
Effects of lauromacrogol injection under contrast-enhanced ultrasound guidance on cesarean scar pregnancy: a prospective cohort study
Introduction
Cesarean scar pregnancy (CSP) is a rare, severe, life-threatening ectopic pregnancy in which the embryo is implanted in a cesarean section scar in the lower uterine segment (1,2). As one of the long-term complications of cesarean delivery, CSP has an incidence ranging from 1 in 1,008 to 2,500 among all cesarean deliveries (1). CSP may cause uterine rupture, life-threatening hemorrhage, high-risk hysterectomy, and maternal mortality (3). However, these can be prevented by early recognition and proper management. Currently, multiple treatment methods are used to manage CSP, including methotrexate (MTX) application (4), uterine artery embolization (UAE) (1), ultrasound-guided local administration of embryocides (5), hysteroscopyic and laparoscopic removal (6), and transvaginal hysterotomy (7).
Nonetheless, for CSP patients with a higher risk of bleeding, pretreatment is suggested before uterine curettage to reduce the risk of massive bleeding during uterine curettage (8). The most commonly used pretreatment method is UAE or UAE combined with MTX (UAEM). As a cytotoxic agent able to inhibit the growth of trophoblasts, MTX has been widely used in the treatment of CSP. However, MTX may induce hepatic and renal dysfunction and myelosuppression (9). UAE combined with curettage may be a favorable choice for CSP in decreasing menstrual blood volume (1), and curettage following combined UAE and MTX pretreatment is also effective in treating CSP (10). The procedures of UAE with or without MTX injection before curettage require catheterization of the uterine artery before injection of MTX (for UAE plus MTX injection) and embolic materials to block the uterine artery for UAE (1,4,10). As such, these methods are not good for women who want to conceive in the future because of the adverse effects, including ovarian dysfunction and quick establishment of collateral circulation (1,4,10).
Lauromacrogol is a novel agent for blood vessel hardening that is widely applied in the sclerotherapy of multiple diseases, including hemangioma, venous malformations, ovarian cysts, uterine fibroids, and varicosity (11-14). It can provoke endothelial maceration and destroy the cellular elements by acting as a detergent on the phospholipid layer of the cell membrane. This medicine has no mutagenic, carcinogenic, or embryotoxic effect and will not produce pain or extravasation necrosis (11-14). Currently, there are only two reports of the use of lauromacrogol in managing CSP as a safe, novel therapeutic approach for CSP (2,14). We hypothesized that contrast-enhanced ultrasound-guided sclerotherapy with lauromacrogol injection (CEUSL) might be as effective and safe in facilitating hysteroscopy curettage for CSP as UAE or UAE combined with MTX. This study was consequently carried out to test this hypothesis. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-190/rc).
Methods
Participants
This prospective cohort study was conducted from January 2018 to December 2020, and approval was obtained from the ethics committee of Zhejiang Provincial People’s Hospital (No. JS2018067). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients or their family members signed the informed consent to participate. The inclusion criteria were patients with CSP confirmed by a history of cesarean delivery, a positive pregnancy test, and sonographic criteria of CSP (Figure 1) (15). Patients were included if they had an average diameter of the gestational sac or mass less than 5.0 cm and high hemorrhagic risk during curettage (total bleeding risk score ≥10) (8). The bleeding risk score was evaluated according to the risk scoring system of intraoperative hemorrhage for CSP designed by Wang et al. (8) based on the risk factors of gestational age, beta human chorionic gonadotropin (β-hCG), maximal CSP mass, and peritrophoblastic perfusion (Table 1). The higher the score is, the higher the risk of bleeding. Patients with a total score ≥10 were identified as being at a high risk of bleeding. The exclusion criteria were patients undergoing direct vaginal removal or laparotomy or laparoscopic removal of the pregnancy; or patients with severe diseases in the liver, kidney, heart, or lung, which precluded surgical procedures. Patients were divided into the UAE group (treated with UAE alone), the UAEM group (treated with UAE combined with MTX), and the contrast-enhanced ultrasound-guided lauromacrogol injection (CEUSL) group based on the desire of the patient. According to the patient’s specific condition, the advantages, disadvantages, and potential risks of different treatment methods were explained to the patients. Moreover, the efficacy and safety of the novel pretreatment approach of CEUSL were explained to the patients (2,14). Following this, the patient voluntarily chose the treatment scheme. The doctor fully respected the patient’s choice without interfering with the patient’s intention. After pretreatment with the above approaches, all patients underwent curettage or hysteroscopy.
