
Ultrasound-assisted carbon nanoparticle labeling of neoadjuvant chemotherapy for breast-conserving surgery in breast cancer
Introduction
Breast cancer is one of the most common cancers in women, accounting for 30% of all cancer cases (1). According to Global Cancer Statistics 2020, breast cancer has eclipsed lung cancer as the top cause of global cancer, with an anticipated 2.3 million new cases (2). The treatment of breast cancer is multidisciplinary. Breast-conserving surgery is frequently used in clinical practice due to patient needs and long-term safety verification (3). Preoperative neoadjuvant chemotherapy can allow some patients to regain the opportunity for surgery and reduce tumor staging in some patients (4). However, tumor regression following chemotherapy can be a concern, as the remaining tumor cells cannot be located even under a microscope in some patients (5). Moreover, illness assessment focuses on axillary lymph node examination after treatment (6). Therefore, to make the incision edge negative in breast-conserving surgery after neoadjuvant chemotherapy, the size and location of the primary tumor are critical. Here, the challenge involves balancing the needs of main tumor labeling and sentinel lymph node biopsy at the same time.
In contrast with other methods such as tumor markers (guide wire and probe) and other dye markers (methylene blue, indocyanine green, gentian purple), which are used to mark nonpalpable breast tumors and sentinel lymph nodes, carbon nanoparticles have good lymphatic tropism as well as lower local diffusion and absorption rates.
Since 2013, based on the advantages and effectiveness of carbon nanoparticles (7), we carried out a new exploration of nanocarbon dyes in precision surgery for gastrointestinal (8,9), thyroid (10), and breast (11) tumors, providing a basis for finding a new and effective staining method. Furthermore, we previously conducted a retrospective analysis of the short-term follow-up of preoperative carbon nanoparticle staining in 16 breast cancer patients and also performed a preliminary study of carbon nanoparticle staining before neoadjuvant chemotherapy in 3 patients. The operative, follow-up, and aesthetic appearance results were all satisfactory (11).
The present study used carbon nanoparticles to label breast cancer before neoadjuvant chemotherapy and sentinel lymph node biopsy. Tracing of the sentinel lymph node and guiding breast-conserving surgery were conducted to minimize postoperative complications. No complications, including cancer recurrence, subcutaneous staining after injection, local skin inflammation or necrosis, or allergic reactions, were observed. We present the following article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-361/rc).
Methods
General patient information
This was a prospective clinical trial (clinical registration number: ChiCTR-OOC-15006844). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee for Drug Clinical Trials of The 900th Hospital of the Joint Logistics Support Force, PLA. Informed consent was obtained from all of the patients.
A total of 68 breast cancer patients confirmed by biopsy between July 2015 and January 2017 were randomly selected from the clinical data. Of these, 32 patients were screened for neoadjuvant chemotherapy, forming a consecutive, random series. We initially performed an ultrasound-guided injection of nanocarbon 0.5 cm from the maximum diameter margin of the transverse and longitudinal axes of the lesion as well as a sentinel lymph node biopsy. Next, 4–6 cycles of neoadjuvant chemotherapy were carried out. Evaluation of the tumor clinical response during chemotherapy was conducted by imaging and ultrasound every 2 cycles. We screened 26 patients who were suitable for breast-conserving surgery after chemotherapy (the patients’ basic information is shown in Table 1). We identified and enrolled patients in the study after obtaining their informed consent. All patients were monitored for side effects at each visit, and all adverse events were reported according to the standard procedures.
Table 1
| Parameter | Result |
|---|---|
| Age (age, ) | 45.7±10.3 |
| Menopause (yes/no) | 11/15 |
| Shortest distance to the nipple (cm, ) | 3.5±0.8 |
| Tumor length (cm, ) | 3.2±1.4 |
| Clinical TNM staging | |
| Stage IIA (example) | 24 |
| Stage IIB (example) | 1 |
| Stage IIIA (example) | 1 |
| Tumor location | |
| Inner and upper quadrant (example) | 6 |
| Inner and lower quadrant (example) | 3 |
| Outer quadrant (example) | 13 |
| Outer and lower quadrant (example) | 4 |
| Pathological type | |
| Invasive ductal carcinoma (example) | 20 |
| Invasive lobular carcinoma (example) | 6 |
| Molecular subtype | |
| Luminal A | 9 |
| Luminal B | 11 |
| HER2 positive | 5 |
| Triple negative | 1 |
| Neoadjuvant chemotherapy | |
| CAF | 7 |
| TC | 9 |
| TAC | 10 |
| Tumor length after NAC (cm, ) | 2.3±1.1 |
Twenty-six patients who completed NAC were assessed as candidates for breast-conserving surgery. The tumor stages were stage II and stage III, and were in 4 directions of the breast. The pathological types were invasive ductal carcinoma and invasive lobular carcinoma. The molecular subtypes were luminal A, luminal B, and HER2-positive. The chemotherapy regimens used were CAF, TC, and TAC. TNM, tumor-node-metastasis; HER2, human epidermal growth factor receptor 2; CAF, cyclophosphamide + adriamycin + fluorouracil; TC, docetaxel + cyclophosphamide; TAC, docetaxel + adriamycin + cyclophosphamide ; NAC, neoadjuvant chemotherapy.
