
Role of the Global Limb Anatomic Staging System in predicting outcomes of chronic limb-threatening ischemia in patients treated by drug-coated balloons
Introduction
Peripheral arterial disease (PAD) is the third most prevalent cardiovascular disease, and there are more than 200 million patients with PAD worldwide (1). Chronic limb-threatening ischemia (CLTI) is the most severe form of PAD and includes a broad group of patients with ischemic rest pain or tissue loss (2). Despite the development in techniques and devices for endovascular therapy (EVT), the prognosis of patients with CLTI is still poor, and anatomic staging is vital for guiding clinical decision-making (3). The Global Limb Anatomic Staging System (GLASS) was proposed in the 2019 Global Vascular Guidelines (GVG) for the management of CLTI (2). The GLASS combines grades for the femoropopliteal and infrapopliteal segments to define anatomic stages based on immediate technical failure (ITF) and 1-year limb-based patency (LBP). The 2019 GVG recommended prioritizing research that could provide better evidence to validate the GLASS, particularly for endovascular strategies in different stages of infrainguinal disease (2).
Several research papers have been published on the validation of GLASS stages in patients with CLTI (4-10). However, the results are conflicting, and a large proportion of the patients were enrolled in studies before 2015. Since 2016, paclitaxel-coated devices have altered the treatment paradigms for PAD, and these may have positive or negative effects in the treatment of those with CLTI (11-13). Therefore, it is crucial to assess the performance of the GLASS in patients with CLTI treated with paclitaxel-coated balloons.
This study aimed to investigate the impact of the GLASS on the outcomes of CLTI with femoropopliteal involvement treated with paclitaxel-coated balloons. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-715/rc).
Methods
Patients
Data for the present study were obtained from the database of a multicenter, real-world cohort study. This database collected information on patients with femoropopliteal lesions treated by drug-coated balloon (DCB) angioplasty using the Orchid DCB (Acotec Scientific Co., Ltd., Beijing, China) from July 2016 to June 2019 at 3 medical centers (Peking University First Hospital, Peking Union Medical College Hospital, and China-Japan Friendship Hospital). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional review boards of Peking University First Hospital, Peking Union Medical College Hospital, and China-Japan Friendship Hospital approved this study. Written informed consent was not needed because this study was retrospective and all the participants’ data were anonymized. All methods were carried out in accordance with the relevant guidelines and regulations. Patients in the database were included in this study if they (I) were aged 18 years or older, (II) had a Rutherford classification category greater than or equal to 4, and (III) had stenotic or obstructive lesions of the femoropopliteal arteries. Patients who were pregnant or planning on pregnancy were excluded from the study. Since this was a pilot study, the sample size was determined by the number of patients enrolled in this cohort.
According to the institutions’ standard protocols, baseline characteristics of patients, including age, gender, cardiovascular risk factors, and Rutherford classification, were collected and recorded in a local database. Patients underwent a preoperative physical examination, ankle-brachial index (ABI) measurements, and vascular duplex ultrasound.
Endovascular procedures
Patients were treated under local anesthesia supplemented with intravenous sedation if necessary. A radiopaque ruler was placed close to the target artery to measure the lesion length. All treatment decisions, including the use of DCB or debulking devices, were made at the discretion of the vascular specialists. In all patients, Orchid DCBs were used for treatment. At least 1 plain balloon was used for predilation before each DCB dilation. The diameter of the DCB was 0.5 or 1 mm larger than the plain balloon. Lesion characteristics, such as reference vessel diameter, degree of stenosis, calcification, and inflow and outflow obstructions, were visually estimated. The implantation of stents was conducted in the case of residual stenosis >30% or flow-limiting dissection. Inflow and outflow lesions were often treated during the same intervention, as determined by the physician. For the tibial arteries, at least 1 patent artery was required at the time of completion angiography. If all 3 tibial arteries had stenosis or occlusion, then the artery with the least severe lesion and a patent outflow tract below the ankle was selected as the target path. Typically, the tibial lesion was crossed intraluminally or subintimally by a 0.014-inch guidewire, which was followed by balloon angioplasty.
