
A rare V-shaped course variant of the posterior right diagonal artery: a case description and literature analysis
Introduction
Most coronary artery anomalies are discovered incidentally using coronary angiography or multidetector-row computed tomography (MDCT) (1,2). Determining the coronary artery anatomy and variations in coronary arterial branching is crucial for percutaneous coronary intervention (PCI) and coronary artery bypass surgery. Coronary artery anomalies in the posterior ventricular wall commonly concentrate on the perfusion arteries of the posterior interventricular septum, which mainly display a combination of multiple coronary branches running along the posterior interventricular sulcus (PIS). The posterior right diagonal artery (PRDA) is an important variant in the branching of the right coronary artery (RCA), which often occurs as a supplement or even a replacement for the posterior descending artery (PDA) and is mainly responsible for the perfusion of the inferior PIS (3). However, due to insufficient data, the origin, course, and identification of the PRDA remain undefined (3).
Herein, we report a rare variant of the V-shaped course of the PRDA that uncharacteristically perfused the superior part of the posterior ventricular septum. This variant appeared to arise from the right marginal artery (RMA) and traversed diagonally across the right ventricular wall to enter the superior PIS, terminating at the left atrioventricular groove.
Case presentation
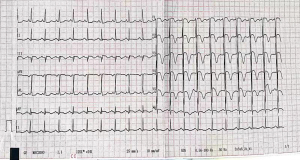
A 52-year-old female patient was admitted to our coronary care unit due to complaints of typical chest pain for 20 hours without any predisposing causes. Clinically suspicious risk factors related to ischemic heart disease were denied in the medical history, individual history, and family history. On admission, the patient was asymptomatic; no positive signs were reported upon physical examination, except for a mildly elevated blood pressure of 151/104 mmHg and an abdominal circumference of 100 cm. Her fasting blood glucose and low-density lipoprotein cholesterol levels were 6.82 and 3.27 mmol/L, respectively, both of which were elevated. Electrocardiography showed sinus rhythm with deep T wave inversion in leads V1–V6, ST-segment depression of 0.05 mV in leads I and aVL, and an ST-segment elevation of 0.1–0.2 mV in leads III and augmented vector foot (aVF) (Figure 1). The left ventricle (LV) end-diastolic diameter and LV ejection fraction were 5.4 cm and 0.615, respectively, and an ultrasound cardiogram showed that there was decreased motion in the inferior wall of the LV near the apex. Based on these clinical findings and a significant elevation in cardiac troponin I (cTNI) levels (11.71 ng/mL; normal 0–0.03 U/L), the patient was initially diagnosed with acute non–ST-segment elevation myocardial infarction (non-STEMI) of the inferior wall of the LV near the apex. She was immediately treated with peroral aspirin, ticagrelor, enoxaparin sodium injection, metoprolol, statins, losartan, and isosorbide mononitrate.
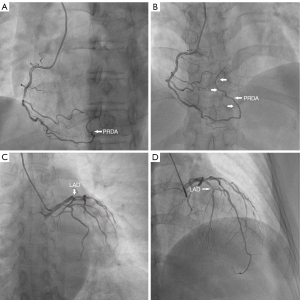
The Global Registry of Acute Coronary Events (GRACE) score of the patient was 194, and she had indications for early coronary angiography. Coronary angiography was performed 2 days after hospitalization, which revealed a V-shaped course variant of the distal RCA, appearing as a rare variation of the PRDA in a RCA “dominant” type. The left coronary arteries were of normal origin and distribution, but there was a critical stenotic lesion in the proximal portion of the LAD. Moreover, stenosis in the ostium lumen of the left circumflex branch and the middle part of the RCA was approximately 70% and 50%, respectively. Percutaneous coronary stent implantation was performed in the stenotic proximal portion of the LAD, which was considered the most suspicious site of myocardial infarction (Figure 2).