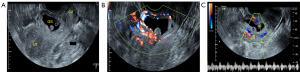
Table 1
| Risk factors | Conditions | Scores |
|---|---|---|
| Gestational age (week) | <8 | 0 |
| ≥8 | 2 | |
| Pretreatment blood β-hCG | <20,000 mIU/mL | 0 |
| ≥20,000 mIU/mL | 1 | |
| Maximal diameter of CSP mass | <5 cm | 0 |
| ≥5 cm | 7 | |
| Muscle thickness at the scar’s thinnest part | >0.15 cm | 0 |
| ≤0.15 cm | 4 | |
| Peritrophoblastic blood flow signals | None | 0 |
| Some | 7 | |
| Effluent | 10 |
β-hCG, beta human chorionic gonadotropin; CSP, cesarean scar pregnancy.
Ultrasound measurement
Transvaginal 2-dimensional ultrasound (Logiq E9/Voluson E8, GE Healthcare, Chicago, IL, USA) was used to routinely measure the diameters of the gestational sac, myometrial thickness at the scar, and embryo length. Color Doppler ultrasound was used to evaluate the blood flow signals in and around the gestational sac (Figure 1).
CEUSL procedure
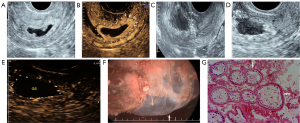
Before sclerotherapy, contrast-enhanced ultrasound (CEUS) was performed to evaluate the blood supply (Figure 2A,2B) and localize the gestational sac for lauromacrogol injection around the gestational sac. Guided by vaginal ultrasound, a 21-gauge 20 cm-long Chiba needle (GALLINI S.R.L Co. Ltd., Italy) was used to puncture the vaginal vault, and a total amount of 8–20 mL of lauromacrogol (10 mL:100 mg; Tianyu Pharmaceutical Co. Ltd., Shanxi, China) was slowly and continuously injected at several points around the gestational sac, with 1–3 mL each time and 4–8 times in total per person to block the blood supply of villi (Figure 2C). There was no definite or standard injection time interval, which ranged from seconds or minutes. If the CEUS was needed to judge the blood perfusion around the gestational sac and locate the injection site, several minutes were necessary. The application principle of lauromacrogol is to reduce the dose and time at each injection as much as possible to achieve the hardening effect while decreasing the risks associated with the injection. The total amount of lauromacrogol required by each patient was different but was applied to completely block the blood supply to the gestational sac, which was related to the number of days of menopause, size of the gestational sac, abundance of blood supply of trophoblast around the gestational sac, and blood β-hCG. During sclerotherapy, CEUS was performed to accurately guide the injection site. When circular enhancement was present around the gestational sac on CEUS to confirm that no contrast medium perfusion was in the villus area, the effect of sclerotherapy was suggested to be significant (Figure 2D,2E), and blood supply was considered to be blocked (16), which indicated the end of the injection. CEUS could clearly show the microcirculation perfusion of CSP from the myometrium to the periphery of the gestational sac, and the decidua enhancement degree at the implantation site (big arrows in Figure 2B) was higher than that at other sites (small arrows in Figure 2B). These characteristics could not be shown by grayscale ultrasound. Although color Doppler ultrasound can show the low-resistance blood flow between the scar and the gestational sac, it cannot accurately judge the relationship between the gestational sac and the scar due to the influence of angle or blood flow velocity.
UAE procedure
UAE was performed with arterial access through the right femoral artery under local anesthesia. After identification of the opening of the internal iliac, uterine, and ovarian artery with angiography, a 5F Yashiro catheter (Terumo Corp., Tokyo, Japan) was navigated into the main uterine artery to check the blood supply of the gestational sac. Then, gelatin sponge particles 1,000–1,400 µm in size (Gelfoam, Pfizer Inc., New York, NY, USA) were used to embolize the left uterine artery with a total dose of 100–150 mg. The contralateral uterine artery was embolized in the same way.
UAEM procedure
After the uterine artery was catheterized, MTX was perfused through a 5-F Yashrio catheter into the uterine artery before embolization using the gelatin sponge particles. The total dose of MTX perfusion in bilateral uterine arteries was 1 mg/kg.
Curettage and hysteroscopy
All patients underwent traditional curettage and hysteroscopy under general anesthesia 24 to 36 hours after CEUSL, UAE, or UAEM. Hysteroscopy was performed to explore the gestational sac position and scar of the previous cesarean section before curettage (Figure 2F). Upon completion of the curettage, a hysteroscopy was used again to check any residue within the uterine cavity, the scar, and possible uterine rupture. Specimens were sent for pathological examination (Figure 2G).