Indications and contraindications of breast-conserving surgery after neoadjuvant chemotherapy
The indications for breast-conserving surgery were the following: (I) maximum tumor diameter of tumor after neoadjuvant chemotherapy less than 3 cm; (II) single tumor with no signs of skin or chest wall involvement; (III) tumor distance from the nipple >2 cm; and (IV) the appropriate tumor: breast ratio, with a good breast shape that could be maintained postoperatively.
The contraindications for breast-conserving surgery were the following: (I) patients who had undergone previous breast or chest wall radiotherapy; (II) the lesions in a wide area or identified as multicentric lesions, for which negative margins would be difficult to achieve; (III) a positive margin after extensive excision of the tumor, or a lack of a guaranteed pathological margin after resection; (IV) patient refusal of breast-conserving surgery; (V) inflammatory breast cancer; (VI) cases in which radiation therapy is needed during pregnancy; and (VII) active connective tissue disease or tolerance to radiotherapy.
Tracer
A carbon nanoparticle suspension injection (Kanalin) was used as a dye, and the carbon nanoparticles (with an average diameter of 150 nm) were evenly distributed throughout the injection. It has a particular interaction with the lymphatic system. When injected into the surrounding tissue of the tumor, the carbon nanoparticle suspension is phagocytized by macrophages, rapidly enters the lymphatic vessels, and makes the lymph nodes black. Meanwhile, the hydrostatic pressure of the tissue pushes it into lymphatic capillaries rather than into the capillaries, where the gaps between endothelial cells are only 30–35 nm.
Preoperative markers and sentinel lymph node biopsy
Sentinel lymph node biopsy is routinely performed before neoadjuvant chemotherapy, as recommended by the 2012 San Antonio Breast Cancer Conference. Patients were placed in the supine position, and the upper limb was abducted. Based on ultrasound-guided confirmation of the maximum horizontal axis of the tumor, 6 injection points were selected at the farthest 0.5 cm away from the outer edge of the tumor, and each injection point was about 60 degrees apart. The carbon nanosuspension was injected into the superficial and deep parts of the gland with a 23-G needle, with 12 points being marked in total (Figure 1). A 0.1 mL nanocarbon suspension (containing 0.05 mg nanoscale suspension and mixed with a 0.9% sodium chloride injection at a 1:1 ratio) was injected into each point.
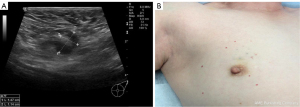
A sentinel lymph node biopsy was subsequently performed. A 3 cm incision was made on the affected side axillary front line and the third fold ribs to explore and excise the lymph nodes and lymphatics, which had been dyed black previously (Figure 2). The excised tissue was sent to the pathology department. The above procedures were carried out by the same group of experienced breast surgeons and color Doppler doctors.

Neoadjuvant chemotherapy
A total of 32 patients with locally advanced breast cancer (no distant metastases were found) who needed downstaging to operate or strongly required downstaging for breast-conserving surgery were selected to complete 4–6 cycles of neoadjuvant chemotherapy preoperatively. The chemotherapy regimens were as follows: (I) cyclophosphamide + adriamycin + fluorouracil (CAF) regimen (cyclophosphamide 100 mg/m2, doxorubicin 30 mg/m2, and fluorouracil 500 mg/m2); (II) docetaxel + adriamycin + cyclophosphamide (TAC) regimen (docetaxel 75 mg/m2, adriamycin 50 mg/m2, and cyclophosphamide 500 mg/m2); and (III) docetaxel + cyclophosphamide (TC) regimen (docetaxel 75 mg/m2 and cyclophosphamide 600 mg/m2). According to the National Comprehensive Cancer Network (NCCN) guidelines, 26 patients who met the indications of breast-conserving surgery were screened out after chemotherapy.
Surgery and postoperative treatment
Operation and incision selection
The patients were placed in the supine position, and the upper extremities were abducted. With the nipple as the center, the breast was divided into two parts: upper and lower. Above the nipple, a nipple-centered arc incision was employed, and below the nipple, a nipple-centered radial incision was used. An axillary incision was designed to be an oblique incision parallel to the axillary fold line. The tissues within 0.5 cm of the tissue stained by carbon nanoparticles were excised during the operation, including the skin of the area and the pectoralis fascia where the tumor was located. The residual cavity and the incision margin of the tumor were examined using frozen pathology (Figure 3). An R0 resection denoted that no tumor cells were found at the endoscopic resection margin. An R1 resection was negative at the macroscopic resection margin, with tumor cells present at the endoscopic resection margin. A positive incision margin required a secondary surgery, and a positive incision margin in secondary surgery was treated with subcutaneous glandectomy. Finally, the operation was completed by routine suture and hemostasis.