Evaluation of the GLASS stages
The GLASS score was evaluated blindly by 2 experts with more than 6 years of experience to reduce bias. The procedure of assessing GLASS followed the 2019 GVG (2). First, high-quality angiographic imaging was obtained. Second, the target arterial path (TAP) was identified. Third, the femoropopliteal (FP) GLASS grade (0–4) and the infrapopliteal (IP) GLASS grade (0–4) were determined. In this step, we evaluated whether there was severe calcification within the FP and IP segments of the TAP. If present, the segment grade was increased by 1. Fouth, FP and IP grades were combined to determine the overall GLASS stage (2).
Pharmacological protocol
Prior to surgery, all patients received dual antiplatelet therapy (aspirin 100 mg/day and clopidogrel 75 mg/day) for at least 7 days or a loading dose (aspirin 300 mg and clopidogrel 300 mg/day) 6 hours before surgery. During the procedure, heparin (5,000 IU) was given for anticoagulation after placement of the long sheath. All patients received dual antiplatelet therapy (aspirin 100 mg/day and clopidogrel 75 mg/day) for at least 6 months after surgery, after which they were switched to administration of a single drug type. For patients with cardiovascular comorbidities, the implementation of antiplatelet therapy was performed at the discretion of the physician. Statins were prescribed for patients with low-density lipoprotein (LDL) cholesterol target levels below 1.8 mmol/L (70 mg/dL) and non-high-density lipoprotein (HDL) cholesterol target levels below 2.6 mmol/L (100 mg/dL).
Follow-up
Follow-up visits were routinely scheduled for 1, 3, 6, and 12 months at the outpatient department. During the visits, symptomatic questioning, physical examination, vascular ultrasound, and ABI were performed. Computed tomography angiography (CTA) was performed if the ABI decreased by more than 20%, if the vascular ultrasound suggested significant restenosis (>50%), or if the patient complained of severe symptoms. The status of patients who did not return for the follow-up was confirmed by telephone contact every 6 months.
Endpoints and definitions
The primary endpoint of the study was the comparison of the 12-month LBP rates across the GLASS stages. The secondary endpoints were freedom from clinically driven target lesion revascularization (CD-TLR) rates, mortality rates, major amputation rates, any amputation rates across the GLASS stages, the overall 12-month primary LBP and freedom from CD-TLR rate, and the risk factors for loss of LBP.
LBP was defined as the maintained patency of a TAP. The TAP was the selected as the continuous route of in-line flow from the groin to the ankle. LBP was considered lost when 50% or more restenosis was found based on CTA or duplex ultrasound scanning or when recurrent symptoms of Rutherford categories 4 to 6 occurred. Primary LBP was defined as uninterrupted patency without procedures performed on the treated limb. The GLASS stage was determined according to femoropopliteal grades, infrapopliteal grades, and calcification modifiers after a review of the preprocedural angiograms, as recommended by the GVG (2). CD-TLR was defined as any percutaneous or open intervention to treat the target limb. CD-TLR was performed when symptoms reoccurred or were not relieved and the patency of TAP was lost according to imaging. A moderate to severe degree of calcification was defined as calcification on 2 sides of the vascular walls, with 1 of the calcifications longer than 5 cm. Technical success was defined as <30% residual stenosis of TAP at completion angiography.
Statistical analysis
Statistical analysis was performed using SPSS 22.0 (IBM Corp., Armonk, NY, USA). Continuous data are shown as means ± SD, and categorical data are shown as numbers with percentages. Normally distributed continuous variables were compared using a 2-sided Student’ t test, and nonnormally distributed continuous variables were compared using the Wilcoxon signed-rank test. Means of baseline and immediate postoperative or follow-up data were compared using paired t tests. Comparisons of categorical variables were performed using a chi-square test or Fisher exact test. Kaplan-Meier survival analysis and the log-rank test were used to estimate LBP and freedom of CD-TLR.
A Cox regression analysis was used to identify independent predictors of restenosis and freedom from CD-TLR. Clinical, angiographic, and procedural variables were entered into the model. The variables associated with the loss of LBP (P<0.1) in the univariate analysis were entered into a multivariable model with the forward stepwise method. Outcomes are reported as the hazard ratio (HR) and 95% confidence interval (CI). Listwise deletion was used to handle missing data. Cases with missing data were omitted, and the remaining data were analyzed. A P value of less than .05 was considered statistically significant.
