
Comparative study of ultrasound-guided microwave ablation and traditional surgery in the treatment of plasma cell mastitis: a multicenter study
Introduction
Plasma cell mastitis (PCM) is a tip of nonlactating mastitis with benign pathological characteristics. PCM is characterized by comedomastitis and the infiltration of a large number of inflammatory cells (plasma cells) and is commonly seen in women aged 30–40 years old. In recent years, the incidence has been rising and has become increasingly observed in younger patients (1). PCM is a difficult and complicated benign breast disease, and its differential diagnosis is challenging, as it can be easily confused with breast carcinoma. PCM is clinically known as immortal cancer. It is frequently associated with redness, swelling, pain, purulence, long-term refractory wounds, and recurrent attacks. In the literature, the incidence of PCM is 4–5% of benign breast diseases (1). The etiology of PCM remains unclear. The main causes are currently thought to be bacterial infections, especially anaerobic infections. As early as the last century, Patey et al. (2) thought nipple inversion was both a clinical manifestation and an important cause of PCM. The nipple inversion was thought to cause an obstruction of the mammary duct, with the endothelial cells of the mammary duct being subsequently damaged, resulting in a large number of aseptic inflammatory substances (3). Kessler et al. (4) believed that the occurrence of PCM was related to its own immune system. In addition, sex hormone disorders (5), long-term use of antipsychotic drugs (6), long-term smoking (7), and obesity (8) were associated with PCM. However, PCM has many clinical manifestations and complex pathophysiological mechanisms, and thus an array of causes can give rise to lesions of the mammary duct. The lesions in turn cause a large number of secretions to form an accumulation of inflammatory tissue under the nipple and areola, resulting in the expansion of the mammary duct and accumulation of inflammatory substances in some breast fibrous tissues, especially plasma cells (9). The treatment of PCM has been considered a major surgical challenge. Currently, the main treatment methods are conservative treatment and traditional surgical treatment. Conservative treatment includes antibiotics (10), antituberculosis drugs (11), immunosuppressants (12), endocrine therapy (13), and traditional Chinese medicine. However, each treatment method has its limitations. Antibiotics are usually ineffective (14,15). Antituberculosis and traditional Chinese medicine treatments have a long course and slow effect (16). Hormone therapy has a rapid effect, but the recurrence rate is high, and the recurrence area after drug withdrawal is larger than the primary lesion (17). A recent German study reported a high-dose (1 mg/kg/day) prednisolone treatment for a duration of 2 to 6 months that yielded a recurrence rate of 15% (17). In the analysis by Lei et al. (18), the recurrence rate of oral steroid therapy was reported to be 20%. Thus far, most scholars in China and abroad still advocate for surgical resection (19), and the surgical methods mainly include mass resection (20), incision and expansion (21), and incision and drainage (21). Some scholars believe that incision and drainage should not be performed on patients in the abscess stage because it may form a delayed sinus tract (22). However, surgical methods continue to improve and now include segmentectomy and suture treatment for patients with abscess in stage I (23). After operation, a suture scar may affect the patient’s appearance and increase the patient’s physical and mental trauma, while surgical complications and the postoperative recurrence rate are still high. The recurrence rate can be up to 79% for treatment with a simple incision and drainage without mastectomy, but the recurrence rate drops to 28% after segmental mastectomy (24). However, this treatment method is not easily accepted by the majority of female patients due to its disadvantages, such as a large area of injury, the length of time required for dressing changes after surgery, loss of function of some glands, and the aesthetic impact of the postoperative incision on the breast (25). Therefore, new therapies are being actively sought to treat patients with PCM.
We integrated microwave ablation into the treatment of PCM. Microwave ablation causes the polar molecules in the lesion area to move at high speed under the action of a microwave field so that the molecular coupling pole of the target tissue is oscillated and rotated, which produces a therapeutic effect through thermal coagulation, tissue dehydration, and necrosis (26). It is mostly used in treating benign breast tumors and has not been reported for the treatment of PCM. The aim of our treatment is to use microwave ablation at high temperature to completely inactivate the necrotic material, inflammatory factors, and the tissue within the infiltration range of the inflammatory tissue in the lesion and abolish the effect of the continued infiltration of inflammatory factors, thereby causing local inflammatory necrosis. The material is slowly absorbed or flows out with the ablation needle so that the lesion is completely healed. We present the following article in accordance with the TREND reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1132/rc).