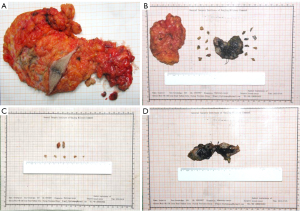
Axillary lymph node dissection
Axillary lymph node biopsy and rapid freezing pathology were carried out in patients with negative sentinel lymph nodes. An axillary lymph node dissection was performed in patients with positive sentinel lymph nodes. Intraoperative removal of lymph nodes was conducted in group I (subaxillary group, lateral breast group, central breast group, subscapular breast group; pectoralis major and intermuscular lymph nodes) and group II (middle axillary group; axillary lymph node of the deep pectoral muscle).
Postoperative chemotherapy
A total of 26 patients underwent 4–6 cycles of systemic chemotherapy at 2–4 weeks postoperatively. According to the clinical evaluation results after neoadjuvant chemotherapy, the original neoadjuvant chemotherapy regimen or a modified regimen was administered. During chemotherapy, gastric mucosa, antiemetic effect, liver protection, and enhanced immunity were routinely protected.
Postoperative radiotherapy
In total, 26 patients were treated with local radiotherapy within 2–4 weeks following the completion of chemotherapy. Radiotherapy was divided into total irradiation (6 MV high-energy X-ray medical accelerator, 1.8–2.0 Gy/time, 5 days per cycle, for a total of 5 sessions of radiotherapy), additional radiation from the primary site (10–16 Gy/time, 7–10 days per cycle, for a total of 5 sessions of radiotherapy), and local radiotherapy in the supraclavicular region (50 Gy/time, 5 days for a cycle, for a total of 5 sessions of radiotherapy).
Endocrine therapy
According to the results of immunohistochemistry and the ages of the patients, endocrine therapy was divided into long-term tamoxifen (nonsteroidal antiestrogen drug) 20 mg/day or long-term letrozole (aromatase inhibitor, synthetic benzyl 3 azole derivative) 2.5 mg/day. We also regularly examined the liver and kidney function of patients and performed routine blood tests during the course of medication.
Evaluation of the curative effect
We assessed the following aspects: tumor cell infiltration, the maximum diameter of the resected tissue, the minimum distance between the tumor margin and the resected margin, the maximum distance between the tumor margin and the resected margin, tumor pathological type, axillary lymph node metastasis, and tumor pathological stage, among others. Time consumption, blood loss, and skin staining were also recorded.
Postoperative follow-up
Physical examination, breast color Doppler ultrasonography, abdominal B ultrasonography, carcinoembryonic antigen (CEA), and cancer antigen (CA) 15-3 were administered every 3 months in the first year postoperatively, and mammography and magnetic resonance imaging (MRI) were performed once annually thereafter. We also conducted a comprehensive evaluation of the patients’ tolerance to follow-up radiotherapy and chemotherapy, ipsilateral breast recurrence, distant metastasis, and survival.
Statistical analysis
Measurement data are expressed as the mean (+) and standard deviation, and count data are expressed by numbers or percentages. The sensitivity (Se), specificity (Sp), positive predictive value (PPV), negative predictive value (NPV), and false-negative rate (FNR) were calculated. SPSS 20 (IBM Corp., Armonk, NY, USA) was used for statistical analyses.
Results
A total of 68 eligible patients were enrolled and participated in the study, and 32 patients received carbon nanoparticle labeling and sentinel lymph node biopsy before neoadjuvant chemotherapy (Figure 4).
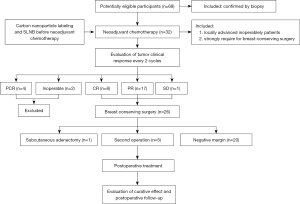
Carbon nanoparticle labeling and sentinel lymph node biopsy
A total of 32 patients received preoperative neoadjuvant chemotherapy, carbon nanoparticle labeling, and sentinel lymph node biopsy. The marking time ranged from 10 to 20 min, with an average of 15.8 min. Black sentinel lymph node staining was found in 29 patients (the sentinel lymph node identification rate was 90.6%), with 0–4 sentinel lymph nodes detected in each patient (average of 1.8). Among the 3 patients without black sentinel lymph node staining, axillary lymph node metastasis was found in 1 patient.
The postoperative pathological examination of axillary lymph nodes revealed that 14 cases were negative and 12 were positive; 11 of the 12 positive cases were detected by sentinel lymph node biopsy. There was 1 false-negative case in which the cancer cells were not found in the black-stained sentinel lymph nodes, but metastatic lymph nodes were found. Table 2 shows the diagnostic values of sentinel nodes using carbon nanoparticles. Based on pathological results (the gold standard), the Se was 91.7% (11/12), the Sp was 100% (14/14), the PPV was 100% (11/11), the NPV was 93.3% (14/15), and the FNR was 3.8% (1/26).
