (20.8, 28.4) | 27.6 (22.3, 31.9) | 24.9 (20.3, 29.5) | 32.2 (26.8, 37.1) | 34.7 (30.5, 41.4) | 33.2 (27.0, 48.1) | 116.778 | <0.001 | |
| 0–12 | 12.3 (11.8, 13.2) | 13.3 (12.7, 13.9) | 15.2 (14.1, 16.1) | 15.4 (15.1, 16.9) | 17.2 (16.1, 18.9) | 17.6 (16.3, 18.9) | 23.0 (19.9, 25.5) | 24.9 (22.8, 27.4) | 28.7 (26.4, 38.3) | 226.253 | <0.001 | |
| Posterior 60 μm | 0–2 | 12.7 (11.7, 13.0) | 12.9 (12.3, 13.3) | 14.1 (13.2, 15.0) | 14.4 (13.1, 14.8) | 15.4 (14.1, 16.9) | 14.8 (13.9, 16.8) | 17.0 (15.8, 19.1) | 18.6 (16.4, 20.3) | 18.9 (16.2, 23.7) | 157.423 | <0.001 |
| 2–6 | 11.7 (10.6, 11.9) | 11.9 (11.6, 12.1) | 12.8 (12.0, 13.7) | 13.3 (12.4, 13.8) | 14.5 (13.6, 15.5) | 13.9 (13.3, 15.7) | 16.8 (15.2, 18.8) | 18.6 (16.8, 20.1) | 19.5 (17.6, 28.4) | 197.280 | <0.001 | |
| 6–10 | 11.4 (10.8, 12.6) | 12.0 (11.5, 12.4) | 13.3 (12.3, 14.2) | 14.4 (13.4, 16.1) | 17.7 (16.3, 20.1) | 19.7 (17.1, 22.4) | 26.7 (22.0, 29.1) | 30.1 (27.8, 31.8) | 31.5 (23.1, 45.3) | 242.802 | <0.001 | |
| 10–12 | 14.4 (11.4, 16.8) | 17.7 (14.8, 19.7) | 18.7 (16.0, 21.4) | 21.8 (18.8, 23.5) | 24.6 (20.5, 27.8) | 23.4 (19.6, 27.0) | 28.9 (25.5, 33.4) | 28.0 (25.9, 34.5) | 28.9 (20.7, 30.5) | 137.644 | <0.001 | |
| 0–12 | 12.1 (11.4, 12.8) | 13.0 (12.1, 13.5) | 14.1 (13.2, 15.0) | 15.1 (14.0, 15.8) | 17.0 (15.7, 18.4) | 17.0 (15.8, 19.5) | 21.9 (19.3, 23.8) | 23.9 (21.5, 26.0) | 24.6 (22.3, 35.4) | 222.576 | <0.001 | |
| Full length | 0–2 | 15.0 (14.5, 15.7) | 15.8 (15.2, 16.3) | 17.5 (16.4, 18.5) | 17.8 (16.6, 18.3) | 18.3 (17.5, 19.3) | 17.9 (17.1, 19.6) | 19.7 (18.2, 21.1) | 20.3 (18.5, 22.3) | 21.5 (19.6, 23.5) | 134.288 | <0.001 |
| 2–6 | 13.5 (12.9, 14.4) | 14.4 (13.9, 14.8) | 15.9 (14.8, 16.7) | 16.0 (15.2, 17.5) | 17.1 (16.3, 18.2) | 16.8 (15.9, 18.6) | 19.4 (17.6, 21.0) | 20.7 (18.9, 22.4) | 23.9 (21.3, 29.9) | 191.934 | <0.001 | |
| 6–10 | 13.4 (11.8, 14.3) | 13.5 (13.0, 14.6) | 15.2 (13.9, 16.9) | 16.6 (14.7, 18.2) | 19.6 (17.7, 22.4) | 22.1 (20.2, 25.6) | 32.2 (25.8, 36.3) | 36.1 (31.3, 39.5) | 43.5 (28.9, 57.3) | 241.862 | <0.001 | |
| 10–12 | 16.2 (13.6, 20.8) | 21.2 (18.9, 25.3) | 25.1 (22.3, 29.5) | 25.4 (21.6, 29.5) | 31.0 (25.8, 37.3) | 27.3 (24.4, 35.4) | 36.5 (30.5, 42.4) | 41.0 (33.4, 45.9) | 40.2 (33.5, 51.8) | 124.176 | <0.001 | |
| 0–12 | 14.1 (13.1, 15.3) | 15.6 (14.8, 16.2) | 17.5 (16.2, 18.6) | 17.7 (17.0, 19.3) | 20.0 (18.8, 21.6) | 20.4 (19.0, 21.7) | 26.1 (22.5, 28.3) | 27.7 (25.5, 30.7) | 32.0 (29.7, 42.0) | 221.406 | <0.001 |
Data are expressed as P50 (P25, P75). All comparison were made using Kruskal-Wallis test. P<0.05 was considered statistically significant. CD, corneal densitometry; y, years.