Methods
General information
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Hebei Province Hospital of Chinese Medicine, and informed consent was obtained from all the patients. A total of 68 patients with PCM confirmed by puncture admitted to Hebei Provincial Hospital of Medicine Traditional Chinese, Yantai Affiliated Hospital of Binzhou Medical University, and Zhejiang Cancer Hospital (tertiary first-class public hospitals) from January 2017 to June 2019 were included in this study. The collection of all data was carried out in accordance with the inclusion and exclusion criteria negotiated by the 3 centers and the postoperative evaluation criteria to minimize deviations. Cases from the 3 centers were integrated and randomly divided into the ablation group and the operation group to compare the clinical value of ultrasound-guided microwave ablation and traditional surgery in treating PCM.
Case selection
Patients were included if they had nonlactating mastitis with breast lumps and clinical features, such as redness and pain or prolonged refractory after rupture; had PCM confirmed by preoperative core needle biopsy; had no severe underlying diseases (e.g., hypertension, coronary heart disease); could tolerate surgery; and voluntarily participated after full notification. Patients were excluded if they had breast cancer, breast nodules, or other diseases; had lactating mastitis; had a severe coagulation disorder and/or important organ dysfunction; or had surgery and/or anesthesia intolerance.
Staging criteria and ultrasonic manifestations
The mass stage (27,28) was considered to be a breast with a local breast mass, unequal size, a tough, unclear boundary, dark or normal skin, breast pain, or axillary lymph node enlargement. Ultrasound manifestations included a low echo mass, a clear boundary, an irregular shape, and no obvious liquid dark area inside. Color Doppler flow imaging (CDFI) criteria included abundant blood flow signals, and those of contrast-enhanced ultrasound included high heterogeneous enhancement.
The abscess stage (27,28) was considered to be a breast with local redness and swelling, pain, fluctuating sensation, body heat, or a long course of disease with pyogenic ulceration, sparse pus juice and slow healing, accompanied by a low fever and axillary lymphadenectasis. Ultrasound manifestations included a low echo area, an unclear boundary, an irregular shape, an internal irregular liquid dark area, and pressure flow. CDFI criteria included little blood flow signal in the surrounding solid tissue, while those of contrast-enhanced ultrasound included scattered low enhancement and no enhancement in large areas.
The sinus phase (27,28) was considered to be a breast with a matured abscess, broken pus, or an incision after pus. It was characterized by pus that was not smooth; long-term nongrowth and formation of holes or channels resulting in permanent nonhealing; presence of sinus; or local stiffness. The ultrasound criteria included a peripheral solid low echo in the central region, a visible dark area of impure liquid, and probe pressure that showed that the pus flowed out of the ulcer.
Treatment and methods
The operation group underwent traditional surgical treatment using segmental resection (10 cases in mass stage; 1 case in sinus stage), incision and drainage (6 cases in abscess stage; 1 case in sinus stage), and breast gland inverted duct splitting + counterpart drainage + partial gland resection (2 cases in mass stage; 9 cases in abscess stage; 1 case in sinus stage). All patients were placed under general anesthesia for the procedures.
Breast gland inverted duct splitting + counterpart drainage + partial gland resection surgical process
The patient was in the supine position, and the upper limbs were abducted. After general anesthesia, routine iodine sterilization was used to lay a sheet. An arc-shaped incision about 10 cm long was taken along the breast skin fold at the outer and lower part of the breast. In the lower edge of the breast, the skin and subcutaneous tissue were cut, the back and anterior spaces of the pectoralis major were entered, and the space was fully freed. the gland was turned from the bottom to the top, and the tissues were probed according to the quadrants.. For multiple necrotic areas, the separation was opened, the necrotic tissue was removed, part of the diseased glands was removed, and the remaining glands were cut along the direction of the milk duct. The dilated milk duct could be observed. When the milk duct was opened, yellow paste-like secretions could be observed, and the secretions were be removed. We checked that there was no active bleeding, then rinsed the affected area with iodophor saline, placed a cross-dwelling drainage tube on the surface of the affected pectoralis major muscle, placed another drainage tube on the opposite side for hedging, fixed it with silk thread, sutured the incision intermittently, and applied a gauze dressing bandage. After these steps, the operation was considered complete. The above operation was performed by the chief physician with more than 10 years of operation experience.