Table 2
| SLNB status | ALND | |||
|---|---|---|---|---|
| cN+ | cN0 | Total | Predict value | |
| Positive | 11 | 0 | 11 | PPV =100% (11/11) |
| Negative | 1 | 14 | 15 | NPV =93.3% (14/15) |
| Total | 12 | 14 | 26 | |
| Predict value | Se =91.7% (11/12) | Sp =100% (14/14) | FNR =3.8% (1/26) | |
Based on the pathological results (the gold standard), the Se was 91.7% (11/12), the Sp was 100% (14/14), the PPV was 100% (11/11), the NPV was 93.3% (14/15), and the FNR was 3.8% (1/26). ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy; Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value; FNR, false-negative rate.
Response to neoadjuvant chemotherapy
Among all 32 patients, there were 4 cases of pathological complete response (pCR; indicating no invasive carcinoma in the primary breast and no carcinoma in the regional lymph nodes). Two cases were inoperable due to a tumor size larger than 3 cm and skin involvement. According to the NCCN guidelines, 26 cases of breast-conserving surgery were selected, including 8 cases of complete response (CR), 17 cases of partial response (PR), 1 case of stable disease (SD), and no cases of progressive disease (PD).
Breast-conserving surgery
A total of 26 cases underwent breast-conserving surgery. Of these, 5 underwent a second operation, as intraoperative frozen pathology examination suggested a positive incision margin, and 1 case underwent subcutaneous adenectomy due to a positive incision margin during the second operation. Excluding 1 case of subcutaneous glandectomy, the diameter of the resected tumors ranged from 2.2 to 4.5 cm (average 3.3 cm). The minimum distance between the resected site and the infiltrated part was 1.0–2.1 cm (average 1.4 cm). The average operation time was 48.4±5.3 minutes. The average blood loss during the operation was 20.8±6.2 mL.
Postoperative follow-up
The follow-up time of the 26 patients who underwent breast-conserving surgery ranged from 6 to 12 months (average 9.2 months). No local recurrence or distant metastasis was observed during the follow-up period. The aesthetic appearance was “good” in 18 patients, “fair” in 5 patients, and “acceptable” in the remaining 3 cases.
Discussion
Neoadjuvant chemotherapy is an important part of breast cancer treatment, and its safety and feasibility have been established (12,13). The primary treatment group includes patients with locally advanced breast cancer and large tumors (>5 cm) or axillary lymph node metastasis, and some patients with early breast cancer with a strong breast-conserving desire (14). The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 clinical study has provided an answer regarding the choice of a neoadjuvant chemotherapy course for breast cancer. Although there was no significant difference between preoperative chemotherapy and postoperative radiotherapy in the disease-free survival (DFS) and overall survival (OS), 4 cycles of neoadjuvant chemotherapy showed greater breast-conserving ability than did postoperative chemotherapy (67.8% vs. 59.8%). Furthermore, the DFS and OS of breast cancer patients with complete pathological remission after neoadjuvant chemotherapy was found to be significantly improved (15,16). Therefore, we chose to perform 4–6 cycles of chemotherapy, which not only satisfies the evaluation of the efficacy of chemotherapy, provides in vivo drug sensitivity results for comprehensive follow-up treatment, and improves breast-conserving opportunities, but also avoids the overtreatment of some patients who are not sensitive to chemotherapy, which delays the treatment cycle.
Based on the results of the present study, the advantages of ultrasound-guided carbon labeling before neoadjuvant chemotherapy in breast-conserving surgery are outlined below.
- Reduction of the recurrence rate. Several large-scale clinical trials have confirmed that the DFS and OS rates of the neoadjuvant chemotherapy are not significantly different from those of postoperative chemotherapy (17). Additionally, these clinical experiments showed that neoadjuvant chemotherapy could significantly improve the breast-conserving surgery rate and reduce the breast incision surgery rate. However, the recurrence rate of ipsilateral breast tumors is higher in patients undergoing breast-conserving surgery at the descending stage than in those who were initially suitable for breast-conserving surgery (18,19). The MD Anderson Cancer Center further studied the factors leading to the recurrence of breast-conserving surgery after neoadjuvant chemotherapy (20), stating 4 points: (i) clinical lymph node status; (ii) residual tumor size; (iii) residual pattern; and (iv) whether there is vascular lymphatic invasion. The precise resection of primary tumors, clear axillary lymph nodes, and the dissection of metastatic lymph nodes are the key factors determining the success of breast-conserving surgery after neoadjuvant chemotherapy (14,21). Ultrasound-guided carbon labeling of tumors and lymph nodes will facilitate the complete removal of lesions during surgery and reduce the rate of postoperative tumor recurrence (7).
- Having unique advantages compared with other preoperative tumor markers.
