
Role of magnetic resonance imaging for preoperative prediction of early biochemical failure in localized prostate cancer
Introduction
The standard care of localized prostate cancer (PCa) relies on surgery, external radiotherapy or brachytherapy. Radical prostatectomy (RP) remains the most common treatment for patients with life expectancy >10 years (1). However, biochemical recurrence at 10 years following RP goes up to 40% (with an associated mortality rate of 6%) (1), and RP can have specific complications (such as incontinence and erectile dysfunction) (2). For these reasons, selection of patients before RP appears essential. This selection is based on D’Amico or National Comprehensive Cancer Network (NCCN) risk classifications, combining histological aggressiveness (Gleason score), anatomical extension [(with digital rectal examination (DRE) or magnetic resonance imaging (MRI)] and PSA level (1).
Multi-parametric MRI (mpMRI) of the prostate is used to optimize cancer detection [Prostate Imaging Reporting and Data System (PIRADS) 2.1 standardized criteria] (3,4), for therapeutic decision guidance and research of local recurrence after curative treatment (5,6). Several MRI prognostic factors are already related with cancer recurrence: PIRADS score (7), apparent diffusion coefficient (ADC) value (8-10), extra-prostatic extension, invasion of seminal vesicles, size of the lesion or apical location (before radiotherapy) (11). Dynamic acquisition also provides useful information for tumor detection and characterization (12).
Plasmatic PSA level is expected to be undetectable within 6 weeks after successful RP (13). Persistently measurable plasmatic PSA in patients following RP is considered as an early biochemical failure (BF) (residual cancer in relation with micrometastases or residual disease in the prostatic fossa). High PSA velocity and unfavorable pathological characteristics tend to point to metastatic disease (1). Nevertheless, no consensus exists, and the majority of patients are treated by salvage radiotherapy alone (1). The recurrence-free survival rate at 5 years is low at 22% but survival in this patient group remains high (95% at 5 years) (1).
Predictive factors of early BF are scarcely described in the literature but are close to those exposed for later recurrence: pre-operative (Gleason score, clinical T stage, body mass index) and pathologic criteria (post-operative stage, extra-prostatic extension, lymph node positivity, positive surgical margin and tumor volume) (8,14,15). However, there is limited knowledge regarding mpMRI predictive factors of early BF.
The aim of our study was to evaluate the preoperative predictive factors of early BF after RP in patients with localized PCa, including clinical, biological, pathological and especially mpMRI factors in order to predict the effectiveness of prostatectomy. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-472/rc).
Methods
Selection of patients
In an observational retrospective cohort study we selected between December 2012 and June 2018 756 patients underwent RP in the University Hospital of Dijon (France) for initial treatment of localized newly diagnosed PCa. Inclusion dates were linked to a database of prostatectomies in our urology department, we searched for corresponding MRIs. From this database, we retrospectively selected patients who had the following inclusion criteria:
- PCa histologically proven by transrectal ultrasound guided biopsy: [at least 12 systematic biopsies more or less a few targeted biopsies (1 to 3) depending on the DRE, ultrasound or MRI].
- Curative treatment by RP ± lymph node dissection (1) after a collegial decision. For patients with intermediate or high risk, staging was performed using CT and bone scintigraphy (1).
- A 3T multiparametric MRI performed in our center before treatment.
Patients were not included if they had one of the following criteria:
- Gleason score <6 on the pathological surgery report.
- A history of PCa or any prostate surgery.
- Lymph node involvement or distant metastasis on initial assessment.
- An initially uncontrolled disease (PSA level detectable despite adjuvant radiotherapy which reflected a probable non-localized disease).
- Poor mpMRI quality (prostate bleeding, metal artifacts mainly related to total hip prosthesis, patient movement or digestive gas, incomplete MRI) or MRI performed in another imaging center.
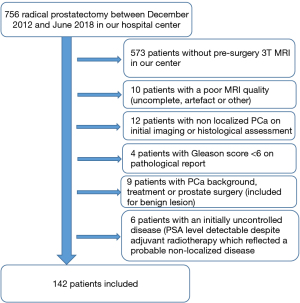
Following this selection process, we identified 142 patients. A flow chart was attached (Figure 1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study obtained ethical approval from the Institutional Review Board of the University Hospital of Dijon (France) and individual consent for this retrospective analysis was waived.
MRI technique and data
All patients underwent MRI on a 3 Tesla magnet (Trio Tim, Siemens Healthcare) with a pelvic antenna (9). The sequences of examination mainly included T2-weighted imaging, diffusion-weighted imaging, and dynamic contrast-enhanced imaging.
Table 1 summarizes the MRI protocol for imaging of the prostate gland. All MRI images were archived using a PACS system (GE Healthcare).
Table 1
| Parameters | T2-weighted | DWI | DCE |
|---|---|---|---|
| Orientation | 3 planes | Axial | Axial |
| TR (ms) | 3,600 | 4,200 | 3, 25 |
| TE (ms) | 75 | 101 | 1, 12 |
| Slice thickness (mm) | 3, 5 | 3, 5 | 3, 5 |
| FOV (mm) | 280 | 240 | 280 |
| B value (s/mm2) | NA | 0, 100, 800 | NA |
| Temporal resolution (s) | NA | NA | 6 |
| Total observation time (s) | NA | NA | 240 |
MRI, magnetic resonance imaging; DWI, diffusion weighted imaging; DCE, dynamic contrast-enhanced on T1 fat sat gadolinium sequence; TR, repetition time; TE, echo time; FOV, field of view.