Ablation process
The microwave ablation under ultrasound guidance was performed in all ablation groups, and all patients had moderate sedation. The ultrasound instrument model was an EPIQ 7 (Philips Ultrasound Co., Ltd., Bothell, WA, USA), and the probe model was L2–5 (Philips Ultrasound Co., Ltd). The microwave instrument model was a KY-2000 (Nanjing Kangyou Medical Equipment Co., Ltd., Nanjing, China), and the ablation needle model was KY-2450A-1.
Patients were placed in the supine position, after which electrocardiogram (ECG) monitoring was initiated. The size, shape, internal echo, blood flow status, and number of lesions were detected by ultrasound, and the lesions were classified, recorded, and marked in detail. A combination of intravenous anesthesia and local anesthesia containing 1% lidocaine was used for anesthesia.
Under ultrasound-guided monitoring, the lesion was isolated from the skin and muscular layer. The pus was extracted, rinsed with saline + ornidazole injection, and placed into a microwave ablation needle at a power of 35 W. The ablation range included about 3 mm around the lesion. The lesions after ablation showed heterogeneous echo changes under 2-dimensional ultrasound. Some patients extruded a tofu-like substance through the ablation needle and sinus orifice after ablation. We checked that there was no residual bleeding. The above operation was performed by the chief physician with more than 10 years of ultrasound intervention experience.
Below is a description of the observation indicators: perioperative indicators were a comparison of the 2 groups based on operation time (minutes), hospital stay (days), wound healing evaluation (29) (14 days after surgery), and intraoperative blood loss (mL). The degree of pain was observed based on the visual analogue score (VAS; 0–10). Painless status (0 points) was characterized by no pain. Mild pain (below 3 points) was characterized by mild pain that the patient could endure. Moderate pain (4–6 points) was characterized by pain that affected sleep but that the patient could still endure. Severe pain (7–10 points) was characterized by gradually intense pain that was intolerable, affected appetite, or affected sleep. To assess the cosmetic effect, the patients’ breast appearance satisfaction was surveyed based on the breast appearance evaluation score table for breast shape effect evaluation (30).
All the enrolled cases were reexamined for postoperative conditions in the outpatient clinic. The routine ultrasound data and clinical manifestation questionnaire scores were obtained before the operation; 1 week after the operation; and 3, 6, and 12 months after the operation. The patients were reviewed with an ordinary ultrasound on the 14th day, 3 and 6 months after the operation, and every 6 months thereafter. According to the efficacy evaluation criteria of solid tumors (31), the effective rate [complete response (CR) + partial response (PR)/total number of cases × 100%], the recurrence rate within 12 months after the operation, and the time to complete disappearance of lesions were evaluated. Initial and final volumes were assessed using ultrasonographic measurements of pretreatment and final follow-up ultrasound results in order to record the complete disappearance time of the lesions.
Statistical method
The data were collected and recorded and input into Excel (Microsoft Corp., Redmond, WA, USA). SPSS 21.0 (IBM Corp., Armonk, NY, USA) was used for data processing. Measurement data are expressed as the mean±standard deviation, and the t test was used for comparison between groups. The enumeration data are expressed as a rate (%), and the χ2 test was used for comparison between groups. Data that did not conform to normality were analyzed with a Z test. A P value less than 0.05 indicated that the difference was statistically significant, a P value less than 0.01 indicated that the difference was extremely significant, and a P value less than 0.05 indicated no significant difference.
Results
Preoperative and postoperative comparison of the 2 groups
According to the different surgical methods, patients were divided into the microwave ablation group (38 cases) and the traditional surgery group (30 cases; Figure 1). The success rate of the operation was 100%. After microwave ablation, a part of the necrotic tissue in the surgical area of patients at the tumor stage and abscess stage was liquefied and discharged through the ablation needle channel or puncture needle suction, and a part of the tissue was absorbed and metabolized.
Statistical comparison between the 2 groups
In this study, 68 patients with PCM confirmed by puncture pathology were enrolled. All patients had unilateral breast disease; there were no patients with double breast disease. There were 72 lesions, including 65 cases of a single lesion, 2 cases of double lesions, and 1 case of triple lesions. The formula for calculating the lesion volume was as follows: V = π/6 × L × W × H. The average lesion volume in the ablation group was 23.40 (range, 7.80–51.09) cm3, and the average lesion volume in the operation group was 20.80 (range 9.36–54.60) cm3. All patients were female. The age range of the operation group was 20–45 years, and the mean age was 32.23±5.72 years. The age range of the ablation group was 20–42 years, and the mean age was 30.45±5.04 years. There was no significant difference in general data between the 2 groups (P>0.05; Table 1).