Preoperative labeling methods for tumors can be divided into 2 categories: surface labeling and tumor body labeling. Surface marking is mainly used to mark the projection of tumors on the skin of the breast using skin tattoos or coordinate paper. The limitation of this method is that the accuracy of the marking is considerably affected by the size and position of the breast. Although tumor body marking, such as guide wire and probe, can accurately determine the original location of the tumors, it cannot guide clinicians to select the range of resected tumors during the operation. At the same time, there is a risk of needle metastasis and labeling displacement. In particular, for residual tumors after neoadjuvant chemotherapy, there is a multifocus pattern, which cannot describe the range of the original tumors, resulting in an increased positive rate of incision margin and secondary operation rate (22). The combination of radioisotopes and blue dye is recommended as the gold standard method for tumor labeling and sentinel lymph node biopsy (23). However, this is difficult to popularize due to safety and economic factors. At present, methylene blue, indocyanine green, gentian purple, superparamagnetic iron oxide nanoparticles, and carbon nanoparticles are effective alternatives. Although staining markers can simultaneously trace sentinel lymph nodes and mark tumors, their retention time in vivo is relatively short, which can easily cause local halo staining of the markers and cannot be applied widely in breast-conserving patients undergoing neoadjuvant chemotherapy (24).
Nanocarbon has good lymphatic tropism and its local diffusion and absorption rates are slower; thus, it remains visible to the naked eye 6 months after labeling. Moreover, compared with traditional dyes, it does not easily affect the surgical field of vision via local halo staining (11,25). Furthermore, the diameter of carbon nanoparticles is too large to enter the blood circulation system, so there is little toxicity and few side effects (26). However, it has been reported that the use of nanocarbon can cause side effects, such as subcutaneous staining after injection, skin inflammation or necrosis inflammatory granuloma, and allergic reactions. No side effects were observed in 26 patients who were followed up by our department.
The nanocarbon suspension used in this study has been maturely applied to the labeling and localization of tumors (such as thyroid cancer, gastric cancer, rectal cancer focus, etc.) and lymph node tracing (27,28). Our previous study showed that nanocarbon exhibits excellent performance in marking breast cancer tumors and sentinel lymph node tracing. In addition, we found that nanocarbon still has a good marking effect intraoperatively after neoadjuvant chemotherapy in 3 patients with breast cancer (11). - Ensuring the sentinel lymph node detection rate of breast-conserving surgery. Numerous clinical trials, such as van Rijk’s 18-month follow-up study (29) on sentinel lymph node biopsy before neoadjuvant chemotherapy, have demonstrated that sentinel lymph node biopsy before chemotherapy can accurately determine the status of axillary lymph nodes. The feasibility of sentinel node biopsy before neoadjuvant chemotherapy was confirmed in a 4-arm, prospective, multicenter cohort study, which showed a sentinel node detection rate of nearly 100% (30). This can avoid the complications and considerable pain caused by axillary lymph node dissection for some patients with early breast cancer. In this study, the sentinel lymph node biopsy identification rate with nanocarbon was 90.6%, the Se was 91.7% (11/12), the Sp was 100% (14/14), the PPV was 100% (11/11), the NPV was 93.3% (14/15), and the FNR was 3.8% (1/26). These results were similar to those reported by studies investigating other methods, which further confirmed the feasibility and validity of this method. Sentinel lymph nodes were not detected in 1 patient with positive lymph nodes in axillary lymph node dissection. In addition to potential problems in the labeling process, some other possible reasons could account for this, such as lymphatic tissue degeneration, lymphatic system injury, lymphatic drainage obstruction, and obesity.
Several studies have shown that sentinel lymph node biopsy accurately stages the axilla following neoadjuvant chemotherapy regardless of the presenting nodal stage (cN0, cN1) (31,32). Moreover, it is as safe to perform as before neoadjuvant chemotherapy and is now standard practice, as patients undergo fewer surgeries and the ypN0 rate is then higher (33). Both of these methods have advantages and disadvantages. Sentinel lymph node biopsy after chemotherapy has become the preferred way to evaluate axillary status in China; however, it should be noted that performing a sentinel lymph node biopsy twice both before and after neoadjuvant chemotherapy is not recommended. - Improving the negative margin rate of breast-conserving surgery. In breast-conserving surgery, a negative margin of incision is the most important factor for surgeons. Complete tumor excision and sufficient normal breast tissue around the tumors to achieve a negative margin is the basic principle and requirement of excision. The pattern of tumor regression after neoadjuvant chemotherapy may be diffuse fragmentation rather than centralization. Therefore, in breast-conserving surgery, it is very important to locate tumors before neoadjuvant chemotherapy (34,35). One study on the margin status of breast-conserving surgery reported that 24.3% of patients after neoadjuvant chemotherapy had tumor-involved margins. Close margins (≤1 mm) were seen in another 111 (17.7%) patients after neoadjuvant chemotherapy. The adjusted odds ratio showed a 3-time higher risk of involved margins compared with primary surgery (36).