Clinical outcome
The following parameters were analyzed: pretreatment time (time from the patient lying on the operating table to leaving the operating table), degree of decline in β-hCG 24 hours before and after pretreatment (before curettage), blood loss during curettage (gauze weighing and volume measurement), duration of hospital stay, hospitalization costs (expenses used directly for CSP treatment, including drugs, clinical procedure, materials, nursing, bed expenses, and patient care), second treatment, and blood transfusion during the treatment period. Adverse events within 24 hours after pretreatment were observed, including fever, nausea/vomiting, and pelvic pain. Changes in white blood cell (WBC) count, hypersensitive C-reactive protein (hs-CRP), and serum liver and kidney function were also recorded.
Study definitions
The CSP was classified into three types. Type I meant that the gestational sac was partially implanted in the uterine scar, with partial or majority protrusion into the uterine cavity, with the uterine myometrium between the gestational sac and the bladder thinned to a thickness of >3 mm. Type II meant the gestational sac was partially implanted in the uterine scar, with partial or majority protrusion into the uterine cavity. with the uterine myometrium between the gestational sac and the bladder having a thickness of ≤3 mm. Type III meant the gestational sac was completely implanted in the myometrium of the uterine scar and protruded toward the bladder, with the uterine myometrium between the gestational sac and the bladder being obviously thinned or even missing, with a thickness of ≤3 mm.
Normal levels of the parameters were as follows: serum β-hCG <5 mIU/mL, body temperature in the mouth <38.0 ℃, WBC count ≤10.0×109/L, hs-CRP level ≤10 mg/L, and creatinine ≤97 µmol/L. When alanine aminotransferase (ALT) or aspartate aminotransferase (AST) was >40 U/L, the serum liver enzyme was considered to be increased. Twenty-four hours after the pretreatment, the degree of pelvic pain was graded with the visual analogue scale (VAS). Mild pain was characterized as pain tolerable in normal life that did not interfere with sleep. Moderate pain was obvious and unbearable pain that required pain drugs for sleep. Severe pain was severe, continuous, and unbearable pain that severely disturbed sleep and required painkillers (17). Massive hemorrhage was present when vaginal bleeding was >200 mL during curettage. A second treatment was defined as uterine arterial embolization, laparoscopy, or laparotomy for the second time. Adverse events were classified according to the Society of Interventional Radiology (SIR) Standards of Practice Committee (18).
Follow-up
During follow-up, serum β-hCG was determined weekly, and changes in WBC count and hs-CRP were recorded every 2 days until they dropped to the normal level. The time of menstruation recovery and changes in first menstrual volume were recorded. Ultrasound was used to monitor residues in the anterior isthmus of the uterus after menstruation recovery. All patients were followed up for menstrual cycle changes, endometrial thickness, and pregnancy within 6 months after treatment.
Statistical analysis
All data are analyzed using the SPSS version 24.0 (IBM Corp., Armonk, NY, USA). Measurement data are presented as mean ± standard deviation if the data were in a normal distribution and were tested using the t-test to compare before and after observations on the same objects or single-factor analysis of variance. If data had a non-normal distribution, the data are presented as median (interquartile range), and the Kruskal-Wallis test was used for comparison. Count data are presented as numbers or percentages (%) and were tested with the chi-square test or Fisher exact test. A P value <0.05 was considered statistically significant.
Results
Demographic and baseline clinical characteristics of enrolled patients
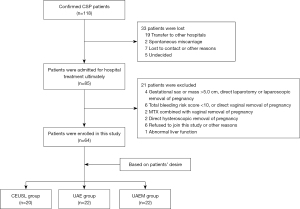
In the study period of 3 years, the total number of pregnancies in our tertiary hospital was 7,868, with a total of 3,454 cesarean deliveries and 120 CSPs. Ultimately, 64 patients were enrolled and divided into the CEUSL (n=20), UAE (n=22), and UAEM (n=22) groups (Table 2). The flow diagram detailing the process of patient enrollment is provided in Figure 3. The 3 groups were matched according to the baseline data, which included the type of CSP (Table 1).