Table 1
| Group | Age, years | Stage | Lesion | Lesion volume | Nipple deformity | Parous | Abnormal leukocytes | Abnormal erythrocyte sedimentation rate | Rheumatoid 3 abnormalities | Abnormal C-reactive protein | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤30 | >30 | Mass | Abscess | Sinus | Left | Right | Yes | None | Yes | None | Yes | None | Yes | None | Yes | None | Yes | None | |||||||||
| Ablation (n=38) | 19 | 19 | 11 | 23 | 4 | 17 | 21 | 23.40 (7.80, 51.09) | 32 | 6 | 33 | 5 | 7 | 31 | 29 | 9 | 1 | 37 | 21 | 17 | |||||||
| Operation (n=30) | 14 | 16 | 12 | 15 | 3 | 15 | 15 | 20.80 (9.36, 54.60) | 26 | 4 | 24 | 6 | 5 | 25 | 25 | 5 | 2 | 28 | 21 | 9 | |||||||
| χ2/Z | 0.075 | 0.942 | 0.186 | –0.056 | 0.081 | 0.579 | 0.036 | 0.505 | 0.647 | 1.542 | |||||||||||||||||
| P | 0.785 | 0.624 | 0.666 | 0.956 | 0.776 | 0.447 | 0.851 | 0.477 | 0.421 | 0.214 | |||||||||||||||||
The hospital stays of the ablation group were significantly shorter than those of the operation group, and the difference was statistically significant (P<0.05). There was a significant difference between the 2 groups in the mass stage and abscess stage (P<0.01), and the difference between the mass stage and sinus stage, abscess stage and sinus stage were statistically significant (P<0.05).
The average operation time in the ablation group was 13.8±10.5 min, and that of the operation group was 78.0±38.09 min. The operation time in the ablation group was significantly shorter than that of the ablation group, and the difference was statistically significant (P<0.05). There was significant difference between the 2 groups in the mass stage and abscess stage (P<0.001), and the difference between the mass stage and sinus stage, abscess stage and sinus stage were statistically significant (P<0.05).
The intraoperative blood loss in the ablation group was significantly less than that of the ablation group, and the difference was statistically significant (P<0.05). There was a significant difference between the 2 groups in the mass stage and abscess stage (P<0.01; Table 2).
Table 2
| Group | Average | MS | AS | SS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospital stays (days) | Operation time (min) | Blood loss (mL) | Hospital stays (days) | Operation time (min) | Blood loss (mL) | Hospital stays (days) | Operation time (min) | Blood loss (mL) | Hospital stays (days) | Operation time (min) | Blood loss (mL) | ||||
| Ablation | 6.55±2.63 | 13.8±10.5 | 2.58±2.89 | 7.18±4.35 | 10.2±8.02 | 2±0.45 | 6.39±1.59 | 14.26±10.44 | 2.43±3.23 | 5.75±0.96 | 21.5±15.61 | 5±4.08 | |||
| Operation | 15.03±6.15 | 78.0±38.09 | 20.17±11.78 | 13.25±5.12 | 83.33±44.38 | 13.33±7.49 | 16.47±6.49 | 76.0±36.46 | 25.33±12.88 | 15±8.66 | 66.6±22.54 | 21.67±7.64 | |||
| 95% CI | (–10.69 to –6.27) | (–77.06 to –51.26) | (–21.54 to –13.64) | (–10.21 to –1.93) | (–101.41 to –44.90) | (–16.05 to –6.62) | (–12.92 to –7.23) | (–78.00 to –45.48) | (–28.57 to –17.23) | (–20.10 to 1.60) | (–81.87 to –8.46) | (–28.00 to –5.33) | |||
| t/Z(Z) | –6.503 | –6.736 | –6.762 | –3.091 | –4.06 | –4.233 | –4.996 | –4.827 | –5.022 | –2.16 | –3.163 | –3.78 | |||
| P | <0.001 | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.031 | 0.025 | 0.013 | |||
MS, mass stage; AS, abscess stage; SS, sinus stage.