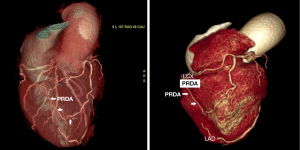
The patient then underwent MDCT to improve our understanding of this abnormal PRDA and determine the spatial relationships between the PRDA and the surrounding structures (Figure 3). We observed that the V-shaped course of the PRDA originated from the RMA and traversed diagonally across the right ventricular wall to enter the superior part of the PIS, terminating at the left atrioventricular groove, where it sent a posterior descending branch. A prolonged left anterior descending (LAD) with severe proximal stenosis was also clearly demonstrated crossing the apex and entering into the inferior third of the PIS. These imaging results suggested that the V-shaped variant of the PRDA along the superior PIS might be associated with insufficient perfusion of the LV inferior wall, particularly in the case of LAD stenosis.
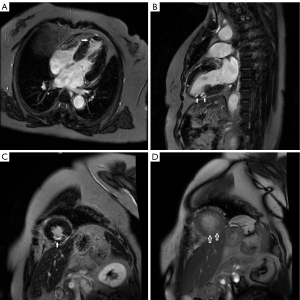
Cardiac magnetic resonance (CMR) revealed significant late gadolinium enhancement (LGE) of gadolinium in part of the apical segment of the left ventricular inferior wall, indicating that the V-shaped course PRDA coupled with stenosis of the LAD might be contributing to myocardial ischemia in the apical segment of the LV inferior wall (Figure 4).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Coronary artery anomalies are common congenital developmental anomalies in the general population. Since most anomalies are comparatively asymptomatic during childhood, they are typically discovered incidentally by coronary angiography or enhanced coronary MDCT examination. The incidence of coronary artery anomalies reported in various series ranges from 0.3% to 1.5% on coronary artery angiogram (4-8) and from 2.33% to 4.96% on MDCT (9,10). The RCA is characterized by a long main trunk and its distal multiple branches, which is significantly different from the bifurcated structure of the left coronary artery. Along the RCA, the sinoatrial nodal artery, cone branch, anterior branch of the right ventricle, RMA, atrioventricular nodal artery, and PDA can be sequentially branched (11). RCA anomalies mainly arise due to the absence, anastomosis, and ectopy of these branches. A rare anatomical variant of the PDA is described as the PRDA, which either originates directly from the RCA between the PDA and RMA or occurs as a continuation of the RMA, traversing diagonally across the posterior wall of the right ventricle to enter the middle third of the PIS with a terminal point near the cardiac apex. This contributes to the perfusion of the inferior part of the diaphragmatic heart face. In this case study, the PRDA was delivered at the RMA and traversed from the apex to the base of the heart, crossing the crux, and terminating at the left atrioventricular groove.
Several studies have investigated the incidence and characteristics of the PRDA. Nerantzis et al. (3) reported the first study investigating the incidence of the PRDA, which revealed 38 PRDAs in a total of 300 cases, with a significant difference among the 3 subgroups. Specifically, the incidence rate of the PRDA was 13.9% (37/266), 16.7% (1/6), and 0% (0/28) in the right dominant type, balanced type, and left dominant type, respectively (3). Margaris et al. (12) reported a PRDA prevalence of 13.7% in 607 patients undergoing coronary angiography. Similar results were also achieved in another study, with the PRDA occurring in 81 (15.1%), 2 (15.4%), and 0 (0%) patients with the right dominant type, balanced type, and left dominant type, respectively. Only 10% of the PRDA occurred as a continuation of the RMA, accounting for 1.32% of total patients (13). In another study by Nerantzis et al., they found that in 14% (7/50) of cases, the distal part of the PIS was occupied by the PRDA (3). Moreover, Ballesteros et al. (11) reported that 221 heart specimens had a slightly higher PRDA incidence of 17.2% in total and 22.1% in the right dominant type. Consistent with previous research, they also confirmed that the origin of the PRDA was predominantly from the RCA (33, 86.8%), while 13.2% were from the RMA, which accounted for only 2.3% of all cases (11).