Results
Patient characteristics
A total of 90 CLTI patients (90 limbs) out of 261 femoropopliteal patients were enrolled in this study. The selection flowchart is shown in Figure 1. Demographic information is presented in Table 1. The mean age of the patients was 70.1±9.5 years, and 60.0% of the patients were male. The prevalence of cardiovascular risk factors was high, of which dyslipidemia, hypertension, and diabetes accounted for 33.3%, 73.3%, and 68.9% of the risk, respectively. Patients with GLASS stage II had dyslipidemia more frequently (P=0.018) than did patients in the other stages. Other demographic and cardiovascular conditions were similar among different GLASS stages. Most of the limbs (91.1%) were Rutherford categories 4 or 5 in the preprocedural evaluation. The preprocedure mean ABI value was 0.42±0.29.
Table 1
| Characteristics | Entire cohort (n=90) | GLASS stage I (n=17) | GLASS stage II (n=12) | GLASS stage III (n=61) | P |
|---|---|---|---|---|---|
| Age, years, means ± SD | 70.1±9.5 | 73.8±9.5 | 71.3±7.7 | 68.7±9.8 | 0.136 |
| Male, n (%) | 54 (60.0) | 14 (82.4) | 6 (50.0) | 34 (55.7) | 0.105 |
| Hypertension, n (%) | 66 (73.3) | 11 (64.7) | 10 (83.3) | 45 (73.8) | 0.531 |
| Dyslipidemia, n (%) | 30 (33.3) | 3 (17.6) | 8 (66.7) | 19 (31.1) | 0.018 |
| Diabetes, n (%) | 62 (68.9) | 9 (52.9) | 9 (75.0) | 44 (72.1) | 0.283 |
| Stroke, n (%) | 24 (26.7) | 5 (29.4) | 3 (25.0) | 16 (26.2) | 0.957 |
| Coronary heart disease, n (%) | 28 (31.1) | 5 (29.4) | 5 (41.7) | 18 (29.5) | 0.698 |
| Renal insufficiency, n (%) | 9 (10.0) | 1 (5.9) | 1 (8.3) | 7 (11.5) | 0.777 |
| Smoking, n (%) | 36 (40.0) | 8 (47.1) | 4 (33.3) | 24 (39.3) | 0.746 |
| Rutherford classification, n (%) | |||||
| 4 | 50 (55.6) | 14 (82.4) | 9 (75.0) | 27 (44.3) | <0.001 |
| 5 | 32 (35.6) | 3 (17.6) | 3 (25.0) | 26 (42.6) | 0.117 |
| 6 | 8 (8.9) | 0 | 0 | 8 (13.1) | 0.124 |
Renal insufficiency was defined as an estimated glomerular filtration rate less than 60 mL/min. GLASS, Global Limb Anatomic Staging System.
Lesion and procedural characteristics
Lesion and procedural characteristics are provided in Table 2. More than 60% of the lesions involved the femoropopliteal arteries only. Total occlusions accounted for 77.8% of all lesions. Most of the lesions were de novo (83.3%). Lesions were classified as GLASS stage I in 18.9%, II in 13.3%, and III in 67.8% of the limbs. The rate of IP involvement was highest in patients with GLASS stage III (6.90% in GLASS I or II vs. 54.1% in GLASS III; P<0.0001). Stents were deployed in 21 limbs (23.3%). The overall technical success rate was 96.7% (87/90), and no difference was found among the different GLASS stages (P=0.478).
Table 2
| Variables | The entire cohort (n=90) |
|---|---|
| Vessel, n (%) | |
| Femoropopliteal only | 55 (61.1) |
| Femoropopliteal and infrapopliteal | 35 (38.9) |
| Moderate or severe calcification | 39 (43.3) |
| Lesion type, n (%) | |
| De novo | 72 (83.3) |
| Presence of ISR | 15 (16.7) |
| CTO | 70 (77.8) |
| Presence of thrombosis | 4 (4.4) |
| Stent deployment | 21 (23.3) |
| BTK intervention, n (%) | 40 (44.4) |
| ATA intervention | 12 (13.3) |
| PA intervention | 20 (22.2) |
| PTA intervention | 13 (14.4) |
| FP score of the GLASS, n (%) | |
| 1 | 6 (6.7) |
| 2 | 15 (16.7) |
| 3 | 15 (16.7) |
| 4 | 54 (60.0) |
| IP score of the GLASS, n (%) | |
| 0 | 55 (61.1) |
| 1 | 0 |
| 2 | 6 (6.7) |
| 3 | 12 (10.3) |
| 4 | 17 (18.9) |
| GLASS stage I, n (%) | 17 (18.9) |
| GLASS stage II, n (%) | 12 (13.3) |
| GLASS stage III, n (%) | 61 (67.8) |
BTK, below the knee; ATA, anterior tibial artery; PA, peroneal artery; PTA, posterior tibial artery; CTO, chronic total occlusion; ISR, in-stent restenosis; FP, femoropopliteal; IP, infrapopliteal; GLASS, Global Limb Anatomic Staging System.