The painless rate in the ablation group was 47.4% (18/38), and that of the surgical group was 13.4% (4/30). The difference between the 2 groups was statistically significant (Z=−4.103; P<0.001). There was a significant difference in the degree of pain between the 2 groups during the abscess period (P<0.01).
The primary healing rate of incision in the ablation group was 78.9% (30/38), and that in the surgical group was 43.4% (13/30). The difference between the 2 groups was statistically significant (Z=356.0; P<0.05). There was a significant difference in the healing of incisions between the 2 groups during the abscess period (P<0.05; Table 3).
Table 3
| Stage | Group | Postoperative pain degree, n [%] | Postoperative wound healing, n [%] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Painless | Mild pain | Moderate pain | Severe pain | Z/χ2 | P | First rate | Second rate | Third rate | Z/χ2 | P | |||
| MS | Ablation | 5 [46] | 4 [36] | 2 [18] | 0 [0] | 3.56 | 0.313 | 11 [100] | 0 [0] | 0 [0] | 3.163 | 0.206 | |
| Operation | 2 [17] | 5 [42] | 3 [25] | 2 [16] | 9 [75] | 2 [17] | 1 [8] | ||||||
| AS | Ablation | 11 [48] | 7 [31] | 4 [17] | 1 [4] | 13.62 | 0.003 | 19 [83] | 3 [13] | 1 [4] | 12.311 | 0.002 | |
| Operation | 2 [13] | 1 [7] | 6 [40] | 6 [40] | 4 [27] | 6 [40] | 5 [33] | ||||||
| SS | Ablation | 2 [50] | 1 [25] | 1 [25] | 0 [0] | 4.96 | 0.175 | 0 [0] | 3 [75] | 1 [25] | 0.058 | 0.809 | |
| Operation | 0 [0] | 0 [0] | 1 [33] | 2 [67] | 0 [0] | 2 [67] | 1 [33] | ||||||
MS, mass stage; AS, abscess stage; SS, sinus stage.
Three months after the operation, the effective rate of the ablation group was 86.8% (33/38), and that of the operation group was 46.7% (14/30). There was a significant difference between the 2 groups (Z=–3.526; P<0.001). The difference between the 2 groups at the abscess stage was more obvious, with statistical significance (P<0.05).
The proportion of “excellent” postoperative appearance in the ablation group was 57.9% (22/38), and that of the surgical group was 23.3% (7/30). The difference between the 2 groups was statistically significant (Z=360.5; P<0.05). There was a significant difference between the 2 groups in the mass stage and abscess stage (P<0.05; Table 4).
Table 4
| Stage | Group | Treatment efficacy, n [%] | Appearance effect, n [%] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CR | PR | SD | PD | Z/χ2 | P | Excellent | Good | Bad | Z/χ2 | P | |||
| MS | Ablation | 9 [82] | 2 [18] | 0 | 0 | 0.093 | 9 [82] | 2 [18] | 0 | 0.036 | |||
| Operation | 5 [42] | 3 [25] | 4 [33] | 0 [0] | 4 [33] | 8 [67] | 0 | ||||||
| AS | Ablation | 15 [66] | 3 [13] | 4 [17] | 1 [4] | 7.82 | 0.02 | 11 [48] | 12 [52] | 0 | 4.661 | 0.031 | |
| Operation | 3 [20] | 2 [13] | 7 [47] | 3 [20] | 3 [20] | 10 [67] | 2 [13] | ||||||
| SS | Ablation | 2 [50] | 2 [50] | 0 | 0 | 0.143 | 2 [50] | 1 [25] | 1 [25] | 2.236 | 0.327 | ||
| Operation | 1 [33] | 0 | 2 [67] | 0 | 0 | 2 [67] | 1 [33] | ||||||
MS, mass stage; AS, abscess stage; SS, sinus stage; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
All patients were followed up for more than 1 year (12–41 months), with an average of 21.35±7.25 months. The recurrence rate of the ablation group was 2.6%. The recurrence rate in the surgical group was 13.3%. The difference was not statistically significant (P>0.05; Table 5).