MRI data analysis
Data analysis was performed using the Image J software. All MR images were retrospectively reviewed by a trained radiologist (not blinded from the initial report). An example of an MRI tumour lesion is attached in Figure 2.
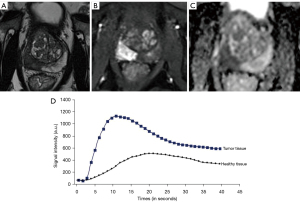
Global prostate data were assessed: prostate volume, ADC value in healthy prostate (transition and peripheral zone), and wash-in calculation (directing coefficient of the ascending slope) from DCE-MRI in healthy prostate.
Each intraprostatic lesion was classified according to the PIRADS algorithm V2.0. The topography and number of significant (PIRADS ≥3) lesions were reported. If more than one significant lesion was present, the index lesion was defined as the one with the highest PIRADS score and the largest diameter. Targets in anterior fibromuscular stroma (AFMS) were then analyzed with transition zone targets.
For the index lesion the following data were assessed: largest diameter (on axial T2 sequence), location, capsular contact (measured along the perimeter of the prostate to avoid a linear distance), tumor wash-in, tumor perfusion curve, ADC value [average region of interest (ROI) including the whole lesion and calculated average of the 10% of the lowest values] and MRI T stage was defined. To estimate presence of an extra-capsular extension (ECE) readers used Likert scale and the European Society of Urogenital Radiology (ESUR) criteria (1).
Clinical, biological and pathological data
Data and medical history were extracted using DxCare software. Clinical and pathological data were:
- Age at diagnosis.
- Time interval between diagnosis and surgery and between MRI and surgery.
- Clinical T stage (DRE).
- Pre-therapeutic and post-surgery PSA plasma level (before and 6 weeks after surgery, any significant PSA level was confirmed at 3 months).
- Any adjuvant treatment (radiotherapy or hormone therapy).
- Data related to biopsies: Gleason score/number of positive biopsies/size of the largest positive biopsy (cancer core length)/MRI performed prior to biopsies or not.
- Data from RP pathological report: prostate weight/number of cancer focus/tumor size/Gleason score/T stage/lymph node dissection and if applicable presence or not of lymph node invasion/ECE/invasion of seminal vesicles/surgical margin.
Other elements have been extrapolated:
- For each cancer described on pathological report: topographic concordance with MRI and biopsies.
- Staging according to NCCN classification. Three variants of this score were determined: the common clinical version (including PSA level, DRE T stage and biopsy Gleason score), the MRI-based version (MRI stage instead of clinical stage) and the pathological version (stage and Gleason of the prostatectomy pathological report).
Follow-up data
Follow-up data were annual PSA level, date of recurrence, survival status and effective follow-up duration in months.
At least annual consultation with a urologist from our center was scheduled. Otherwise, the patient was followed by his general practitioner and referred to the urologist if necessary. For patients with a follow-up less than 5 years in our database, the investigator attempted to know the recurrence’s status in 2020 by contacting the patient’s general practitioner.
Statistical analysis
Qualitative data were described using number (percentage) and quantitative data using median and [interquartile range]. Patients were categorized according to absence or presence of early BF, defined as a PSA level >0.10 ng/mL 6 weeks after surgery (16). For continuous variables, comparisons were performed after testing for variances homogeneity, test used depending on the normality of the distribution.
In detail, we used Wilcoxon test for age, PSA level, time between biopsies and surgery, number of positive biopsies, biopsy core length, time between MRI and surgery, MRI prostate volume, PSA density, MRI tumor size, length of MRI capsular contact, MRI wash in and postoperative index lesion size, We used Fisher test for clinical T stage, biopsies Gleason score, clinical NCCN, number of MRI lesions, MRI T stage, NCCN MRI risk, MRI tumor location, presence of an MRI capsular contact, postoperative number of lesions, postoperative Gleason score, postoperative T stage, postoperative NCCN risk, postoperative lymph node invasion and postoperative invasion of seminal vesicles. We used Student t-test for ADC values, the Kruskal-Wallis test for prostate weight and the Chi-square test for MRI performed before biopsies or not, PIRADS score, MRI perfusion curve, surgical lymph node dissection, postoperative ECE and post-operative margins. The χ2 test or the Fisher exact test was used for categorical variables. Finally, we add in Table 2 the analysis of correlation between independent variables. We recall that P tests the correlation coefficient against 0, i.e., two variables are correlated if the result is greater than 0.5.