Follow-up outcomes
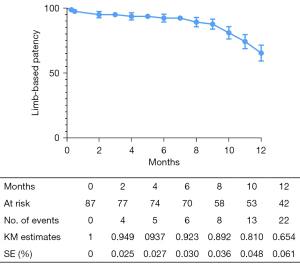
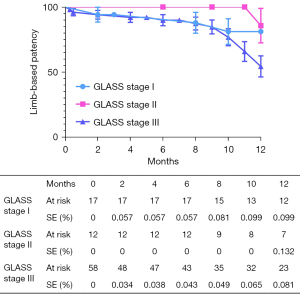
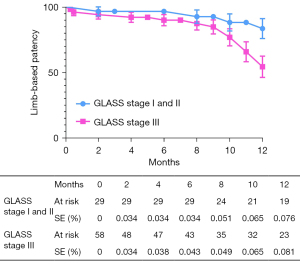
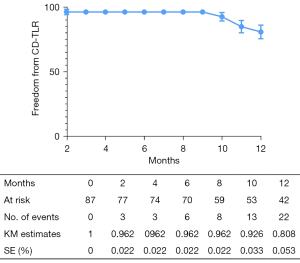
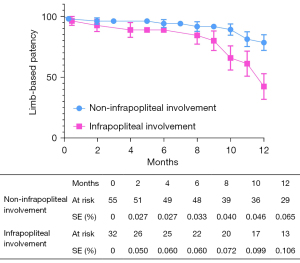
The overall follow-up rate was 90.8% (79/87), and the mean follow-up time was 370.1±198.7 days. According to the Kaplan-Meier estimate at 12 months, the LBP rate was 65.4% for the entire cohort (Figure 2). The primary 12-month LBP rates among the GLASS stages were not significantly different (stage I 81.1%; stage II 85.2%; stage III 54.4%; P=0.080; Figure 3). However, the differences were significant when GLASS stages I and II were classified as 1 category and compared with stage III patients (stage I and II 83.5%; stage III 54.4%; P=0.027; Figure 4). In the entire cohort, severe FP lesion (GLASS scores 3 or 4) was not a risk factor for loss of patency (P=0.329). In patients involving only the femoropopliteal artery, a severe FP lesion was also not a risk factor for loss of patency (P=0.893). The freedom from CD-TLR calculated by the Kaplan-Meier estimate was 80.8% at 12 months for the entire cohort (Figure 5). The difference in the freedom from CD-TLR rate was significant in the dichotomous classification (stage I and II 96.6%; stage III 70.4%; P=0.030) but not in the original GLASS classification (stage I 94.1%; stage II: 100%; stage III 70.4%; P=0.088). Overall, the 12-month mortality rate was 9.2%, which was similar among the GLASS stages (P=0.919). The rates of any amputation and major amputation at 12 months were 8.0% and 1.1%, respectively, with no significant differences among the GLASS stages (P=0.149 and P=0.777). The primary 12-month LBP rate was significantly higher in the patients without infrapopliteal involvement than in those with infrapopliteal involvement ( 78.5% vs. 42.4%; P=0.005; Figure 6). The mean ABI value at follow-up was 0.78±0.35, which was an improvement on the baseline (P<0.001).
As shown in Table 3, the univariate analysis determined that possible risk factors for loss of LBP were renal function impairment, coronary artery disease, and GLASS stage III (P<0.1). The Cox multivariate analysis showed that coronary heart disease (HR =2.397; 95% CI: 1.038–5.535; P=0.041) and GLASS stage III (HR =3.022, 95% CI: 1.021–8.944; P=0.046) were independent risk factors for the loss of 12-month LBP.