Table 5
| Group | Cases | Recurrence | No recurrence | 95% CI | χ2 | P |
|---|---|---|---|---|---|---|
| Ablation | 38 | 1 (3%) | 37 (97%) | (0.019–1.663) | 2.818 | 0.162 |
| Operation | 30 | 4 (13%) | 26 (87%) |
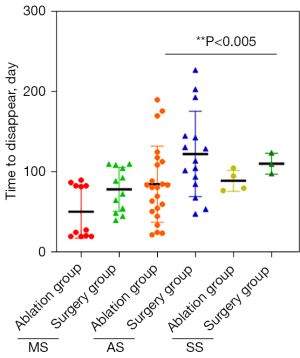
The time for the complete disappearance of lesions in the ablation group was 20–190 days, with an average of 75.55±43.59 days. The average time for mass phase was 50.82±32.88 days, that for the abscess phase was 85±47.42 days, and that for the sinus phase was 89.25±13.12 days.
The time of complete disappearance of lesions in the operation group was 40–227 days, with an average of 103.87±45.98 days. The average for the mass stage was 78.58±27.26 days, that for the abscess stage was 122.73±53.21 days, and that for the sinus stage was 110.67±13.01 days. The time of lesion volume reduction in the ablation group was significantly shorter than that of the operation group, and there was a significant difference between the 2 groups (Z=−2.761; P<0.006; Figure 2).
Discussion
In recent years, ultrasound-guided microwave ablation for benign breast nodules (32-34) and malignant breast tumors (35,36) has been more intensively applied and achieved good therapeutic effects, but the treatment of PCM with ultrasound-guided microwave ablation has not been reported. In this study, 38 patients with PCM treated with ultrasound-guided microwave ablation were compared with 30 patients with PCM treated with traditional surgery to compare the therapeutic effect.
The results of this study showed that PCM was more common in younger women aged 26–30 years with a lactation history. The average hospital stay of the ablation group was 6.55±2.63 days, and the average hospital stay of the traditional operation group was 15.03±6.15 days. This result showed that the duration of the acute phase and postoperative stress response in the ablation group was significantly shorter than that of the open surgical resection group. This suggests that there was less trauma and more stability of the postoperative course in the ablation group, which could save medical costs and patient family support time. The average operation time of the ablation group was 14.5±10.78 min, while that of the operation group was 76.43±38.89 min. The average bleeding volume of the ablation group was 2.58±2.89 mL, while that of the operation group was 20.17±11.78 mL. The painless rate in the ablation group was 47.4% (18/38), and that of the operation group was 13.4% (4/30). Statistically significant differences were found in all of the measures mentioned above. Compared with the clinical stage group, there was a very significant difference in the degree of pain between the 2 groups in the abscess stage (P<0.05). The reason for this might be that the sample size was insufficient, and there was no significant difference in statistics due to the inability to form a clear comparison. Three months after the operation, the effective rate of the ablation group was 86.8% (33/38), and that of the surgical group was 46.7% (14/30). There was a statistically significant difference between the 2 groups, and that of the abscess stage was more obvious. The satisfaction of the ablation group was better than that of the surgical group. The comparison of the time used for complete absorption of lesion volume showed that the overall course of disease in the ablation group was much shorter than that of the surgical group, and the healing time was shorter for the ablation group than for the surgical group. Compared with traditional surgery, in the ablation group, the healing and recovery time of the disease was greatly shortened, and the physical and mental pain of the patients was reduced.
When microwave ablation therapy is integrated into the treatment of PCM, the polar molecules are induced to move at high speed under the action of the microwave field so that the molecular coupling pole of the target tissue oscillates and rotates to generate thermal coagulation, which removes the necrotic substances in the lesions. Inflammatory factors and tissues within the infiltration range of inflammatory tissue are completely inactivated. They lose the effect of inflammatory factors that continue to infiltrate and are slowly absorbed as local inflammatory necrotic substances or flow out with the ablation needle; then, granulation tissue grows in to refill the abscess. Microwave ablation therapy has the advantage of maintaining the shape of the breast after the lesions are completely healed. Moreover, compared with traditional surgery, microwave ablation is less traumatic, has a shorter operation time, and has a more accurate curative effect.
However, the sample size observed in this study was small, and the follow-up time of individual patients was short. There is no evidence of the theory of microwave ablation in mastitis treatment based on pathology and physiology. Therefore, to better guide clinical practice, future research needs to expand the sample size, find better observation and evaluation indicators, pay attention to the observation of long-term efficacy and recurrence rate, conduct basic research, and improve the pathophysiological theoretical basis of microwave ablation in the treatment of PCM.