Table 2
| Variables | CEUSL (n=20) | UAE (n=22) | UAEM (n=22) | P |
|---|---|---|---|---|
| Patient age (years) | 30.95±4.66 | 33.72±3.94 | 32.67±4.04 | 0.107 |
| Number of previous cesarean sections | 1.00 (1.00, 3.00) | 1.00 (1.00, 2.00) | 1.00 (1.00, 2.00) | 0.982 |
| Interval time from recent cesarean section (years) | 3.50 (1.00, 14.00) | 4.00 (1.00, 14.00) | 6.00 (1.00, 15.00) | 0.334 |
| Gestational age (d) | 45.50 (36.00, 65.00) | 48.00 (34.00, 79.00) | 46.00 (35.00, 90.00) | 0.897 |
| Length of the embryo (cm) | 0.50 (0.00, 1.60) | 0.30 (0.00, 1.50) | 0.20 (0.00, 4.50) | 0.201 |
| Classification of CSP | 0.904 | |||
| Type I | 2 (10.00%) | 4 (18.20%) | 4 (17.40%) | |
| Type II | 15 (75.00%) | 16 (72.70%) | 17 (73.90%) | |
| Type III | 3 (15.00%) | 2 (9.10%) | 2 (8.70%) | |
| β-hCG (mIU/mL) | 47,417.00 (7,892.16, 201,904.00) | 36,739.80 (6,457.00, 146,461.00) | 38,301.23 (6,500.00, 120,673.70) | 0.159 |
| Body temperature (℃) | 36.60±0.45 | 36.52±0.34 | 36.47±0.31 | 0.493 |
| WBC counts (×109/L) | 6.87±1.33 | 7.38±1.51 | 7.74±1.98 | 0.232 |
| hs-CRP (mg/L) | 1.85 (0.50, 4.00) | 2.75 (0.50, 12.60) | 3.00 (1.00, 9.00) | 0.056 |
| ALT (U/L) | 20.50 (10.00, 35.00) | 15.00 (8.20, 33.00) | 18.20 (8.00, 34.00) | 0.065 |
| AST (U/L) | 15.00 (10.00, 35.00) | 14.00 (8.00, 29.00) | 15.00 (10.00, 20.00) | 0.423 |
| Cr (μmol/L) | 44.95 (38.10, 68.90) | 52.10 (30.00, 65.93) | 49.10 (38.10, 65.26) | 0.219 |
Data are presented as mean ± standard deviation if in normal distribution or as median (interquartile range) if not in normal distribution. Count data are presented as n (%). CSP, cesarean scar pregnancy; CEUSL, contrast-enhanced ultrasound-guided lauromacrogol injection; UAE, uterine artery embolization; UAEM, uterine artery embolization combined with the application of methotrexate; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Cr, creatinine.
General clinical outcomes with different pretreatment methods
The pretreatment and curettage were all technically successful, with no incidences of uncontrollable massive hemorrhage, uterine rupture, or emergency hysterectomy.
The time for pretreatment was significantly decreased (P<0.001) in the CEUSL group (35.60±7.87 min) compared with that in the UAE (56.77±7.28 min) and UAEM (55.87±6.85 min) group (Table 3). The hospitalization cost was significantly decreased (P<0.001) in the CEUSL group (CNY ¥8,457.78) compared with that in the UAE (CNY ¥14,034.02) and UAEM (CNY ¥12,313.00) groups. The β-hCG percentage decrease 24 hours after the pretreatment and hospital stay were significantly decreased (P<0.05) in the CEUSL group (0.68±0.06 and 4 days, respectively) compared with those in the UAE (0.71±0.08 and 5 days, respectively) and UAEM (0.65±0.07 and 5 days, respectively) group.
Table 3
| Variables | CEUSL (n=20) | UAE (n=22) | UAEM (n=22) | P |
|---|---|---|---|---|
| Pretreatment time (min) | 35.60±7.87 | 56.77±7.28* | 55.87±6.85* | <0.001 |
| β-hCG decrease (%) | 0.68±0.06 | 0.71±0.08 | 0.65±0.07# | 0.022 |
| Blood loss (mL) | 15.00 (5.00, 200.00) | 12.50 (5.00, 200.00) | 10.00 (5.00, 200.00) | 0.794 |
| Hospital stay (d) | 4.00 (3.00, 7.00) | 5.00 (3.00, 8.00) | 5.00 (3.00, 9.00)* | 0.026 |
| Hospitalization costs (RMB) | 8,457.78 (6,567.79, 11,542.51) | 14,034.02 (10,163.06, 20,470.16)* | 12,313.00 (8,562.47, 17,687.13)*# | <0.001 |
| Second treatment, n (%) | 0 (0.00) | 0 (0.00) | 0 (0.00) | – |
| Menstruation recovery in 5 weeks, n (%) | 17 (85.00%) | 19 (86.40%) | 20 (90.90%) | 0.982 |
| β-hCG normalization in 5 weeks, n (%) | 17 (85.00%) | 20 (90.90%) | 20 (90.90%) | 0.837 |
| Thickness of endometrium (mm) | 9.14±1.82 | 9.93±1.67 | 9.11±1.69 | 0.300 |
Data are presented as mean ± standard deviation if in normal distribution or as median (interquartile range) if not in normal distribution. Count data are presented as n (%). *, P<0.05 vs. CEUSL group; #, P<0.05 vs. UAE group. Thickness of endometrium: average thickness during the luteal phase of multiple menstrual cycles after treatment. CEUSL, contrast-enhanced ultrasound-guided lauromacrogol injection; UAE, uterine artery embolization; UAEM, uterine artery embolization combined with the application of methotrexate; β-hCG, beta human chorionic gonadotropin.