Table 3
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| P value | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | ||
| Age (≥65) | 0.172 | 0.557 (0.241–1.290) | |||
| Gender | 0.441 | 0.720 (0.312–1.662) | |||
| Hypertension | 0.818 | 0.896 (0.350–2.290) | |||
| Dyslipidemia | 0.365 | 0.648 (0.253–1.658) | |||
| Diabetes | 0.859 | 1.089 (0.426–2.784) | |||
| Stroke | 0.676 | 1.203 (0.505–2.870) | |||
| Renal function impairment | 0.043 | 2.649 (1.033–6.792) | 0.165 | N/A | |
| Smoking | 0.812 | 1.109 (0.474–2.596) | |||
| Coronary artery disease | 0.034 | 2.467 (1.069–5.696) | 0.041 | 2.397 (1.038–5.535) | |
| Restenosis | 0.440 | 1.398 (0.597–3.275) | |||
| CTO | 0.445 | 1.526 (0.516–4.516) | |||
| Calcium (Ca+) | 0.568 | 0.770 (0.313–1.890) | |||
| GLASS stage 3 | 0.041 | 3.104 (1.049–9.181) | 0.046 | 3.022 (1.021–8.944) | |
| Stent deployment | 0.577 | 1.306 (0.511–3.342) | |||
CD-TLR, target lesion revascularization; CI, confidence interval; CTO, chronic total occlusion; GLASS, Global Limb Anatomic Staging System; N/A, not applicable.
Discussion
In this retrospective analysis of the CLTI subgroup in a multicenter database, 12-month results for 90 CLTI limbs treated with DCB were examined. Lesions were classified as GLASS stage I in 18.9%, II in 13.3%, and III in 67.8% of the limbs. The overall 12-month LBP was 65.4%, and differences in LBP and freedom from CD-TLR were not found among the 3 GLASS stages. However, the LBP and freedom from CD-TLR rates of stage III were lower than the combined LBP stages of I and II. Moreover, when stages I and II were defined as a single category, GLASS stage III was the independent risk factor for loss of 12-month LBP in univariate and multivariate analyses.
The performance of the GLASS has been investigated in several studies since it was first proposed in the 2019 GVG (4-9). Analysis of the Bypass versus Angioplasty in Severe Ischemia of the Leg (BASIL)-1 cohort elucidated the association of the GLASS with ITF, amputation-free survival, limb salvage, and freedom from major adverse limb events following EVT (9). Most of the lesions in the EVT group of the BASIL-1 cohort were located in the femoropopliteal segments (femoropopliteal alone 74.6%; femoropopliteal and infrapopliteal 25.4%), which is similar to the present study. A recent single-center, retrospective study found similar results, with the technical success lower in the GLASS stage III group and GLASS stage III able to predict ITF through multivariable analysis (7). Another single-center, retrospective study found that GLASS stages II and III were associated with a higher risk of restenosis in first-time revascularization at the 1- and 5-year follow-up (4). Conflicting results were obtained from other studies. Results of a study focusing on patients with CLTI on hemodialysis showed that GLASS staging could not predict wound healing or amputation-free survival (5). A retrospective study that analyzed patients with CLTI presenting with infrapopliteal lesions revealed that the GLASS stage was not predictive of wound healing (8). Another study found that 1-year LBP could also not be predicted after tibial EVT in diabetic patients with CLTI (6). In the present study, which enrolled femoropopliteal-involved patients with CLTI, original GLASS stages were not associated with 1-year LBP (P=0.080). It is assumed that the marginally negative result was caused by the small sample size, especially in stages I and II. Therefore, we grouped stages I and II to enhance the statistical power. Stage III was an independent risk factor for loss of 1-year LBP when stages I and II were defined as a single category. The differential performance of the GLASS in different studies may be a result of the different balloon designs, receptors, and paclitaxel doses in various DCBs. The current evidence, although limited, suggests that the performance of the GLASS was better in femoropopliteal than in infrapopliteal lesions for determining limb events. This phenomenon may be explained by the fact that risk factors of restenosis for FP lesions are better known than those for IP lesions. Therefore, the predictions of FP lesions based on these studies are more effective. In the present study, patients without IP involvement had better 12-month LBP than did patients with IP involvement. Patients with different FP lesion severities had similar 12-month LBP results. The results suggest that the severity of IP lesions has a greater impact on the outcome of CLTI than it has on the outcome of FP lesions. This could be partially explained by the fact that the restenosis rates of IP lesions were higher than those of FP lesions.