Conclusions
Ultrasound-guided microwave ablation is highly efficient in treating PCM. It can greatly shorten the hospitalization time and treatment time of patients, and it can achieve better cosmetic results. It is especially suitable for patients at the mass stage of PCM and is thus worthy of extensive promotion and application in the clinic.
Acknowledgments
Funding: This study was funded by the Zhejiang Provincial Natural Science Foundation of China (No. LSD19H180001).
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-21-1132/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1132/coif). All authors report that this study was funded by the Zhejiang Provincial Natural Science Foundation of China (No. LSD19H180001). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Hebei Province Hospital of Chinese Medicine, and informed consent was obtained from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang YQ. Observation on the effect of surgery combined with Shengyang Yiwei Decoction and Yanghe Decoction in the treatment of plasma cell mastitis. Henan Journal of Surgery 2018;24:74-5.
- Patey DH, Thackray AC. Pathology and treatment of mammary-duct fistula. Lancet 1958;2:871-3. [Crossref] [PubMed]
- Li LY, Yang L, Zhu M, Wang CJ, Han B. Therapeutic effect of suppurative plasma cell mastitis patients with acute infection undergoing incision and drainage. Chinese Journal of Nosocomiology 2016;26:905-7.
- Kessler E, Wolloch Y. Granulomatous mastitis: a lesion clinically simulating carcinoma. Am J Clin Pathol 1972;58:642-6. [Crossref] [PubMed]
- Jung Y, Chung JH. Mammary duct ectasia with bloody nipple discharge in a child. Ann Surg Treat Res 2014;86:165-7. [Crossref] [PubMed]
- Zhu DS, Zhai Z, Zhao LN, Li TH, Zhong X, Li W, Li YL, Wang HL, Wang T. Study on Ten Cases of Antipsychotics Associated Plasma Cell Mastitis. World Journal of Integrated Traditional and Western Medicine 2014;9:753-5.
- Liu L, Zhou F, Wang P, Yu L, Ma Z, Li Y, Gao D, Zhang Q, Li L, Yu Z. Periductal Mastitis: An Inflammatory Disease Related to Bacterial Infection and Consequent Immune Responses? Mediators Inflamm 2017;2017:5309081. [Crossref] [PubMed]
- Ma TF, Wang XT, Lin HX, Qu Y, Zhao HP. Meta-analysis of the evaluation of the effect of the operation combined with serum milk formula on plasma cell mastitis. Guangming Journal of Chinese Medicine 2017;32:1827-31.
- Guo L, Du B. Analysis of the effect of incision and drainage in the treatment of purulent plasma cell mastitis. World Latest Medicine Information 2017;17:22+24.
- Wang Q. Attention should be paid to the diagnosis, treatment and research of non-lactating mastitis. Chin J Breast Dis 2013;7:154-6. (Electronic Version).
- Zhu LB, Li PF, Zhang PB. Progress in the diagnosis and treatment of plasma cell mastitis. J Clin Surg 2019;41:497.
- Omranipour R, Mohammadi SF, Samimi P. Idiopathic granulomatous lobular mastitis - report of 43 cases from iran; introducing a preliminary clinical practice guideline. Breast Care (Basel) 2013;8:439-43. [Crossref] [PubMed]
- Zhao HJ, Ma JX, Li JY, Yang WM, Yang HY, Li XL. Study on Kangfuxin Liquid Combined with Tamoxifen in Treating Duct Fistula of Plasma Cell Mastitis in Nipple Depression. Modern Journal of Integrated Traditional Chinese and Western Medicine 2015;24:3451-3.
- Elzahaby IA, Khater A, Fathi A, Hany I, Abdelkhalek M, Gaballah K, Elalfy A, Hamdy O. Etiologic revelation and outcome of the surgical management of idiopathic granulomatous mastitis; An Egyptian centre experience. Breast Dis 2016;36:115-22. [Crossref] [PubMed]
- Bashir MU, Ramcharan A, Alothman S, Beaugris S, Khan SA, Sbeih MA, Engdahl R. The enigma of granulomatous mastitis: A series. Breast Dis 2017;37:17-20. [Crossref] [PubMed]
- Lamy PJ, Roques S, Viglianti C, Fabbro M, Montels F. HE4, a novel marker for epithelial ovarian cancer: evaluation of analytical performances. Ann Biol Clin (Paris) 2010;68:325-9. [PubMed]
- Keller K, Meisel C, Petzold A, Wimberger P. Granulo-matöse Mastitis- möglicher diagnostischer und thera-peutischer Ablauf anhand von Fallbeispielen. Senolo-gie 2018;15:e23.