Adverse reactions after the pretreatment
After pretreatment, there were significantly fewer patients (P<0.05) with pelvic pain, increased WBC count, and hs-CRP elevation in the CEUSL group than in the UAE or UAEM groups (Table 4). There was no significant difference in the number of patients with fever, nausea/vomiting, elevated ALT/AST, or decreased menstruation among the three groups (P>0.05). Low fever was primarily present in the 3 groups, with the highest temperature being 38.1 ℃ in the CEUSL group, 38.5 ℃ in the UAE group, and 38.7 ℃ in the UAEM group, all of which returned to normal within 2 days. One patient in the CEUSL group experienced mild to moderate pelvic pain, which was relieved after bed rest. Additionally, 1 patient in the UAE group and 2 patients in the UAEM group experienced severe pain that required medication. Twenty-four hours after pretreatment, the WBC count and CRP levels were significantly lower (P<0.05) in the CEUSL group [(7.94±1.45)×109/L and 6.65 mg/L, respectively] than those in the UAE group [(10.35±2.63)×109/L and 21.25, respectively] and the UAEM group [(9.37±2.57)×109/L and 25.28, respectively; Table 5], and the levels of these parameters returned to normal within 5 days. Based on the SIR classification of adverse events (18), the CEUSL group experienced only minor complications, whereas the UAE and UAEM groups experienced not only minor but also major complications (severe pain requiring medication).
Table 4
| Variables | CEUSL (n=20) (%) | UAE (n=22) (%) | UAEM (n=22) (%) | P |
|---|---|---|---|---|
| Fever | 2 (10.00) | 10 (45.5) | 11 (50.00) | 0.017 |
| Nausea/vomiting | 0 (0.00) | 1 (4.55) | 2 (9.09) | 0.399 |
| Pelvic pain | 1 (5.00) | 8 (34.78) | 7 (31.82) | 0.045 |
| Increased WBC count | 2 (10.00) | 11 (50.00) | 7 (31.82) | 0.026 |
| Elevated hs-CRP | 4 (20.00) | 16 (72.73) | 15 (65.18) | 0.001 |
| Elevated ALT/AST | 0 (0.00) | 1 (4.55) | 1 (4.55) | 0.632 |
| Elevated Cr | 0 (0.00) | 0 (0.00) | 0 (0.00) | – |
| Decreased menstruation | 7 (35.00) | 6 (27.27) | 9 (40.91) | 0.697 |
CEUSL, contrast-enhanced ultrasound-guided lauromacrogol injection; UAE, uterine artery embolization; UAEM, uterine artery embolization combined with the application of methotrexate; hs-CRP, hypersensitive C-reactive protein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Cr, creatinine.
Table 5
| Variables | CEUSL (n=20) | UAE (n=22) | UAEM (n=22) | P |
|---|---|---|---|---|
| Body temperature (℃) | 37.07±0.36 | 37.30±0.67 | 37.30±0.58 | 0.301 |
| WBC counts (×109/L) | 7.94±1.45 | 10.35±2.63* | 9.37±2.57 | 0.009 |
| hs-CRP (mg/L) | 6.65 (1.30, 13.80) | 21.25 (1.80, 87.60)* | 25.28 (1.28, 69.10)* | <0.001 |
Data are presented as mean ± standard deviation if in normal distribution or as median (interquartile range) if not in normal distribution. *, P<0.05 vs. CEUSL group. CEUSL, contrast-enhanced ultrasound-guided lauromacrogol injection; UAE, uterine artery embolization; UAEM, uterine artery embolization combined with application of methotrexate.