DCB has been proven to consistently reduce late lumen loss, binary restenosis, and target lesion revascularization compared with angioplasty alone in the treatment of femoropopliteal disease (14,15). Recent studies including patients with infrapopliteal CLTI revealed that DCB could improve both angiographic and clinical outcomes compared with angioplasty alone (11,16,17). Although concerns about mortality and amputation risk exist (12,18), the use of DCB in patients with CLTI will likely become more popular in the future. To the best of our knowledge, the present study is the first to focus on the validation of the GLASS staging in patients with CLTI treated with DCB. The rates of 1-year LBP and freedom from CD-TLR were lower in GLASS stage III limbs than in GLASS stage I and stage II limbs. The GLASS classification is promising for predicting outcomes in patients with CLTI treated with DCB.
There are potential limitations in the present study. First, since the study was a retrospective analysis of a multicenter database, it was a nonrandomized and noncomparative study with a limited sample size. Second, some data were not available in the database for the present study, including data concerning, the limb status (wound, ischemia, and foot infection score), and the pedal modifier (19). Analysis based on these factors was impossible, and bias could have been introduced.
In conclusion, GLASS stage III was associated with lower rates of 1-year LBP and freedom from CD-TLR in CLTI patients with femoropopliteal lesions treated with DCB. GLASS stage III was an independent risk factor for loss of 12-month LBP. The present study suggests that GLASS stage III could be used to predict the prognosis of patients with CLTI. Further studies with a larger number of samples and a longer duration of follow-up are warranted.
Acknowledgments
Funding: This work was supported by National High Level Hospital Clinical Research Funding (Interdisciplinary Clinical Research Project of Peking University First Hospital) (No. 2022CR44), the National Key R&D Program of China (No. 2017YFC0109105), the Scientific Research Seed Fund of Peking University First Hospital (No. 2018SF023), the Youth Clinical Research Project of Peking University First Hospital (No. 2018CR16), and the Interdisciplinary Clinical Research Project of Peking University First Hospital (No. 2018CR33).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-715/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-715/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional review boards of Peking University First Hospital, Peking Union Medical College Hospital, and China-Japan Friendship Hospital approved this study. Written informed consent was not needed because this study was retrospective and all the participants’ data were anonymized.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013;382:1329-40. [Crossref] [PubMed]
- Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, et al. Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. Eur J Vasc Endovasc Surg 2019;58:S1-S109.e33.
- Almasri J, Adusumalli J, Asi N, Lakis S, Alsawas M, Prokop LJ, Bradbury A, Kolh P, Conte MS, Murad MH. A systematic review and meta-analysis of revascularization outcomes of infrainguinal chronic limb-threatening ischemia. J Vasc Surg 2018;68:624-33. [Crossref] [PubMed]
- Liang P, Marcaccio CL, Darling JD, Kong D, Rao V, St John E, Wyers MC, Hamdan AD, Schermerhorn ML. Validation of the Global Limb Anatomic Staging System in first-time lower extremity revascularization. J Vasc Surg 2021;73:1683-1691.e1. [Crossref] [PubMed]
- Tokuda T, Oba Y, Koshida R, Kagase A, Matsuda H, Suzuki Y, Murata A, Ito T. Validation of Global Limb Anatomical Staging System (GLASS) in patients with hemodialysis and Chronic Limb-Threatening Ischemia after endovascular treatment. Heart Vessels 2021;36:809-17. [Crossref] [PubMed]