- Lei X, Chen K, Zhu L, Song E, Su F, Li S. Treatments for Idiopathic Granulomatous Mastitis: Systematic Review and Meta-Analysis. Breastfeed Med 2017;12:415-21. [Crossref] [PubMed]
- Bani-Hani KE, Yaghan RJ, Matalka II, Shatnawi NJ. Idiopathic granulomatous mastitis: time to avoid unnecessary mastectomies. Breast J 2004;10:318-22. [Crossref] [PubMed]
- Yang XX, Wu B. Advances in diagnosis and treatment of plasma cell mastitis. Chinese Journal of Breast Disease 2015;9:115-8. (Electronic Edition).
- Wang YL, Zhao QF, Li Q, Wang S. Clinical study on operation scheme selection and the MRI site type of plasma cell mastitis. Chinese Imaging Journal of Integrated Traditional and Western Medicine 2015;13:598-600.
- Gao YJ, Ma XJ, Wang J, He XP, Gao HF, Yan ZQ. Non-surgical treatment of abscess, sinus and fistula plasma cell mastitis. Chin J Breast Dis 2013;7:379-80. (Electronic Edition).
- Zheng XH, Huang HL, Zhang SB. Analysis of the curative effect of super-segment resection and first-stage suture in the treatment of abscessed plasma cell mastitis. Chin J Curr Adv Gen Surg 2018;21:302-4.
- Versluijs-Ossewaarde FN, Roumen RM, Goris RJ. Subareolar breast abscesses: characteristics and results of surgical treatment. Breast J 2005;11:179-82. [Crossref] [PubMed]
- Yu Q, Wang GL. Study on the clinical diagnosis and treatment of non-lactating mastitis. J Clin Surg 2019;27:201-3.
- He W, Huang PT, Zhan WW, Zhang W, Wang ZL, Zhang C, Zhang HX, Jin ZQ, Cai WJ. Interventional Ultrasound of Breast and Thyroid. People's Heal Publig Hous 2018;11.
- Cui JJ, Lin Z, Chen S, Wang S, Ma J. Treatment of 35 Cases of Plasma Cell Mastitis by Syndrome Differentiation and Physical Therapy. Journal of Changchun University of Chinese Medicine 2013;29:689-90.
- Li CY, Lin HJ, Hu JQ, Lei Jing, Hu Y. Ultrasonographic manifestations of nonpuerperal mastitis in 42 women. Jiangsu Med J 2012;38:1554-6.
- Zeng Y, Huang H. Combined application of mameton circumcision,tamoxifen and dexamethasone shock therapy for plasma cell mastitis. Journal of Mudanjiang Medical University 2020;41:107-9.
- Zhu Y, Tang X, Xing JL, Wu SL. Clinical Observation on 30 Cases of Plasma Cell Mastitis Treated by Staged Operation of Traditional Chinese Medicine. Guiding Journal of Traditional Chinese Medicine and Pharmacy 2020;26:69-72.
- Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer 2006;42:1031-9. [Crossref] [PubMed]
- Wu H, Chen BH, Han ZY, Zhang J, Yu J, Liang P. Therapeutic effect of ultrasound-guided percutaneous microwave ablation for benign breast lesions. Acad J Chin PLA Med Sch 2017;38:917-921+934.
- Zhou N, Qian LY. Current status and progress of microwave ablation therapy for benign breast lesions. Chinese Journal of Minimally Invasive Surgery 2020;20:369-72.
- Li Y, Zhao S, Ji L, Wang XL, He HB, Shang N, Zhou Y. Ultrasound-guided Microwave Ablation Versus Traditional Open Surgery for Multiple-sited Benign Breast Nodules. Chinese Journal of Minimally Invasive Surgery 2018;18:911-914+919.
- Zhang C, Wang ZB, Ding Q. Minimally and non-invasive ablative treatment of early-stage breast cancer. Chin J Surg Oncol 2020;12:329-33.
- Yang XQ, Qin CX, Zhou SJ. Microwave ablation in treatment of locally advanced breast cancer. Journal of Regional Anatomy and Operative Surgery 2018;27:845-7.