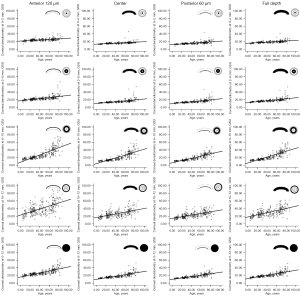
Correlations of CD with age and corneal topography parameters
An increase in CD with age was observed, and CD in different corneal layers and regions were significantly correlated with age (all P values <0.001; Table 3, Figure 3). This correlation gradually increased from 0–2, to 2–6, to 6–10 mm (ρ=0.659, ρ=0.790, ρ=0.900; all P values <0.001), while the CD at 10–12 mm had the lowest correlation with age (ρ=0.628; P value <0.001). The CD at 6–10 and 10–12 mm had a significant correlation with K1 (all P values <0.05); however, there was no correlation between CD and CCT or TCT (all P values >0.05; Table 3). CD in different corneal layers and regions were significantly correlated with spherical aberration (all P values <0.001; Table 3), and the correlation gradually increased from 0–2, to 2–6, to 6–10 mm (ρ=0.215, ρ=0.253, ρ=0.342; all P values <0.001), while it decreased again at 10–12 mm (ρ=0.299; P value <0.001).
Table 3
| Parameters | Cornea layer | 0–2 mm | 2–6 mm | 6–10 mm | 10–12 mm | 0–12 mm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ρ | P | ρ | P | ρ | P | ρ | P | ρ | P | ||||||
| Age | Anterior 120 μm | 0.514 | <0.001 | 0.696 | <0.001 | 0.880 | <0.001 | 0.552 | <0.001 | 0.813 | <0.001 | ||||
| Center layer | 0.671 | <0.001 | 0.800 | <0.001 | 0.902 | <0.001 | 0.597 | <0.001 | 0.861 | <0.001 | |||||
| Posterior 60 μm | 0.713 | <0.001 | 0.799 | <0.001 | 0.899 | <0.001 | 0.660 | <0.001 | 0.857 | <0.001 | |||||
| Full length | 0.659 | <0.001 | 0.790 | <0.001 | 0.900 | <0.001 | 0.628 | <0.001 | 0.855 | <0.001 | |||||
| K1 | Anterior 120 μm | 0.059 | 0.305 | 0.056 | 0.332 | 0.176 | 0.002 | 0.189 | 0.001 | 0.158 | 0.006 | ||||
| Center layer | 0.093 | 0.107 | 0.104 | 0.070 | 0.190 | 0.001 | 0.221 | <0.001 | 0.170 | 0.003 | |||||
| Posterior 60 μm | 0.146 | 0.011 | 0.156 | 0.006 | 0.234 | <0.001 | 0.283 | <0.001 | 0.226 | <0.001 | |||||
| Full length | 0.105 | 0.067 | 0.108 | 0.060 | 0.195 | 0.001 | 0.235 | <0.001 | 0.182 | 0.001 | |||||
| K2 | Anterior 120 μm | 0.048 | 0.407 | 0.031 | 0.585 | 0.110 | 0.055 | 0.111 | 0.057 | 0.097 | 0.091 | ||||
| Center layer | 0.043 | 0.450 | 0.059 | 0.304 | 0.117 | 0.041 | 0.146 | 0.012 | 0.100 | 0.081 | |||||
| Posterior 60 μm | 0.071 | 0.215 | 0.094 | 0.102 | 0.157 | 0.006 | 0.191 | 0.001 | 0.147 | 0.010 | |||||
| Full length | 0.062 | 0.282 | 0.066 | 0.254 | 0.125 | 0.030 | 0.151 | 0.009 | 0.114 | 0.047 | |||||
| CCT | Anterior 120 μm | 0.130 | 0.024 | 0.104 | 0.070 | 0.055 | 0.343 | −0.035 | 0.553 | 0.048 | 0.405 | ||||
| Center layer | 0.022 | 0.704 | 0.047 | 0.415 | 0.023 | 0.690 | 0.006 | 0.914 | 0.028 | 0.623 | |||||