- Hicks CW, Zhang GQ, Canner JK, Weaver ML, Lum YW, Black JH, Abularrage CJ. The Global Anatomic Staging System Does Not Predict Limb Based Patency of Tibial Endovascular Interventions. Ann Vasc Surg 2021;75:79-85. [Crossref] [PubMed]
- Tokuda T, Oba Y, Koshida R, Suzuki Y, Murata A, Ito T. Prediction of the Technical Success of Endovascular Therapy in Patients with Critical Limb Threatening Ischaemia Using the Global Limb Anatomical Staging System. Eur J Vasc Endovasc Surg 2020;60:696-702. [Crossref] [PubMed]
- Hata Y, Iida O, Takahara M, Asai M, Masuda M, Okamoto S, Ishihara T, Nanto K, Kanda T, Tsujimura T, Okuno S, Matsuda Y, Mano T. Infrapopliteal Anatomic Severity and Delayed Wound Healing in Patients With Chronic Limb-Threatening Ischemia in the Era of the Global Limb Anatomic Staging System. J Endovasc Ther 2020;27:641-6. [Crossref] [PubMed]
- Kodama A, Meecham L, Popplewell M, Bate G, Conte MS, Bradbury AW. Editor's Choice - Relationship Between Global Limb Anatomic Staging System (GLASS) and Clinical Outcomes Following Revascularisation for Chronic Limb Threatening Ischaemia in the Bypass Versus Angioplasty in Severe Ischaemia of the Leg (BASIL)-1 Trial. Eur J Vasc Endovasc Surg 2020;60:687-95. [Crossref] [PubMed]
- Utsunomiya M, Takahara M, Iida O, Soga Y, Hata Y, Shiraki T, Nagae A, Kato T, Kobayashi N, Suematsu N, Tasaki J, Horie K, Uchida D, Kodama A, Azuma N, Nakamura M. Limb-Based Patency After Surgical vs Endovascular Revascularization in Patients with Chronic Limb-Threatening Ischemia. J Endovasc Ther 2020;27:584-94. [Crossref] [PubMed]
- Liistro F, Angioli P, Ventoruzzo G, Ducci K, Reccia MR, Ricci L, Falsini G, Scatena A, Pieroni M, Bolognese L. Randomized Controlled Trial of Acotec Drug-Eluting Balloon Versus Plain Balloon for Below-the-Knee Angioplasty. JACC Cardiovasc Interv 2020;13:2277-86. [Crossref] [PubMed]
- Katsanos K, Spiliopoulos S, Teichgräber U, Kitrou P, Del Giudice C, Björkman P, Bisdas T, de Boer S, Krokidis M, Karnabatidis D. Editor's Choice - Risk of Major Amputation Following Application of Paclitaxel Coated Balloons in the Lower Limb Arteries: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Eur J Vasc Endovasc Surg 2022;63:60-71. [Crossref] [PubMed]
- Mohapatra A, Saadeddin Z, Bertges DJ, Madigan MC, Al-Khoury GE, Makaroun MS, Eslami MH. Nationwide trends in drug-coated balloon and drug-eluting stent utilization in the femoropopliteal arteries. J Vasc Surg 2020;71:560-6. [Crossref] [PubMed]
- Caradu C, Lakhlifi E, Colacchio EC, Midy D, Bérard X, Poirier M, Ducasse E. Systematic review and updated meta-analysis of the use of drug-coated balloon angioplasty versus plain old balloon angioplasty for femoropopliteal arterial disease. J Vasc Surg 2019;70:981-995.e10. [Crossref] [PubMed]
- Zhang B, Yang M, He T, Li X, Gu J, Zhang X, Dai X, Li X, Lu X, Lang D, Hu H, Chen X, Yang B, Gu H, Zhang X, Zou Y. Twelve-Month Results From the First-in-China Prospective, Multi-Center, Randomized, Controlled Study of the FREEWAY Paclitaxel-Coated Balloon for Femoropopliteal Treatment. Front Cardiovasc Med 2021;8:686267. [Crossref] [PubMed]
- Jia X, Zhuang B, Wang F, Gu Y, Zhang J, Lu X, Dai X, Liu Z, Bi W, Liu C, Wang S, Liistro F, Guo W. Drug-Coated Balloon Angioplasty Compared With Uncoated Balloons in the Treatment of Infrapopliteal Artery Lesions (AcoArt II-BTK). J Endovasc Ther 2021;28:215-21. [Crossref] [PubMed]
- Liistro F, Weinberg I, Almonacid Popma A, Shishehbor MH, Deckers S, Micari A. Paclitaxel-coated balloons versus percutaneous transluminal angioplasty for infrapopliteal chronic total occlusions: the IN.PACT BTK randomised trial. EuroIntervention 2022;17:e1445-54. [Crossref] [PubMed]
- Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc 2018;7:e011245. [Crossref] [PubMed]
- Shao J, Ma J, Lai Z, Yu X, Li K, Xu L, Chen J, Wang C, Cao W, Liu X, Yuan J, Liu B. Impaired pedal arch affects the treatment effect in patients with single tibial artery revascularization demonstrated by intraoperative perfusion. Quant Imaging Med Surg 2022;12:3204-12. [Crossref] [PubMed]