Effect of CSP types on outcomes and adverse reactions
No significant differences were detected in the hospitalization cost, time of pretreatment, bleeding amount during curettage, pelvic pain after pretreatment, β-hCG percentage change, WBC count, fever, or nausea/vomiting among the three groups (P>0.05).
Outcomes at follow-up
Follow-up was performed in all patients. At follow-up, all patients reported resuming normal menstruation within 8 weeks of treatment, with no residual gestational sac. Seventeen (85.00%) patients in the CEUSL group resumed normal menstruation and normal β-hCG five weeks after treatment (Table 3), which was not significantly different (P>0.05) from that in the UAE (86.40% and 90.90%, respectively) and UAEM (90.90% and 90.90%, respectively) groups. Seven (35.00%) patients in the CEUSL, 6 (27.27%) in the UAE, and 9 (40.91%) in the UAEM groups experienced reduced menstrual volume after menstruation recovery, with no significant differences (P>0.05) between any two of the groups.
At the 6-month follow-up, no significant change (P>0.05) was found in the menstrual cycle or endometrium thickness during the luteal phase. All patients had contraception within 6 months after CSP treatment because they had no desire for a future pregnancy or were worried about the recurrence of CSP. No pregnancies occurred at 6 months of follow-up.
Discussion
In this study, we investigated the clinical efficacy of sclerotherapy with lauromacrogol for CSP under CEUS guidance in comparison with the classic pretreatment methods of UAE and UAEM. We found that the effect of sclerotherapy with lauromacrogol under the guidance of CEUS was equivalent to that of UAE or UACE in the pretreatment of CSP. Moreover, compared with UAE and UAEM, the sclerotherapy with lauromacrogol was safer, with a lower incidence of adverse effects and a significantly decreased hospital cost.
Because of an increasing number of cesarean deliveries and the improved ultrasound diagnostic accuracy of ectopic pregnancy (19), the incidence of CSP has risen significantly to 6.1% among all ectopic pregnancies in people who have previously had a cesarean delivery. For CSP, surgical treatment entails the risk of postoperative adhesions impairing future fertility, long hospital stay and recovery, and future placental previa/accrete. Blind dilation and curettage, as well as isolated suction curettage, may cause a life-threatening hemorrhage. Currently, there is no consensus on the standardized therapy for CSP in clinical practice (20). UAE has now been widely used to control hemorrhage and preserve the uterus in the pretreatment of CSP (19), and UAE combined with uterine curettage may be a safe and effective approach for CSP (4,19). In UAE, using MTX infusion through the arterial catheter to the gestational sac before embolization could effectively eliminate residual villi in the scar tissue. Because MTX alone followed by suction curettage necessitates a longer hospital stay and greater bleeding volume compared with UAE (21), and UAE combined with MTX (50 mg) before curettage may be an effective and safe approach in the management of CSP.
Curettage may be performed under guidance of ultrasonography, which can more clearly show the endometrium, gestational sac location, and myometrium thickness between the bladder and the gestational sac (19). In our study, sclerotherapy with lauromacrogol injection was performed under CEUS guidance, which, compared with UAE or UAEM, realized the precision treatment by super selectively blocking the blood supply to the gestational sac. CEUSL does not affect the blood supply of the uterus or uterine artery branches. Therefore, post-embolization is less likely to occur, and the ovarian function will not be affected in CEUSL as it may be with the UAE or UAEM approaches. In the literature, the feasibility and effectiveness of lauromacrogol injection in the pretreatment of CSP were reported, and lauromacrogol injection was considered safe, feasible, and effective in the pretreatment of CSP (2,14). Nonetheless, these studies were of a retrospective design or case reports with lower efficacy, and they did not conduct an in-depth investigation into the safety and efficacy of lauromacrogol injection in the pretreatment of CSP (2,14). In our prospective study with three groups of patients treated with three different modalities, the pretreatment effect was similar in all three groups, with no significant difference in blood loss during curettage or in the need for intraoperative blood transfusion or secondary treatment. The pretreatment time in the CEUSL group was significantly shortened, and patients could get out of bed after 0.5 to 1 hour of rest if no other discomfort was present, which avoided the discomfort caused by bandage compression, prolonged bed immobilization, and other limitations of sore limbs and pain in UAE or UAEM (22). The CSP type may affect the outcome of pretreatment. Nonetheless, the components of CSP types were similar in the three groups (P>0.05), and no significant differences were detected in the pretreatment outcome and adverse effects among the 3 groups. This indicates that the outcome and adverse effects in the CEUSL group are comparable with those in the UAE or UAEM groups. However, it is important to point out that UAE and UAEM necessitated higher technical requirements with an increased treatment cost compared with that of the CEUSL group (P<0.001).