In our study, the negative rates of the first and second incision margins were 77% and 96%, respectively. Only 1 case underwent subcutaneous mastectomy because the second incision margin was still positive. Reasonable labeling methods and good physicochemical properties of nanocarbon materials ensure a higher cutting-edge negative rate. Through our labeling method, the 3-dimensional stereotaxic localization of the tumors can provide a visual reference for clinicians. The labeling distance before neoadjuvant chemotherapy was 0.5 cm, while the resection of the tumors was about 0.5 cm away from the black-stained area during surgery, which not only ensured the safe margin of incision but also removed the black-stained tissues as far as possible to avoid local complications caused by nanocarbon residues. We observed that the minimum margin distance between the resected and infiltrated parts of 25 specimens was 1.0–2.1 cm (average 1.4 cm), which was a safe distance for breast-conserving surgery. Moreover, 5 of the 26 patients underwent a second excision, and 1 patient underwent a modified radical mastectomy for breast cancer due to a positive incision margin. A previous meta-analysis based on 21 studies reported that the local recurrence risk is 2.42 times for patients with positive initial margins compared to those with negative initial margins (P<0.001) (37). Therefore, we believe that the surgical procedure should be changed when the secondary margin is still positive. The average diameter of the resected tumors was 3.3 cm. The normal tissues were preserved as much as possible while ensuring complete resection of the tumors. - Improving cosmetic outcomes. Combined with improvements in the CR rate brought about by neoadjuvant chemotherapy, the application of carbon nanoparticles can enable clinicians to accurately excise and preserve normal tissues as much as possible. Considering that Asian women generally have smaller breasts, this plays an important role in the recovery of the breast and the preservation of breast shape. The cosmetic outcomes after breast cancer treatment will also reduce patients’ association with cancer in life and improve their quality of life (38).
Some expectations for carbon nanoparticles
The amount of accumulation of the carbon nanoparticle suspension in tumor tissues and lymph nodes is not yet known. 13C skeleton isotope labeling of carbon nanoparticles have been developed with the same properties as commercial CNP suspension injections. Also, the 13C-content determinations of samples by isotope-ratio mass spectrometry can be used for mapping and quantification in tumor-draining lymph nodes (39). This will benefit safety evaluation and clinical practice guidance.
Study limitations
Some limitations to this study should be addressed. First, the sample number of patients involved in this study was too small to draw a definitive conclusion. We will further verify our findings by expanding the number of clinical trial participants in the future. Second, tumor reconstructive technology may be needed to improve the appearance and symmetry of the breasts after breast-conserving surgery. In 2018, the application of oncoplastic surgery (OPS) in breast cancer was described in the diagnosis and treatment specifications in China and many other countries. Third, tumor regression after neoadjuvant chemotherapy is not a completely central regression. Without staining, tumor resection will be blind. Due to the irregular staining range of carbon nanoparticles, the resection margin was expanded to 0.5 cm from the staining margin; that is, 1.0 cm from the tumor to achieve R0 resection. This can also avoid local complications caused by nanocarbon residues. Lastly, classical clinical trials, such as NSABP B-18, B-27, and an Italian consensus on neoadjuvant therapy for operable breast cancer, recommend that puncture biopsy should be performed after 2 cycles of neoadjuvant therapy (17,19,40). Due to economic constraints and patient compliance, we did not perform a puncture biopsy in this study. We hope that the follow-up can be included in the standardized treatment process.
Conclusions
The application of nanocarbon in breast-conserving surgery with neoadjuvant chemotherapy can directly guide surgeons in the resection of primary tumors, improve the negative rates of surgical margins, and reduce the difficulty of the procedure. At the same time, nanoparticles serving as sentinel lymph node tracers can improve the detection rate of metastatic lymph nodes, accurately determine the stage of axillary lymph nodes, and guide axillary lymph node dissection. However, further study is needed regarding the staging of axillary lymph nodes after neoadjuvant chemotherapy. Therefore, the application of carbon nanoparticles has been preliminarily verified as safe and reliable in breast-conserving surgery with neoadjuvant chemotherapy.
Acknowledgments
The authors would like to thank all participants who contributed to the study.