Table 2
| Pearson correlation coefficient | ||||||||
|---|---|---|---|---|---|---|---|---|
| GS | NPB | BCL | Clinical NCCN | MRI NCCN | MLCC | MTS | MRI T stage | |
| GS | 1.00000; –; 142 | 0.29046; 0.0005; 142 | 0.41429; <0.0001; 142 | 0.78709; <0.0001; 142 | 0.64433; <0.0001; 142 | 0.30134; 0.0008; 123 | 0.24629; 0.0044; 132 | 0.34779; <0.0001; 142 |
| NPB | 0.29046; 0.0005; 142 | 1.00000; –; 142 | 0.59218; <0.0001; 142 | 0.47213; <0.0001; 142 | 0.43468; <0.0001; 142 | 0.43092; <0.0001; 123 | 0.39133; <0.0001; 132 | 0.38795; <0.0001; 142 |
| BCL | 0.41429; <0.0001; 142 | 0.59218; <0.0001; 142 | 1.00000; –; 142 | 0.53712; <0.0001; 142 | 0.51830; <0.0001; 142 | 0.39425; <0.0001; 123 | 0.43469; <0.0001; 132 | 0.46064; <0.0001; 142 |
| Clinical NCCN | 0.78709; <0.0001; 142 | 0.47213; <0.0001; 142 | 0.53712; <0.0001; 142 | 1.00000; –; 142 | 0.76257; <0.0001; 142 | 0.37612; <0.0001; 123 | 0.34573; <0.0001; 132 | 0.41589; <0.0001; 142 |
| MRI NCCN | 0.64433; <0.0001; 142 | 0.43468; <0.0001; 142 | 0.51830; <0.0001; 142 | 0.76257; <0.0001; 142 | 1.00000; –; 142 | 0.51227; <0.0001; 123 | 0.57324; <0.0001; 132 | 0.79835; <0.0001; 142 |
| MLCC | 0.30134; 0.0008; 121 | 0.43092; <0.0001; 121 | 0.39425; <0.0001; 121 | 0.37612; <0.0001; 121 | 0.51227; <0.0001; 121 | 1.00000; –; 123 | 0.79728; <0.0001; 121 | 0.57213; <0.0001; 121 |
| MTS | 0.24629; 0.0044; 132 | 0.39133; <0.0001; 132 | 0.43469; <0.0001; 132 | 0.34573; <0.0001; 132 | 0.57324; <0.0001; 132 | 0.79728; <0.0001; 123 | 1.00000; –; 132 | 0.63766; <0.0001; 132 |
| MRI T stage | 0.34779; <0.0001; 142 | 0.38795; <0.0001; 142 | 0.46064; <0.0001; 142 | 0.41589; <0.0001; 142 | 0.79835; <0.0001; 142 | 0.57213; <0.0001; 123 | 0.63766; <0.0001; 132 | 1.00000; –; 142 |
0.29046: Pearson correlation coefficient; 0.0005: confidence interval; 142: population covered by the analysis. GS, Gleason score on biopsies; NPB, number of positive biopsies; BCL, biopsy core length in mm; NCCN, National Comprehensive Cancer Network; MRI, magnetic resonance imaging; MLCC, MRI length of capsular contact in mm; MTS, MRI tumor size in mm.
Logistic regression analysis was performed to test for predictors of early BF. All variables were tested by univariate analysis. Because of the small number of events, only variables with a P value less than 0.01 in univariate analysis were selected for the multivariate analysis. Odds ratio (OR) were presented with their 99% confidence interval (99% CI). All the tests were 2-sided and a P value <0.01 was considered significant. Analyses were performed using SAS software version 9.4.
Results
Characteristics of patients
Preoperative clinical and biological characteristics of patients are reported in Table 3. The topographical concordance of the biopsies with pathological data was 84% (103/122 patients) in no early BF group versus 95% (19/20 patients) in early BF group (P=0.31).
Table 3
| Parameter | All patients (n=142) | No early BF (n=122) | Early BF (n=20) | P | Test |
|---|---|---|---|---|---|
| Age (years) | 65 [61–68] | 64 [60–67] | 66 [63–69] | 0.21 | Wilcoxon |
| PSA (ng/mL) | 7.3 [5.6–9.6] | 7.1 [5.5–9.0] | 9.3 [7.4–14.5] | <0.001 | Wilcoxon |
| Clinical T stage | 0.24 | Fisher | |||
| T1c | 73 | 66 | 7 | ||
| T2a | 45 | 38 | 7 | ||
| T2b | 16 | 12 | 4 | ||
| T2c | 6 | 5 | 1 | ||
| T3a | 2 | 1 | 1 | ||
| Time between biopsies and surgery (months) | 3.5 [2.4–4.4] | 3.5 [2.4–4.6] | 3.0 [2.3–4.3] | 0.18 | Student |
| Gleason score | <0.001 | Fisher | |||
| 3+3 | 63 | 60 | 3 | ||
| 3+4 | 47 | 43 | 4 | ||
| 4+3 | 16 | 9 | 7 | ||
| 4+4 | 10 | 6 | 4 | ||
| >4+4 | 6 | 4 | 2 | ||
| Number of positive biopsies | 4.0 [2.0–6.0] | 4.0 [2.0–6.0] | 6.0 [4.0–9.0] | <0.001 | Student |
| Biopsy core length (mm) | 7.0 [4.0–10.0] | 6.5 [4.0–10.0] | 10.5 [7.5–13.5] | <0.001 | Student |
| Clinical NCCN risk classification | <0.001 | Fisher | |||
| (Very) low risk | 40 | 38 | 2 | ||
| Intermediate favorable | 40 | 38 | 2 | ||
| Intermediate unfavorable | 42 | 32 | 10 | ||
| (Very) high | 20 | 14 | 6 |
Results are expressed as number for categorical variables and median [interquartile range] for continuous variables. BF, biochemical failure; PSA, prostate specific antigen; NCCN, National Comprehensive Cancer Network.