| Posterior 60 μm | 0.031 | 0.586 | 0.037 | 0.522 | 0.005 | 0.936 | −0.019 | 0.741 | 0.011 | 0.854 | |||||
| Full length | 0.063 | 0.273 | 0.057 | 0.321 | 0.031 | 0.586 | −0.019 | 0.741 | 0.032 | 0.575 | |||||
| TCT | Anterior 120 μm | 0.124 | 0.031 | 0.112 | 0.050 | 0.075 | 0.190 | 0.001 | 0.987 | 0.069 | 0.229 | ||||
| Center layer | 0.030 | 0.597 | 0.061 | 0.289 | 0.047 | 0.417 | 0.047 | 0.422 | 0.053 | 0.355 | |||||
| Posterior 60 μm | 0.048 | 0.403 | 0.059 | 0.305 | 0.028 | 0.629 | 0.021 | 0.725 | 0.035 | 0.541 | |||||
| Full length | 0.072 | 0.211 | 0.054 | 0.347 | 0.019 | 0.749 | 0.055 | 0.335 | −0.015 | 0.800 | |||||
| Spherical aberration | Anterior 120 μm | 0.124 | 0.030 | 0.203 | <0.001 | 0.333 | <0.001 | 0.259 | <0.001 | 0.295 | <0.001 | ||||
| Center layer | 0.269 | <0.001 | 0.272 | <0.001 | 0.342 | <0.001 | 0.276 | <0.001 | 0.324 | <0.001 | |||||
| Posterior 60 μm | 0.245 | <0.001 | 0.248 | <0.001 | 0.359 | <0.001 | 0.358 | <0.001 | 0.339 | <0.001 | |||||
| Full length | 0.215 | <0.001 | 0.253 | <0.001 | 0.342 | <0.001 | 0.299 | <0.001 | 0.321 | <0.001 | |||||
P<0.05 was considered statistically significant. CD, corneal densitometry; ρ, Spearman correlation coefficients; K1, flat-axis keratometry; K2, steep-axis keratometry; CCT, central corneal thickness; TCT, thinnest corneal thickness.
Discussion
This study documents normal CD values in 347 eyes from 181 Chinese participants aged 5–90 years and their relationship with age, sex, and corneal parameters, including corneal keratometry, corneal thickness, and corneal spherical aberration. Several studies have reported normal CD at different ages (6,15-17). A previous comparative study reported normal values of corneal Scheimpflug densitometry in 794 eyes of 445 healthy White individuals aged 20.2–84.2 years, and showed that CD was associated with age only in the peripheral cornea (16). Alzahrani et al. reported the CD measured by Oculus Pentacam in 192 eyes of 97 healthy participants grouped by age (10–70 years), and the results suggested that there may be other factors affecting CD in addition to age (17). Pakbin et al. reported CD values increased with age until 35 years and decreased thereafter in 261 healthy photorefractive keratectomy candidates aged 21–40 years (6). Garzón et al. studied normative CD values and their relationship with keratometry, corneal thickness, and spherical equivalent in 338 healthy participants 20–52 years of age (15). This study examined normal CD data and its correlation with corneal parameters corresponding to the maximum age range, to the best of our knowledge, ever assessed in an Asian population.