Funding: This work was supported by the Fujian Province Science and Technology Plan Project Natural Science Foundation in 2020 (No. 2020J011141) and the Fujian Province Guiding Project in 2021 (No. 2021Y0061).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-361/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-361/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Chinese Clinical Trial Registry (clinical registration number: ChiCTR-OOC-15006844). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee for Drug Clinical Trials of The 900th Hospital of Joint Logistics Support Force, PLA. Informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. Lancet 2021;397:1750-69. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Moo TA, Sanford R, Dang C, Morrow M. Overview of Breast Cancer Therapy. PET Clin 2018;13:339-54. [Crossref] [PubMed]
- Asaoka M, Gandhi S, Ishikawa T, Takabe K. Neoadjuvant Chemotherapy for Breast Cancer: Past, Present, and Future. Breast Cancer (Auckl) 2020;14:1178223420980377. [Crossref] [PubMed]
- Reis J, Thomas O, Lahooti M, Lyngra M, Schandiz H, Boavida J, Gjesdal KI, Sauer T, Geisler J, Geitung JT. Correlation between MRI morphological response patterns and histopathological tumor regression after neoadjuvant endocrine therapy in locally advanced breast cancer: a randomized phase II trial. Breast Cancer Res Treat 2021;189:711-23. [Crossref] [PubMed]
- Citgez B, Yigit B, Yetkin SG. Management of the Axilla and the Breast After Neoadjuvant Chemotherapy in Patients with Breast Cancer: A Systematic Review. Sisli Etfal Hastan Tip Bul 2021;55:156-61. [Crossref] [PubMed]
- Zhang L, Huang Y, Yang C, Zhu T, Lin Y, Gao H, Yang M, Cheng M, Wang K. Application of a carbon nanoparticle suspension for sentinel lymph node mapping in patients with early breast cancer: a retrospective cohort study. World J Surg Oncol 2018;16:112. [Crossref] [PubMed]
- Wang Y, Ma J, Huang S, Wang R, Jiang C, Yu L, Lin N, Yang W, Wang W. Safety and efficacy of preoperative tattooing with charcoal nanoparticles for laparoscopic resection of gastric tumors. J Nanosci Nanotechnol 2016;16:7290-4. [Crossref]
- Lin N, Yu C, Zhu Y, Wang Y. Place titanium clips and place metal sheets at the anus to help with rectal cancer surgery. Asian J Surg 2020;43:385-6. [Crossref] [PubMed]
- Zhang Z, Wang Y. Is carbon nanoparticle useful in thyroid surgery regardless of surgery extent and experience? Otolaryngol Head Neck Surg 2014;150:503. [Crossref] [PubMed]
- Jiang Y, Lin N, Huang S, Lin C, Jin N, Zhang Z, Ke J, Yu Y, Zhu J, Wang Y. Tracking nonpalpable breast cancer for breast-conserving surgery with carbon nanoparticles: implication in tumor location and lymph node dissection. Medicine (Baltimore) 2015;94:e605. [Crossref] [PubMed]
- Bear HD. Neoadjuvant chemotherapy for operable breast cancer: individualizing locoregional and systemic therapy. Surg Oncol Clin N Am 2010;19:607-26. [Crossref] [PubMed]
- Adamson K, Chavez-MacGregor M, Caudle A, Smith B, Baumann D, Liu J, Schaverien M. Neoadjuvant Chemotherapy does not Increase Complications in Oncoplastic Breast-Conserving Surgery. Ann Surg Oncol 2019;26:2730-7. [Crossref] [PubMed]
- Teshome M, Kuerer HM. Breast conserving surgery and locoregional control after neoadjuvant chemotherapy. Eur J Surg Oncol 2017;43:865-74. [Crossref] [PubMed]
- Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil SR, Tamkus D, King KM, Pajon ER, Wright MJ, Robert J, Paik S, Mamounas EP, Wolmark N. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 2008;26:778-85. [Crossref] [PubMed]
- Ishitobi M, Komoike Y, Motomura K, Koyama H, Inaji H. Early response to neo-adjuvant chemotherapy in carcinoma of the breast predicts both successful breast-conserving surgery and decreased risk of ipsilateral breast tumor recurrence. Breast J 2010;16:9-13. [Crossref] [PubMed]
- Fisher ER, Wang J, Bryant J, Fisher B, Mamounas E, Wolmark N. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer 2002;95:681-95. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 2018;19:27-39. [Crossref] [PubMed]
- Mamounas EP, Anderson SJ, Dignam JJ, Bear HD, Julian TB, Geyer CE Jr, Taghian A, Wickerham DL, Wolmark N. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol 2012;30:3960-6. [Crossref] [PubMed]
- Chen AM, Meric-Bernstam F, Hunt KK, Thames HD, Oswald MJ, Outlaw ED, Strom EA, McNeese MD, Kuerer HM, Ross MI, Singletary SE, Ames FC, Feig BW, Sahin AA, Perkins GH, Schechter NR, Hortobagyi GN, Buchholz TA. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol 2004;22:2303-12. [Crossref] [PubMed]