Patients with early BF had higher preoperative PSA level, Gleason score according to biopsies, number of positive biopsies, tumor biopsy core length and higher NCCN risk (P<0.001 for all). In contrast, there was no difference among the groups concerning age, time interval between diagnosis and surgery and clinical T stage.
Preoperative MRI
Preoperative MRI data are reported in Table 4 (global characteristics) and Table 5 (index lesion characteristics).
Table 4
| Parameter global characteristics (n=142) | All patients (n=142) | No early BF (n=122) | Early BF (n=20) | P | Test |
|---|---|---|---|---|---|
| Time interval between MRI and surgery (months) | 2.6 [1.4–4.1] | 2.6 [1.4–4.1] | 2.6 [1.3–4.3] | 0.86 | Fisher |
| MRI performed before biopsies | 33 (23.2%) | 25 (20.5%) | 8 (40.0%) | 0.08 | Fisher |
| Prostate volume (mm3) | 45 [35–62] | 43 [34–61] | 55 [46–66] | 0.05 | Wilcoxon |
| PSA density | 0.13 [0.10–0.18] | 0.13 [0.09–0.17] | 0.16 [0.12–0.27] | 0.05 | Wilcoxon |
| Number of lesions | 0.26 | Fisher | |||
| 0 | 10 | 10 | 0 | ||
| 1 | 106 | 92 | 14 | ||
| 2 | 22 | 17 | 5 | ||
| 3 | 4 | 3 | 1 | ||
| MRI T stage | <0.001 | Fisher | |||
| T2a | 44 | 43 | 1 | ||
| T2b | 8 | 8 | 0 | ||
| T2c | 42 | 39 | 3 | ||
| T3a | 22 | 16 | 6 | ||
| T3b | 15 | 5 | 10 | ||
| T4 | 1 | 1 | 0 | ||
| MRI NCCN risk classification | <0.001 | Fisher | |||
| (Very) low | 28 | 28 | 0 | ||
| Intermediate favorable | 23 | 22 | 1 | ||
| Intermediate unfavorable | 42 | 40 | 2 | ||
| (Very) high | 49 | 32 | 17 |
Results are expressed as number or number (percentage) for categorical variables and median [interquartile range] for continuous variables. MRI, magnetic resonance imaging; BF, biochemical failure; PSA, prostate specific antigen; NCCN, National Comprehensive Cancer Network.
Table 5
| Parameter index lesion characteristics (n=132) | All patients (n=132) | No early BF (n=112) | Early BF (n=20) | P | Test |
|---|---|---|---|---|---|
| PIRADS V 2.0 | 0.03 | Chi-Square | |||
| 3 | 4 | 4 | 0 | ||
| 4 | 50 | 47 | 3 | ||
| 5 | 78 | 61 | 17 | ||
| Tumor location | 0.24 | Fisher | |||
| Peripheral zone | 97 | 79 | 18 | ||
| Central zone | 32 | 30 | 2 | ||
| Anterior fibro-muscular stroma | 3 | 3 | 0 | ||
| Tumor size (mm) | 16 [12–21] | 15 [11–21] | 20 [14–37] | <0.001 | Wilcoxon |
| Capsular contact (yes) | 123 (93.2%) | 103 (92.0%) | 20 (100.0%) | 0.35 | Fisher |
| Length of capsular contact (mm) | 13 [9–18] | 12 [9–16] | 21 [15–38] | <0.001 | Wilcoxon |
| ADC value (mm2/s) | 808 [701–933] | 826 [713–936] | 760 [636–860] | 0.06 | Student |
| Average of the lowest 10% of ADC’s value (mm2/s) | 591 [453–742] | 602 [468–747] | 566 [409–679] | 0.22 | Student |
| Ratio ADC lesion/ADC healthy area | 0.5 [0.4–0.6] | 0.6 [0.5–0.6] | 0.5 [0.4–0.6] | 0.06 | Student |
| Wash in coefficient | 200 [156–267] | 197 [152–259] | 222 [190–272] | 0.09 | Student |
| Type of perfusion curve | 0.96 | Chi-Square | |||
| 1 | 6 | 5 | 1 | ||
| 2 | 43 | 36 | 7 | ||
| 3 | 83 | 71 | 12 |
Results are expressed as number or number (percentage) for categorical variables and median [interquartile range] for continuous variables. MRI, magnetic resonance imaging; BF, biochemical failure; PIRADS, Prostate Imaging Reporting and Data System; ADC, apparent diffusion coefficient.
In most of the patients (109, 77%), diagnostic biopsies were performed before MRI. The topographical concordance of MRI with pathological data was 88% (125 patients) with 105 patients (86%) in no early BF group and 20 patients (100%) in early BF group. Ten (7%) of the 142 patients didn’t have target lesion visible on MRI, none of them were in the early BF group. There was no difference among the groups concerning all these parameters.
MRI T stage and MRI NCCN risk classifications were significantly different among the groups (P<0.001 for both). Ten out of 20 patients (50%) in early BF group had a seminal vesicles invasion on MRI (stage 3b) versus 5 out of 112 patients (4.5%) in the other group.