The results of this study demonstrated that CD in the 0–2, 2–6, 6–10, and 10–12 mm zones, and in the anterior, middle, or posterior layers gradually increased with age. Interestingly, this is not entirely consistent with previous study reports (15,17). Indeed, a previous study found that CD was significantly correlated with age for all layers and corneal regions with a total diameter of 0–10 mm, except for 0–2 mm (17). Another study also showed that age was significantly correlated with CD in all 3 layers; however, only the corneal zone at 6–10 mm was significantly correlated with age (15). Pakbin et al. found that patients aged 30–35 years had higher densitometry values and denser peripheral corneas than did those aged 20–25 years (6). Wang et al. reported no correlation between central 0–4 mm CD measured by swept source AS-OCT (SS-AS-OCT) and age (7). A previous study revealed that CD in the peripheral zones increased significantly with age; however, there were no significant age-related changes in the central area, which was attributed to age-related limbal degeneration, such as arcus senilis (15). The cornea may be smaller than 12 mm in some individuals, and the limbus and sclera may increase the measured CD, so the correlation with age in the 10–12 mm region may be compromised. The present study showed that CD was significantly correlated with age, not only in the peripheral area but also in the central area; the correlation between CD and age gradually increased from the central 0–2 mm to the peripheral 6–10 mm area, but then decreased at 10–12 mm.
This study provided evidence that the highest CD values were in the anterior 120 µm, and the lowest CD values were in the posterior corneal layer, which is consistent with previous studies of CD measured by Pentacam (10,15,16). The highest densitometry value obtained by SS-AS-OCT was in the midstromal layer, and the lowest was in the anterior layer (7). The authors concluded that 4 factors, including refractive index, hydration gradient status of the entire cornea, keratocyte distribution density, and structural organization of the lamella, impacted heterogeneous corneal backscatter in normal corneas (7). Meanwhile, a study using in vivo confocal reported the corneal backscatter to be the highest in the posterior layer and lowest in the midstromal layer (18). This discrepancy may be due to the differences in the imaging methods of Pentacam, SS-AS-OCT, and confocal microscopy. Both Pentacam and SS-AS-OCT consist of examinations that do not require any patient contact, and the reflection of the tear film layer may affect the measurement of its optical density. In contrast, confocal microscopy is a contact examination that eliminates the effect of the tear film layer. Pentacam uses the Scheimpflug technique to obtain 25–50 corneal slit images on different meridians with a blue light source of 475 mm wavelength, which can effectively yield three-dimensional stereoscopic images of the anterior segment. SS-AS-OCT uses a 1,050-nm wavelength light source with a depth resolution of 8 µm and lateral resolution of 20 µm to obtain superior image quality. However, SS-AS-OCT images were obtained using a manually photographed model, and densitometry assessed only the horizontal meridian 0–180° and 0–4 mm pupil center corneal regions of this cross-section (7). In another study, confocal microscopy was used to scan the central cornea in full-thickness mode with 72% light intensity and a 6-µm scan step (8). The CD was expressed differently among the 3 devices: SS-AS-OCT scale (0–255 GSU), Pentacam scale (0–100 GSU), and confocal microscopy in scattering units (SU). Different image analyses and reading scales render it impossible to directly and quantitatively compare the 3 CD measurements. Thus, the results cannot be compared reliably or converted consistently across different devices.
A previous study reported that the lowest CD was in the 2–6 mm zone and that the highest was in the periphery in individuals aged 20–50 years (15). Another study revealed that CD was lowest in the central 6 mm and highest in the periphery, with no difference between the central area and the surrounding 2–6 mm annulus (16). Alzahrani et al. reported that CD at the 0–2 and 2–6 mm of the anterior layer were higher in the 10–19 and 40–49 years age groups than in the 20–29 years age group, and the authors suggested that hormonal changes occurring in different age groups may be responsible for the densitometric differences (17). In our study, the lowest CD was 6–10 mm at 5–29 years, 2–6 mm at 30–69 years, and 0–2 mm at 70–89 years, and the highest CD was 10–12 mm at 5–79 years and 6–10 mm at 80–90 years. These results suggest that the peripheral cornea is more transparent in young people, while the peripheral transparency of the cornea gradually decreases with age. We speculate that corneas close to the limbus are more vulnerable to oxidative stress factors in the blood, thus affecting corneal transparency. However, the specific mechanisms require further investigation.