At follow-up, patients experienced different degrees of reduction of menstrual volume after menstruation recovery in all 3 groups, which might have been caused by mechanical injury of curettage to the endometrium. Moreover, endometrial ischemia and inflammation caused by arterial embolization may affect endometrial function and increase the risk of uterine adhesion (23). Besides denaturing protein in the vascular endothelial cells, lauromacrogol can also damage the endometrial and glandular epithelial cells, resulting in adhesion and fibrosis of the uterus (24). Therefore, the dose of lauromacrogol should be reduced to minimize the risk of possible injury.
Compared with UAE or UAEM, CEUSL in the pretreatment of CSP has similar efficacy, shortened pretreatment time, decreased hospitalization cost, no radiation exposure during the sclerotherapy process, and no post-embolization syndrome caused by UAE or UAEM, all of which increase the rate of patient acceptance. These facts suggest that sclerotherapy with lauromacrogol is an ideal choice for pretreatment before curettage for patients with CSP at high risk of hemorrhage during curettage. In addition, this technique is simple, requires no high-end equipment, and may be a preferred alternative to UAE and UACE.
Some limitations existed in this study, including a small cohort of patients, enrolment of only Chinese patients, a single-center study design, and no long-term follow-up, all of which might reduce the generalizability of the outcomes. Future large-scale, multi-centered, randomized clinical trials with long-term follow-up need to be performed to resolve these issues.
Conclusions
In conclusion, the pretreatment procedures were all technically successful, resulting in good outcomes for all patients. Compared with UAE, with or without MTX injection, CEUSL injection may be as safe, convenient, and effective for the pretreatment of CSP, with fewer adverse effects and decreased pretreatment time and hospital stay.
Acknowledgments
Funding: This work was supported by the Medical and Health Science and Technology Project of Zhejiang Province (No. 2022KY554).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-190/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-190/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Our study protocol was approved by the ethics committee of Zhejiang Provincial People’s Hospital (No. JS2018067). All patients signed informed consent to participate.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li X, Niu H, Li J, Zhang L, Qu Q. Clinical assessment of uterine artery embolization combined with curettage when treating patients with cesarean scar pregnancy: A retrospective study of 169 cases. J Obstet Gynaecol Res 2020;46:1110-6. [Crossref] [PubMed]
- Wei SS, Li DH, Zhang ZF, Sun WC, Jia CL. Type II caesarean scar pregnancy management by ultrasound-guided local lauromacrogol injection in combination with suction curettage: A case report. Medicine (Baltimore) 2020;99:e19743. [Crossref] [PubMed]
- Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson CJ. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound Obstet Gynecol 2003;21:220-7. [Crossref] [PubMed]
- Xiao Z, Cheng D, Chen J, Yang J, Xu W, Xie Q. The effects of methotrexate and uterine arterial embolization in patients with cesarean scar pregnancy: A retrospective case-control study. Medicine (Baltimore) 2019;98:e14913. [Crossref] [PubMed]
- Hegde CV. Cesarean Scar Pregnancy: Some Management Options. J Obstet Gynaecol India 2017;67:153-6. [Crossref] [PubMed]
- Pirtea L, Balint O, Secosan C, Grigoras D, Ilina R. Laparoscopic Resection of Cesarean Scar Ectopic Pregnancy after Unsuccessful Systemic Methotrexate Treatment. J Minim Invasive Gynecol 2019;26:399-400. [Crossref] [PubMed]
- Chen H, Zhou J, Wang H, Tan W, Yao M, Wang X. The Treatment of Cesarean Scar Pregnancy with Uterine Artery Embolization and Curettage as Compared to Transvaginal Hysterotomy. Eur J Obstet Gynecol Reprod Biol 2017;214:44-9. [Crossref] [PubMed]