There were 157 target lesions identified in 132 patients. The MRI lesion index was most often localized in the peripheral zone [97 out of 132 patients (73.5%)]. A PIRADS 5 score was most frequently found for the index lesion (78 patients–59 %). Most of the target lesions had a capsular contact (n=123; 93%), the prevalence of these last criteria weren’t significantly different between our groups. There was also no significant difference for the median ADC value of the index lesion (P=0.06), the mean value of the lowest 10% ADC (P=0.22), neither for the ratio ADC target lesion/healthy prostate (P=0.06). There was no difference either concerning DCE-MRI parameters such as wash-in coefficient (P=0.09) or type of perfusion curve (P=0.96). Only two MRI parameters of the index lesion were significantly different among the groups: the length of the capsular contact and the tumor size that were longer for patients with early BF (P<0.001 for both).
Postoperative pathological data
Postoperative pathological data are reported in Table 6.
Table 6
| Parameter | All patients (n=142) | No early BF (n=122) | Early BF (n=20) | P | Test |
|---|---|---|---|---|---|
| Prostate weight (g) | 55 [46–71] | 54 [46–70] | 57 [52–80] | 0.10 | Wilcoxon |
| Number of lesions | 0.23 | Fisher | |||
| 1 | 57 | 49 | 8 | ||
| 2 | 59 | 52 | 7 | ||
| 3 | 25 | 21 | 4 | ||
| 4 | 1 | 0 | 1 | ||
| Gleason score | <0.001 | Fisher | |||
| 3+3 | 20 | 19 | 1 | ||
| 3+4 | 81 | 78 | 3 | ||
| 4+3 | 21 | 13 | 8 | ||
| 4+4 | 13 | 8 | 5 | ||
| >4+4 | 7 | 4 | 3 | ||
| Pathological T stage | <0.001 | Fisher | |||
| T2a | 17 | 17 | 0 | ||
| T2b | 1 | 1 | 0 | ||
| T2c | 67 | 64 | 3 | ||
| T3a | 36 | 31 | 5 | ||
| T3b | 20 | 9 | 11 | ||
| T4 | 1 | 0 | 1 | ||
| Pathological NCCN classification | <0.001 | Fisher | |||
| (Very) low | 4 | 4 | 0 | ||
| Intermediate favorable | 19 | 18 | 1 | ||
| Intermediate unfavorable | 55 | 53 | 2 | ||
| (Very) high | 64 | 47 | 17 | ||
| Lymph node dissection | 60 (42.3) | 44 (36.1) | 16 (80.0) | <0.001 | Chi-Square |
| Lymph node invasion proven by histology | 13 (21.7) | 2 (4.5) | 10 (50.0) | <0.001 | Fisher |
| Size of the index lesion (mm) | 22 [16–28] | 20 [16–26] | 35 [28–46] | <0.001 | Student |
| ECE | <0.001 | Chi-Square | |||
| Not invaded | 39 | 39 | 0 | ||
| Invaded not exceeded | 48 | 45 | 3 | ||
| Invaded exceeded | 55 | 38 | 17 | ||
| Invasion of seminal vesicles | 21 (14.8) | 9 (7.4) | 12 (60.0) | <0.001 | Fisher |
| Margins | <0.001 | Chi-Square | |||
| R0 | 104 | 97 | 7 | ||
| R1 | 38 | 25 | 13 |
Results are expressed as number or number (percentage) for categorical variables and median [interquartile range] for continuous variables. BF, biochemical failure; NCCN, National Comprehensive Cancer Network; ECE, extra-capsular extension.
There were 254 tumor lesions in our 142 patients, many of MRI unseen foci were subcentimetric with a Gleason score ≤3+3 (87 out of the 97 unseen tumor foci). Between 1 and 4 tumor foci were identified by patients. There was no significant difference among the groups concerning these criteria. Patients with early BF more frequently had lymph node involvement (P<0.001—11 out of 20 early BF with proven lymph node involvement).
As with the pre-operative data, Gleason score/T stage/NCCN risk and the size of the main tumor focus were also higher in patients with early BF (P<0.001 for all). Pathological ECE, surgical margin (R1) and seminal vesicle invasion were also significantly higher (P<0.001).
Post-operative data and follow-up
Following surgery, 33 patients received adjuvant treatment including all patients in the early BF group and 13 patients in no early BF group. Twelve patients received only radiotherapy, 2 only hormone therapy and 19 both treatments [including 15 patients (75%) in early BF group] after a collegial decision. In the early BF group, the median PSA level 6 weeks after surgery was 0.48 (0.19–1.21) ng/mL.
The median follow-up time was 62.7 (29.6–79.4) months with 79 patients (56%) with an effective follow-up >5 years. No death related to PCa was observed. At the end of the follow-up, a total of 30 patients (21%) had recurrence of their PCa including 9 (45%) in the early BF group (P=0.014). Time before recurrence was 39 (25.0–60.0) months without significant difference between groups.
Predictive factors of early BF
Logistic regression analysis was performed to determine predictive factors of early BF. All parameters listed in Tables 4,5 were tested by univariate analysis. Significant criteria were:
- Gleason score ≥4+3: OR =9.6 (2.5–36.8); P<0.001; AUC =0.75.