Previous studies have reported that corneal parameters—including K1 and K2—are positively correlated with age, but that CCT and TCT are negatively correlated with age (19,20). However, the relationship between spherical aberration and age has remained rather inconsistent. A positive correlation has been found between spherical aberration and age (21,22); however, another study found no correlation between age and spherical aberrations (19). Some investigators believe that the annual decrease in corneal endothelial cell density leads to an increase in corneal thickness and may increase CD (17). Our study showed that K1, K2, and spherical aberrations were positively correlated with age and CD; however, neither CCT or TCT was correlated with age and CD. Our study suggests that changes in K1, K2, and spherical aberrations with age may underpin their correlation with CD.
The relationship between sex and CD remains unclear. One previous study reported no correlation between sex and CD measured using a SS-AS-OCT device (7). CD measured using in vivo confocal microscopy was 3.5% higher in men than in women (8). In line with some previous studies, the present study found that sex did not affect CD at 0–10 mm measured using the Oculus Pentacam (15,16). The lower CD value of 10–12 mm in the anterior 120 µm corneal layer seen in men may be due to differences in white-to-white (WTW) length although the specific reasons require further investigation.
This study had some limitations. The sample size was not equal for each age group, with notably fewer participants over 80 years of age. Previous literature has reported that a CD value at the 10–12 mm region has the weakest repeatability and reproducibility (16); thus, there is controversy regarding the inclusion of 10–12 mm CD values, as partial limbus and sclera may increase CD values in people with WTW diameters <12 mm. This study failed to investigate the repeatability and reproducibility of CD measurements across different corneal zones in different age groups.
Conclusions
This study presents age-related changes in Scheimpflug CD values in Asians across a wide range of ages, along with the association of these CD values to corneal topographic measurements. Both peripheral and central CD values increased significantly with age. As age increased, the lowest CD moved from the periphery to the center. Keratometry and spherical aberration gradually increased with age, whereas there was no significant age-related change in corneal thickness.
Acknowledgments
We would like to thank Editage (https://www.editage.cn/) for English language editing.
Funding: This work was supported by Basic and Applied Basic Research Fund of Guangdong Province (No. 2021A1515111012), Shenzhen Science and Technology Program (No. JCYJ20210324142800001), the Medical Scientific Research Foundation of Guangdong Province of China (No. B2021216), and the SanMing Project of Medicine in Shenzhen (No. SZSM201812091).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-486/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-486/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the principles of Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Shenzhen Eye Hospital, and informed consent was taken from all individual participants or their legal guardians before data collection.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Meek KM, Knupp C. Corneal structure and transparency. Prog Retin Eye Res 2015;49:1-16. [Crossref] [PubMed]
- Qazi Y, Wong G, Monson B, Stringham J, Ambati BK. Corneal transparency: genesis, maintenance and dysfunction. Brain Res Bull 2010;81:198-210. [Crossref] [PubMed]
- Kashizuka E, Yamaguchi T, Yaguchi Y, Satake Y, Shimazaki J. Corneal Higher-Order Aberrations in Herpes Simplex Keratitis. Cornea 2016;35:1562-8. [Crossref] [PubMed]
- Fantes FE, Hanna KD, Waring GO 3rd, Pouliquen Y, Thompson KP, Savoldelli M. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol 1990;108:665-75. [Crossref] [PubMed]