The PRDA, as one of the perfusion arteries, is responsible for supplying blood to the posterior ventricular septum together with the PDA and the distal portion of the LAD crossing the apex. When present, the PDRA serves as a bridge between the RCA and the left anterior descending artery (12). The incidence of PRDA is significantly associated with the development of the PDA, which occurs more frequently with a short PDA, and partially or completely replaces the perfusion effect of the PDA on the inferior posterior interventricular septum (3). In contrast to the previous studies, our case study revealed an uncharacteristic course of the PRDA that was close to the letter V. Thus far, this kind of variant had not been reported in the literature, so we will temporarily refer to this as V-shaped PRDA. The V-shaped PRDA completely replaced the PDA by supplying retrograde perfusion of the upper posterior interventricular septum from the apex to the base of the heart along the PIS. To the best of our knowledge, this is the first report of this new variant of the PRDA.
Coronary artery anomalies are rarely identified throughout an individual’s life and are most often recognized in an autopsy, primarily because these anomalies are difficult to diagnose by routine examination or clinical testing. Selective coronary angiography is an appropriate imaging approach for accurate visualization of the coronary artery system. However, the ability of coronary angiography to assess the relationship between coronary arteries and their surrounding structures is relatively inadequate. MDCT is a powerful tool for assessing the spatial relationships between a coronary artery variant and the surrounding structures, avoiding unnecessary invasive coronary interventions (14,15). In the absence of the PRDA, the posterior right ventricular wall is perfused by small branches arising from the RCA. Notably, the PRDA has been confused with an accessory interventricular branch in some angiographic studies (16). Therefore, MDCT may be a good alternative diagnostic method; in our case study, the V-shaped course of the PRDA was well demonstrated using MDCT, as were the relationships among the arteries alone PIS (Figure 3).
Approximately 80% of congenital coronary artery anomalies are benign and clinically asymptomatic, while 20% are clinically significant (17,18). Identifying anomalies is significant in cases of interventions, including surgical or interventional revascularization. The fact that the PRDA, along with the anterior interventricular artery, perfuses the inferior PIS and its adjacent areas has important practical significance. Several observations underscore the important function of the PRDA and suggest that in the presence of an occluded PDA, bypass grafts should be anastomosed to the artery occupying the inferior part of the PIS, which could likely be the PRDA, rather than to the PDA (12). In the presence of anterior interventricular artery occlusion, the PRDA provides a bridge that can perfuse the inferior third of the ventricular septum area, thereby limiting the potential of myocardial ischemic progression (11). Unfortunately, the V-shaped course of the PRDA might contribute to myocardial ischemia of the apical segment of the inferior LV wall due to the absence of the bridge function. Thus, when analyzing coronary arteriography, it is important to look for the PRDA and consider that, depending on the type of PDA, the PRDA could be used to perfuse the inferior part of the posterior interventricular sulcus and the adjacent area (12).
Conclusions
To the best of our knowledge, this is the first report of a new variant of the PRDA with a characteristic V-shaped course perfusing the superior part of the posterior ventricular septum. The PRDA may play an important role in compensatory perfusion to the apical segment of the LV inferior wall. Thus, a V-shaped course of the PRDA may be a potential risk factor for myocardial ischemia in the apical segment of the LV inferior wall because it reduces the possibility of collateral circulation, particularly that related to LAD stenosis.