- Wang Q, Ma H, Peng H, He L, Bian C, Zhao X. Risk factors for intra-operative haemorrhage and bleeding risk scoring system for caesarean scar pregnancy: a case-control study. Eur J Obstet Gynecol Reprod Biol 2015;195:141-5. [Crossref] [PubMed]
- Ash A, Smith A, Maxwell D. Caesarean scar pregnancy. BJOG 2007;114:253-63. [Crossref] [PubMed]
- Lou T, Gao Y, Feng Y, Lu J, Zhang Z, Bai H. Reproductive outcomes of cesarean scar pregnancies pretreated with methotrexate and uterine artery embolization prior to curettage. Taiwan J Obstet Gynecol 2020;59:381-6. [Crossref] [PubMed]
- Du C, Chai N, Linghu E, Li H, Feng X, Ning B, Wang X, Tang P. Long-term outcomes of EUS-guided lauromacrogol ablation for the treatment of pancreatic cystic neoplasms: 5 years of experience. Endosc Ultrasound 2022;11:44-52. [Crossref] [PubMed]
- Li JX, Zhang HL, Xu HX, Yu SY. Contrast-enhanced ultrasound evaluation of a refractory ovarian endometrial cyst and ultrasound-guided aspiration sclerotherapy using urokinase and lauromacrogol. Clin Hemorheol Microcirc 2021;78:391-400. [Crossref] [PubMed]
- Qin S, Liu Y, Ning H, Tao L, Luo W, Lu D, Luo Z, Qin Y, Zhou J, Chen J, Jiang H. EUS-guided lauromacrogol ablation of insulinomas: a novel treatment. Scand J Gastroenterol 2018;53:616-20. [Crossref] [PubMed]
- Wu Q, Liu X, Zhu L, Zhu Y, Mei T, Cao S, Shen Y, Ding J, Lin T. Clinical Assessment of Ultrasound-Guided Local Lauromacrogol Injection Combined With Curettage and Hysteroscopy for Cesarean Scar Pregnancy. Front Pharmacol 2020;11:601977. [Crossref] [PubMed]
- Timor-Tritsch IE, Monteagudo A, Calì G, D'Antonio F, Kaelin Agten A. Cesarean Scar Pregnancy: Diagnosis and Pathogenesis. Obstet Gynecol Clin North Am 2019;46:797-811. [Crossref] [PubMed]
- Wang S, Li BH, Wang JJ, Guo YS, Cheng JM, Ye H, Zang CY, Zhang Y, Duan H, Zhang XY. The safety of echo contrast-enhanced ultrasound in high-intensity focused ultrasound ablation for abdominal wall endometriosis: a retrospective study. Quant Imaging Med Surg 2021;11:1751-62. [Crossref] [PubMed]
- Thong ISK, Jensen MP, Miró J, Tan G. The validity of pain intensity measures: what do the NRS, VAS, VRS, and FPS-R measure? Scand J Pain 2018;18:99-107. [Crossref] [PubMed]
- Khalilzadeh O, Baerlocher MO, Shyn PB, Connolly BL, Devane AM, Morris CS, Cohen AM, Midia M, Thornton RH, Gross K, Caplin DM, Aeron G, Misra S, Patel NH, Walker TG, Martinez-Salazar G, Silberzweig JE, Nikolic B. Proposal of a New Adverse Event Classification by the Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol 2017;28:1432-1437.e3. [Crossref] [PubMed]
- Feng Y, Chen S, Li C, Zhang X, Duan H, Sooranna S, Johnson MR, Li J. Curettage after uterine artery embolization combined with methotrexate treatment for caesarean scar pregnancy. Exp Ther Med 2016;12:1469-75. [Crossref] [PubMed]
- Birch Petersen K, Hoffmann E, Rifbjerg Larsen C, Svarre Nielsen H. Cesarean scar pregnancy: a systematic review of treatment studies. Fertil Steril 2016;105:958-67. [Crossref] [PubMed]
- Zhuang Y, Huang L. Uterine artery embolization compared with methotrexate for the management of pregnancy implanted within a cesarean scar. Am J Obstet Gynecol 2009;201:152.e1-152.e1523.
- Xiao J, Shi Z, Zhou J, Ye J, Zhu J, Zhou X, Wang F, Zhang S. Cesarean Scar Pregnancy: Comparing the Efficacy and Tolerability of Treatment with High-Intensity Focused Ultrasound and Uterine Artery Embolization. Ultrasound Med Biol 2017;43:640-7. [Crossref] [PubMed]
- Tsikouras P, Manav B, Koukouli Z, Trypsiannis G, Galazios G, Souftas D, Souftas V. Ovarian reserve after fibroid embolization in premenopausal women. Minim Invasive Ther Allied Technol 2017;26:284-91. [Crossref] [PubMed]
- Sato D, Kurita M, Ozaki M, Kaji N, Takushima A, Harii K. Extravascular injection of sclerotic agents does not affect vessels in the rat: experimental implications for percutaneous sclerotherapy of arteriovenous malformations. Eur J Vasc Endovasc Surg 2012;44:73-6. [Crossref] [PubMed]