- Tumor biopsy core length >7 mm: OR =4.3 (1.1–17.1); P=0.006; AUC =0.71.
- Clinical NCCN ≥ intermediate unfavorable: OR =6.0 (1.4–26.0); P<0.002; AUC =0.71.
- MRI NCCN ≥ high risk: OR =13.9 (2.8–69.5); P<0.001; AUC =0.79.
- MRI T stage ≥3: OR =22.3 (4.4–114.5); P<0.001; AUC =0.83.
- Size of capsular contact >20 mm: OR >8.7 (2.1–36.3); P<0.001; AUC =0.70.
Pre-surgery PSA level (thresholds of 10 or 20 ng/mL as used in NCCN classification), having at least 4 positive biopsies, a cut off at 17 mm for index lesion size or a cut off at 10 mm for capsular contact length were not significant [whereas the cut off at 20 mm (16) was significant].
For multivariate analysis we tested pairwise combinations of pre-operative, MRI or mixed data. Only relevant analyses are reported (P<0.01/AUC >0.80):
- Gleason ≥4+3 and capsular contact length >20 mm: OR =6.6 (1.6–28.3) and 6.3 (1.3–29.7)/P<0.001 and P=0.002/AUC =0.80 (99% CI: 0.64–0.95).
- Gleason ≥4+3 and MRI T stage ≥3: OR 6.8 (1.4–32.5) and 17.4 (3.2–94.9)/P=0.002 and P<0.001/AUC =0.89 (99% CI: 0.77–1.00).
- NCCN IRM ≥ high risk and capsular contact length >20 mm: OR =8.1 (2.2–106.3) and 4.82 (1.1–22.7)/P=0.001 and P=0.009/AUC =0.83 (99% CI: 0.69–0.96).
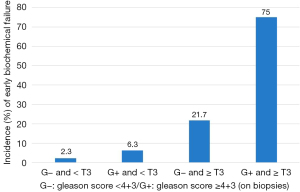
The best association for prediction of a radical prostatectomy early BF was preoperative Gleason score ≥4+3 and MRI T stage ≥3. Presence of these two factors permitted to predict early BF after RP with a sensitivity of 60%, a specificity of 97%, a positive predictive value of 75%, a negative predictive value of 94%, and an accuracy of 91% (Figure 3).
Discussion
RP remains the treatment of choice for eradication of localized PCa. A high risk of extra-prostatic extension is a usual contraindication for nerve sparing. European Association of Urology (EAU) has established guidelines for the evaluation of tumor extension before RP, recently updated (17), mpMRI may be helpful for selecting a nerve-sparing approach because it has good specificity but low sensitivity for detecting pT3a stages. Previous studies have shown the interest of mpMRI for prediction of recurrence following RP (10,11,18). By focusing on prediction of persistence of detectable post-operative PSA, defined in our study as early BF, our result emphasizes the crucial role of mpMRI before RP. In addition to Gleason score on biopsies, MRI capsular contact length and MRI T stage confirm their major role in post-operative PSA significant level prediction.
Indeed, the combination of significant cancer on biopsy (Gleason ≥4+3) and suspected extracapsular extension/seminal vesicle invasion (stage ≥ T3a) is 75% predictive of incomplete surgery (80% for the combination of Gleason score and capsular contact >20 mm), all with negative predictive values >90%. A multivariate prognostic score combining these different factors should be refined by a larger cohort in order to predict the effectiveness of surgery especially since patients with early BF have a high risk of recurrence and progression disease. A potential predictive MRI/Gleason score could counter-indicate RP in patients identified at high risk of early BF, avoiding surgical complications and directing patients to alternative treatment (radiotherapy and/or hormone therapy).
The distribution of index lesions didn’t show any topographical predominance outside the usual distribution of cancers between peripheral zone (about 70%) and transition zone (about 30%) (1) in contrast to other studies (14). The number of target lesions on MRI didn’t influence the risk of early BF. The patient’s prognosis appears to be largely driven by the index lesion in the case of a RP.
T3 or higher suspected T-stage on MRI is the best threshold to predict early PSA failure despite the relative subjectivity of ECE on MRI. Pathological analysis and MRI bring an up-staging of NCCN classification compared to clinical data as in other studies (19). The size of the target lesion is also associated with the risk of early BF.
In our study, PIRADS score is not associated with the risk of early BF, in contrast to its well-established role for prediction of recurrence (20) and characterization of malignant lesions (21). Our data also shows a limit of significance of the target/healthy area ADC ratio, despite the interest of ADC value in detecting significant lesions and the estimation of the aggressiveness having already been demonstrated (4,22). Others ADC data weren’t significant. Similarly, evaluation of perfusion parameters (wash-in and type of perfusion curve) weren’t predictors of early BF in our study, despite the role of quantitative perfusion in the prediction of significant lesion/tumor aggressiveness (5,23).
In contrast, the length of the capsular contact is one of the most interesting MRI data for prediction of early BF. We studied 2 cut offs: the commonly used 10 mm threshold and 20 mm according to the recent study by Mendez et al. (24). A threshold of 20 mm seems more relevant than 10 mm to predict an early BF (50% of patients in group 2 for 20 mm). Definition of significant capsular contact should be redefined in the future. According to our results, it is clear that mpMRI improves the tumor T classification and modifies the prognostic of PCa even if it remains dependent and limited by the radiologist’s experience.