- Consejo A, Jiménez-García M, Rozema JJ. Age-Related Corneal Transparency Changes Evaluated With an Alternative Method to Corneal Densitometry. Cornea 2021;40:215-22. [Crossref] [PubMed]
- Pakbin M, Khabazkhoob M, Pakravan M, Fotouhi A, Jafarzadehpur E, Aghamirsalim M, Hashemi H. Corneal Scheimpflug Densitometry in Photorefractive Keratectomy Candidates. Cornea 2020;39:1381-8. [Crossref] [PubMed]
- Wang XY, Zhang TQ, Rachwani AR, Blanco-Domínguez I, Rocha de Lossada C, Adán-Civiera AM, Peraza-Nieves J. New algorithm for corneal densitometry assessment based on anterior segment optical coherence tomography. Eye (Lond) 2022;36:1675-80. [Crossref] [PubMed]
- Hillenaar T, Cals RH, Eilers PH, Wubbels RJ, van Cleynenbreugel H, Remeijer L. Normative database for corneal backscatter analysis by in vivo confocal microscopy. Invest Ophthalmol Vis Sci 2011;52:7274-81. [Crossref] [PubMed]
- Wei R, Li M, Yang W, Shen Y, Zhao Y, Fu D, Shang J, Zhang J, Choi J, Zhou X. Corneal Densitometry After Small Incision Lenticule Extraction (SMILE) and Femtosecond Laser-Assisted LASIK (FS-LASIK): 5-Year Prospective Comparative Study. Front Med (Lausanne) 2020;7:521078. [Crossref] [PubMed]
- Morales-Fernández L, Benito-Pascual B, Pérez-García P, Perucho-González L, Sáenz-Francés F, Santos-Bueso E, García-Bella J, Sánchez-Jean R, García-Feijoo J, Martínez-de-la-Casa JM. Corneal densitometry and biomechanical properties in patients with primary congenital glaucoma. Can J Ophthalmol 2021;56:364-70. [Crossref] [PubMed]
- Çağlayan M, Öncül H, Alakus MF, Dag U. Corneal and lens densitometry with Pentacam HR in children with vernal keratoconjunctivitis. Clin Exp Optom 2021;104:156-61. [Crossref] [PubMed]
- Shen Y, Han T, Jhanji V, Shang J, Zhao J, Li M, Zhou X. Correlation Between Corneal Topographic, Densitometry, and Biomechanical Parameters in Keratoconus Eyes. Transl Vis Sci Technol 2019;8:12. [Crossref] [PubMed]
- Shen Y, Jian W, Sun L, Li M, Han T, Son J, Zhou X. One-Year Follow-Up of Changes in Corneal Densitometry After Accelerated (45 mW/cm2) Transepithelial Corneal Collagen Cross-Linking for Keratoconus: A Retrospective Study. Cornea 2016;35:1434-40. [Crossref] [PubMed]
- Zhao J, Yang W, Zhao J, Shen Y, Sun L, Han T, Wang X, Yao P, Zhou X. A four-year observation of corneal densitometry after implantable collamer lens V4c implantation. Ann Transl Med 2021;9:536. [Crossref] [PubMed]
- Garzón N, Poyales F, Illarramendi I, Mendicute J, Jáñez Ó, Caro P, López A, Argüeso F. Corneal densitometry and its correlation with age, pachymetry, corneal curvature, and refraction. Int Ophthalmol 2017;37:1263-8. [Crossref] [PubMed]
- Ní Dhubhghaill S, Rozema JJ, Jongenelen S, Ruiz Hidalgo I, Zakaria N, Tassignon MJ. Normative values for corneal densitometry analysis by Scheimpflug optical assessment. Invest Ophthalmol Vis Sci 2014;55:162-8. [Crossref] [PubMed]
- Alzahrani K, Carley F, Brahma A, Morley D, Hillarby MC. Corneal clarity measurements in healthy volunteers across different age groups: Observational study. Medicine (Baltimore) 2017;96:e8563. [Crossref] [PubMed]
- Patel S, McLaren J, Hodge D, Bourne W. Normal human keratocyte density and corneal thickness measurement by using confocal microscopy in vivo. Invest Ophthalmol Vis Sci 2001;42:333-9. [PubMed]
- Ma R, Liu Y, Zhang L, Ma J, Cui T, Lei Y, Hou J, Shen Z, Yi X, Liang G, Wang Y. Changes in Corneal Morphology with Age in Asian Population: A Multicenter Study of 30,618 Cases. Adv Ther 2021;38:5763-76. [Crossref] [PubMed]
- Daxer A, Misof K, Grabner B, Ettl A, Fratzl P. Collagen fibrils in the human corneal stroma: structure and aging. Invest Ophthalmol Vis Sci 1998;39:644-8. [PubMed]
- Al-Sayyari TM, Fawzy SM, Al-Saleh AA. Corneal spherical aberration in Saudi population. Saudi J Ophthalmol 2014;28:207-13. [Crossref] [PubMed]
- Elkitkat RS, Fouad YA, Shams A, Hamza I. Normative Values of Corneal Spherical Aberration, Pupil Size, and Other Key Refractive and Topographic Parameters in a Large Cohort of Egyptian Cataract Surgery Candidates. Clin Ophthalmol 2020;14:4571-7. [Crossref] [PubMed]