Acknowledgments
Funding: This study was supported by the Research Foundation of Beijing Friendship Hospital, Capital Medical University (No. yyqdkt2017-34).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-805/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ojha V, Pandey NN, Kumar S, Ramakrishnan S, Jagia P. Anomalous origin of left main coronary artery from pulmonary artery: Patient characteristics and imaging associations on multidetector computed tomography angiography. J Card Surg 2021;36:4043-53. [Crossref] [PubMed]
- Şahin T, Ilgar M. Investigation of the Frequency of Coronary Artery Anomalies in MDCT Coronary Angiography and Comparison of Atherosclerotic Involvement between Anomaly Types. Tomography 2022;8:1631-41. [Crossref] [PubMed]
- Nerantzis CE, Gribizi JE, Margaris NG, Antonelis JP, Salahas TI, Koroxenidis GT. Posterior right diagonal artery. Anat Rec 1994;238:528-32. [Crossref] [PubMed]
- Angelini P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation 2002;105:2449-54. [Crossref] [PubMed]
- Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary angiography. Cathet Cardiovasc Diagn 1990;21:28-40. [Crossref] [PubMed]
- Garg N, Tewari S, Kapoor A, Gupta DK, Sinha N. Primary congenital anomalies of the coronary arteries: a coronary arteriographic study. Int J Cardiol 2000;74:39-46. [Crossref] [PubMed]
- Aydinlar A, Ciçek D, Sentürk T, Gemici K, Serdar OA, Kazazoglu AR, Kumbay E, Cordan J. Primary congenital anomalies of the coronary arteries: a coronary arteriographic study in Western Turkey. Int Heart J 2005;46:97-103. [Crossref] [PubMed]
- Alexander RW, Griffith GC. Anomalies of the coronary arteries and their clinical significance. Circulation 1956;14:800-5. [Crossref] [PubMed]
- Karabay KO, Yildiz A, Geceer G, Uysal E, Bagirtan B. The incidence of coronary anomalies on routine coronary computed tomography scans. Cardiovasc J Afr 2013;24:351-4. [Crossref] [PubMed]
- Graidis C, Dimitriadis D, Karasavvidis V, Dimitriadis G, Argyropoulou E, Economou F, George D, Antoniou A, Karakostas G. Prevalence and characteristics of coronary artery anomalies in an adult population undergoing multidetector-row computed tomography for the evaluation of coronary artery disease. BMC Cardiovasc Disord 2015;15:112. [Crossref] [PubMed]
- Ballesteros LE, Ramirez LM, Quintero ID. Right coronary artery anatomy: anatomical and morphometric analysis. Rev Bras Cir Cardiovasc 2011;26:230-7. [Crossref] [PubMed]
- Margaris NG, Kostopoulos KG, Nerantzis CE, Filippatos GS, Kardaras FG, Salahas AI, Antonellis JP, Ifandis GP, Kranidis AI, Tavernarakis A. Posterior right diagonal artery. An angiographic study. Angiology 1997;48:673-7. [Crossref] [PubMed]
- Nerantzis CE, Lefkidis CA, Smirnoff TB, Agapitos EB, Davaris PS. Variations in the origin and course of the posterior interventricular artery in relation to the crux cordis and the posterior interventricular vein: an anatomical study. Anat Rec 1998;252:413-7. [Crossref] [PubMed]
- Kunimoto S, Sato Y, Kunimasa T, Kasamaki Y, Takayama T, Matsumoto N, Kasama S, Yoda S, Saito S, Hirayama A. Double left anterior descending artery arising from the left and right coronary arteries:depiction at multidetector-row computed tomography. Int J Cardiol 2009;132:e54-6. [Crossref] [PubMed]
- Gao X, Li H, Chen H. Myocardial ischemia due to a type IV dual LAD with the long LAD arising from the right sinus of valsalva: a case report and literature review. Intern Med 2015;54:2619-23. [Crossref] [PubMed]
- James TN. Anatomy of the coronary arteries in health and disease. Circulation 1965;32:1020-33. [Crossref] [PubMed]
- Spindola-Franco H, Grose R, Solomon N. Dual left anterior descending coronary artery: angiographic description of important variants and surgical implications. Am Heart J 1983;105:445-55. [Crossref] [PubMed]
- Ku L, Lv H, Yu Z, Ma X. A hitherto unreported combination of pulmonary stenosis, single coronary artery anomaly, and coronary sinus to left atrial communication. J Card Surg 2022;37:2842-4. [Crossref] [PubMed]