The functional parameters of the index tumor (such as ADC value or perfusion profile), which help to characterize its aggressiveness, are interesting markers of the risk of recurrence, but fail in our study to predict early BF following RP.
According to the literature, Gleason score is associated with ADC value. In our study, the ADC is at the limit of significance (P=0.06) probably due to the small study population.
In contrast, our results confirm that the morphological characteristics of the index tumor (size of the lesion, length of capsular contact, presence of an ECE) are more relevant in predicting early BF.
Our study has several limitations. The sample size, as well as the retrospective nature of this single institution study, limits the generalizability of our results. The majority of the biopsies weren’t directed by a prior MRI. This common practice during the patient inclusion period is no longer recommended nowadays according to the superiority of mpMRI associated with targeted biopsies for the detection of significant cancers (25). Our MRI detection sensitivity could have been reduced because of these conditions. Furthermore, we are not able to ensure that patients with early BF did not have metastatic or lymphatic disease prior to surgery (despite our exclusion criteria).
Conclusions
Combination of preoperative Gleason score and T stage based on mpMRI permits to predict persistence of detectable PSA (early BF) following RP for patients with localized PCa, with a high accuracy (positive predictive value of 75% and negative predictive value of 94%). These results highlight the major importance of mpMRI in the initial staging of PCa, for lesion detection but also for prognostic stratification, in order to optimize therapeutic strategies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-472/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-472/coif). AC serves as an unpaid editorial board member of Quantitative Imaging in Medicine and Surgery; RL serves as an unpaid deputy editor of Quantitative Imaging in Medicine and Surgery. The other authors have no conflicts of interest to declare.
Ethcial Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study obtained ethical approval from the Institutional Review Board of the University Hospital of Dijon (France) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rozet F, Hennequin C, Beauval JB, Beuzeboc P, Cormier L, Fromont-Hankard G, Mongiat-Artus P, Ploussard G, Mathieu R, Brureau L, Ouzzane A, Azria D, Brenot-Rossi I, Cancel-Tassin G, Cussenot O, Rebillard X, Lebret T, Soulié M, Renard Penna R, Méjean A. French ccAFU guidelines - Update 2018-2020: Prostate cancer. Prog Urol 2018;28:R81-132. Retraction in: Prog Urol 2018;28:S79-130. [Crossref] [PubMed]
- Redondo C, Rozet F, Velilla G, Sánchez-Salas R, Cathelineau X. Complications of radical prostatectomy. Arch Esp Urol 2017;70:766-76. [PubMed]
- Ullrich T, Quentin M, Oelers C, Dietzel F, Sawicki LM, Arsov C, Rabenalt R, Albers P, Antoch G, Blondin D, Wittsack HJ, Schimmöller L. Magnetic resonance imaging of the prostate at 1.5 versus 3.0T: A prospective comparison study of image quality. Eur J Radiol 2017;90:192-7. [Crossref] [PubMed]
- Boesen L. Multiparametric MRI in detection and staging of prostate cancer. Dan Med J 2017;64:B5327. [PubMed]
- Panebianco V, Barchetti F, Sciarra A, Musio D, Forte V, Gentile V, Tombolini V, Catalano C. Prostate cancer recurrence after radical prostatectomy: the role of 3-T diffusion imaging in multi-parametric magnetic resonance imaging. Eur Radiol 2013;23:1745-52. [Crossref] [PubMed]
- Renard-Penna R, Michaud L, Cormier L, Bastide C, Beuzeboc P, Fromont G, Hennequin C, Mongiat-Artus P, Peyromaure M, Rozet F, Richaud P, Salomon L, Soulié M. membres du Comité de cancérologie de l’AFU. Imagery of treated prostate cancer. Prog Urol 2015;25:128-37. [Crossref] [PubMed]
- Gündoğdu E, Emekli E, Kebapçı M. Evaluation of relationships between the final Gleason score, PI-RADS v2 score, ADC value, PSA level, and tumor diameter in patients that underwent radical prostatectomy due to prostate cancer. Radiol Med 2020;125:827-37. [Crossref] [PubMed]
- Winkel DJ, Breit HC, Shi B, Boll DT, Seifert HH, Wetterauer C. Predicting clinically significant prostate cancer from quantitative image features including compressed sensing radial MRI of prostate perfusion using machine learning: comparison with PI-RADS v2 assessment scores. Quant Imaging Med Surg 2020;10:808-23. [Crossref] [PubMed]