- Karakatsanis A, Tasoulis MK, Wärnberg F, Nilsson G, MacNeill F. Meta-analysis of neoadjuvant therapy and its impact in facilitating breast conservation in operable breast cancer. Br J Surg 2018;105:469-81. [Crossref] [PubMed]
- Mathieu MC, Bonhomme-Faivre L, Rouzier R, Seiller M, Barreau-Pouhaer L, Travagli JP. Tattooing breast cancers treated with neoadjuvant chemotherapy. Ann Surg Oncol 2007;14:2233-8. [Crossref] [PubMed]
- Lyman GH, Giuliano AE, Somerfield MR, Benson AB 3rd, Bodurka DC, Burstein HJ, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol 2005;23:7703-20. [Crossref] [PubMed]
- Zhou Y, Liang Y, Zhang J, Feng Y, Li X, Kong X, Ma T, Jiang L, Yang Q. Evaluation of Carbon Nanoparticle Suspension and Methylene Blue Localization for Preoperative Localization of Nonpalpable Breast Lesions: A Comparative Study. Front Surg 2021;8:757694. [Crossref] [PubMed]
- Li J, Jia S, Wang Y, Zhang Y, Kong L, Cao Y, Liu Y, Zhang Y, Chen B. Long-term tracing and staining of carbon nanoparticles for axillary lymph nodes in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy. Asian J Surg 2022;45:89-96. [Crossref] [PubMed]
- Figarol A, Pourchez J, Boudard D, Forest V, Akono C, Tulliani JM, Lecompte JP, Cottier M, Bernache-Assollant D, Grosseau P. In vitro toxicity of carbon nanotubes, nano-graphite and carbon black, similar impacts of acid functionalization. Toxicol In Vitro 2015;30:476-85. [Crossref] [PubMed]
- Liu Y, Li L, Yu J, Fan YX, Lu XB. Carbon nanoparticle lymph node tracer improves the outcomes of surgical treatment in papillary thyroid cancer. Cancer Biomark 2018;23:227-33. [Crossref] [PubMed]
- Liu P, Tan J, Tan Q, Xu L, He T, Lv Q. Application of Carbon Nanoparticles in Tracing Lymph Nodes and Locating Tumors in Colorectal Cancer: A Concise Review. Int J Nanomedicine 2020;15:9671-81. [Crossref] [PubMed]
- van Rijk MC, Nieweg OE, Rutgers EJ, Oldenburg HS, Olmos RV, Hoefnagel CA, Kroon BB. Sentinel node biopsy before neoadjuvant chemotherapy spares breast cancer patients axillary lymph node dissection. Ann Surg Oncol 2006;13:475-9. [Crossref] [PubMed]
- Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, Lebeau A, Liedtke C, von Minckwitz G, Nekljudova V, Schmatloch S, Schrenk P, Staebler A, Untch M. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 2013;14:609-18. [Crossref] [PubMed]
- Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013;310:1455-61. [Crossref] [PubMed]
- Mamounas EP, Brown A, Anderson S, Smith R, Julian T, Miller B, Bear HD, Caldwell CB, Walker AP, Mikkelson WM, Stauffer JS, Robidoux A, Theoret H, Soran A, Fisher B, Wickerham DL, Wolmark N. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2005;23:2694-702. [Crossref] [PubMed]
- Pilewskie M, Morrow M. Axillary Nodal Management Following Neoadjuvant Chemotherapy: A Review. JAMA Oncol 2017;3:549-55. [Crossref] [PubMed]
- Dalberg K, Mattsson A, Rutqvist LE, Johansson U, Riddez L, Sandelin K. Breast conserving surgery for invasive breast cancer: risk factors for ipsilateral breast tumor recurrences. Breast Cancer Res Treat 1997;43:73-86. [Crossref] [PubMed]
- Bouzón A, Acea B, García A, Iglesias Á, Mosquera J, Santiago P, Seoane T. Risk factors for positive margins in conservative surgery for breast cancer after neoadjuvant chemotherapy. Cir Esp 2016;94:379-84. [PubMed]
- Volders JH, Haloua MH, Krekel NM, Negenborn VL, Barbé E, Sietses C, Jóźwiak K, Meijer S, van den Tol MP. “the nationwide network and registry of histo- and cytopathology in the Netherlands (PALGA)”. Neoadjuvant chemotherapy in breast-conserving surgery - Consequences on margin status and excision volumes: A nationwide pathology study. Eur J Surg Oncol 2016;42:986-93. [Crossref] [PubMed]
- Houssami N, Macaskill P, Marinovich ML, Dixon JM, Irwig L, Brennan ME, Solin LJ. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer 2010;46:3219-32. [Crossref] [PubMed]
- Volders JH, Negenborn VL, Spronk PE, Krekel NMA, Schoonmade LJ, Meijer S, Rubio IT, van den Tol MP. Breast-conserving surgery following neoadjuvant therapy-a systematic review on surgical outcomes. Breast Cancer Res Treat 2018;168:1-12. [Crossref] [PubMed]
- Xie P, Xin Q, Yang ST, He T, Huang Y, Zeng G, Ran M, Tang X. Skeleton labeled 13C-carbon nanoparticles for the imaging and quantification in tumor drainage lymph nodes. Int J Nanomedicine 2017;12:4891-9. [Crossref] [PubMed]
- Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 2001;(30):96-102.