- Park J, Rho MJ, Moon HW, Kim J, Lee C, Kim D, Kim CS, Jeon SS, Kang M, Lee JY. Dr. Answer AI for Prostate Cancer: Predicting Biochemical Recurrence Following Radical Prostatectomy. Technol Cancer Res Treat 2021;20:15330338211024660. [Crossref] [PubMed]
- Yoon MY, Park J, Cho JY, Jeong CW, Ku JH, Kim HH, Kwak C. Predicting biochemical recurrence in patients with high-risk prostate cancer using the apparent diffusion coefficient of magnetic resonance imaging. Investig Clin Urol 2017;58:12-9. [Crossref] [PubMed]
- Woo S, Han S, Kim TH, Suh CH, Westphalen AC, Hricak H, Zelefsky MJ, Vargas HA. Prognostic Value of Pretreatment MRI in Patients With Prostate Cancer Treated With Radiation Therapy: A Systematic Review and Meta-Analysis. AJR Am J Roentgenol 2020;214:597-604. [Crossref] [PubMed]
- Cristel G, Esposito A, Briganti A, Damascelli A, Brembilla G, Freschi M, Ambrosi A, Montorsi F, Del Maschio A, De Cobelli F. MpMRI of the prostate: is there a role for semi-quantitative analysis of DCE-MRI and late gadolinium enhancement in the characterisation of prostate cancer? Clin Radiol 2019;74:259-67. [Crossref] [PubMed]
- Kim JK, Jeong CW, Ku JH, Kim HH, Kwak C. Prostate specific antigen (PSA) persistence 6 weeks after radical prostatectomy and pelvic lymph node dissection as predictive factor of radiographic progression in node-positive prostate cancer patients. J Cancer 2019;10:2237-42. [Crossref] [PubMed]
- Harmon SA, Gesztes W, Young D, Mehralivand S, McKinney Y, Sanford T, Sackett J, Cullen J, Rosner IL, Srivastava S, Merino MJ, Wood BJ, Pinto PA, Choyke PL, Dobi A, Sesterhenn IA, Turkbey B. Prognostic Features of Biochemical Recurrence of Prostate Cancer Following Radical Prostatectomy Based on Multiparametric MRI and Immunohistochemistry Analysis of MRI-guided Biopsy Specimens. Radiology 2021;299:613-23. [Crossref] [PubMed]
- Al Hussein Al Awamlh B, Marks LS, Sonn GA, Natarajan S, Fan RE, Gross MD, Mauer E, Banerjee S, Hectors S, Carlsson S, Margolis DJ, Hu JC. Multicenter analysis of clinical and MRI characteristics associated with detecting clinically significant prostate cancer in PI-RADS (v2.0) category 3 lesions. Urol Oncol 2020;38:637.e9-637.e15. [Crossref] [PubMed]
- Seyedin SN, Watkins JM, Mayo Z, Snow AN, Laszewski M, Russo JK, Mott SL, Tracy CR, Smith MC, Buatti JM, Caster JM. A Recursive Partitioning Analysis Demonstrating Risk Subsets for 8-Year Biochemical Relapse After Margin-Positive Radical Prostatectomy Without Adjuvant Hormone or Radiation Therapy. Adv Radiat Oncol 2021;6:100778. [Crossref] [PubMed]
- Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2021;79:243-62. [Crossref] [PubMed]
- Capogrosso P, Vertosick EA, Benfante NE, Sjoberg DD, Vickers AJ, Eastham JA. Can We Improve the Preoperative Prediction of Prostate Cancer Recurrence With Multiparametric MRI? Clin Genitourin Cancer 2019;17:e745-50. [Crossref] [PubMed]
- Kumar A, Samavedi S, Mouraviev V, Bates AS, Coelho RF, Rocco B, Patel VR. Predictive factors and oncological outcomes of persistently elevated prostate-specific antigen in patients following robot-assisted radical prostatectomy. J Robot Surg 2017;11:37-45. [Crossref] [PubMed]
- Yang X, Shi Y, Lin Y, Tian Y. Efficacy of radical prostatectomy on prostate cancer patients and analysis of risk factors for biochemical recurrence after radical prostatectomy. J BUON 2020;25:2623-8. [PubMed]
- Loffroy R, Chevallier O, Moulin M, Favelier S, Genson PY, Pottecher P, Crehange G, Cochet A, Cormier L. Current role of multiparametric magnetic resonance imaging for prostate cancer. Quant Imaging Med Surg 2015;5:754-64. [PubMed]
- Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, Rouviere O, Logager V, Fütterer JJ. ESUR prostate MR guidelines 2012. Eur Radiol 2012;22:746-57. [Crossref] [PubMed]
- Kir G, Arikan EA, Seneldir H, Ankarali H, Oznergiz S, Olgun ZC, Yildirim A. Determining the cut-off values of tumor diameter, degree of extraprostatic extension, and extent of surgical margin positivity with regard to biochemical recurrence of prostate cancer after radical prostatectomy. Ann Diagn Pathol 2020;44:151431. [Crossref] [PubMed]
- Mendez G, Foster BR, Li X, Shannon J, Garzotto M, Amling CL, Coakley FV. Endorectal MR imaging of prostate cancer: Evaluation of tumor capsular contact length as a sign of extracapsular extension. Clin Imaging 2018;50:280-5. [Crossref] [PubMed]
- Briganti A, Joniau S, Gontero P, Abdollah F, Passoni NM, Tombal B, Marchioro G, Kneitz B, Walz J, Frohneberg D, Bangma CH, Graefen M, Tizzani A, Frea B, Karnes RJ, Montorsi F, Van Poppel H, Spahn M. Identifying the best candidate for radical prostatectomy among patients with high-risk prostate cancer. Eur Urol 2012;61:584-92. [Crossref] [PubMed]


