
Muscle perfusion and the effect of compression garments in delayed-onset muscle soreness assessed with arterial spin labeling magnetic resonance imaging
Introduction
Skeletal muscle injuries are the most prevalent injuries in recreational and elite sports, contributing for up to 55% of all injuries sustained, and often prevent athletes from participation in training or competition (1,2). Unaccustomed exercises or high-load eccentric muscle contractions often lead to delayed-onset muscle soreness (DOMS), which is one of the most common reasons for complaints in sports and impaired muscle performance (3). This is connected to structural changes as well as damage to the skeletal muscle and loss of myofibrillar integrity with Z-band streaming and disruption(4), changes in vascular permeability (5), inflammatory processes (6), and increased muscle proteins and enzymes in the systemic bloodstream (7). These complex immune responses, which start immediately after training and continue over several days, play a key role in the regeneration of exercise-induced muscle damage (EIMD) such as DOMS; little is known about potential changes in muscle perfusion during these cascading processes (6).
Wearing compression garments during and after exercise for improved regeneration and recovery is increasingly popular to reduce exercise-related impairments and injury risk as well as to enhance performance (8). However, it is controversial how much impact compression garments have on DOMS when worn during or after exercise (8,9). Several results indicate that compression therapy is effective in enhancing recovery after exercise; however, the relationship between regeneration and wear time has not been defined clearly, and the underlying mechanisms are not fully understood (8,9). One explanation might be the differences in the compression levels applied among studies (10), as little is known about the relationship between compression levels and microvascular perfusion of the affected musculature during recovery from high-intensity exercise. Sperlich et al. reported no change in muscle perfusion at rest applying compression of 37 mmHg to the thigh muscles, whereas reduced muscle perfusion occurred 15 min after a high-intensity cycling exercise (11). In contrast, Bochmann et al. described an increased arterial inflow in the forearm when using 13 to 23 mmHg of external compression (12).
In a previously published study using intravoxel incoherent motion (IVIM) MR imaging, Riexinger et al. reported a short-term effect of increased muscle perfusion (30 min after eccentric exercise inducing DOMS) with normalization during regeneration after 6–48 h (13). Further, compression garments (21–22 mmHg) did not alter microvascular muscle perfusion at rest, nor did they have any significant effect during the regeneration phase of DOMS (13). IVIM comes along with several technical limitations and the results may be contradictory to the known inflammatory and immune responses in the context of DOMS, which start immediately after training and occur over several days (6,13).
Arterial spin labeling (ASL) is a non-invasive magnetic resonance imaging (MRI) technique to measure perfusion which was applied successfully in certain muscle perfusion studies (14-17). It allows a quantitative, non-invasive measurement of local perfusion without the need of contrast agents. ASL is based on the inversion of the blood magnetization by radiofrequency (RF) pulses, which can be used as an endogenous tracer. ASL can be divided into three categories: continuous ASL (CASL), pulsed ASL (PASL), and pseudo-continuous ASL (PCASL). Among those approaches, PCASL is most commonly recommended due to its high signal-to-noise ratio compared to PASL and clinical applicability compared to CASL, which is not compatible with modern body coil RF transmission hardware (18). Selectivity to a specific muscle exercise as well as to muscle effort has been demonstrated (15,17,19). Therefore, PCASL MRI seems to be an optimal technique to determine locally changes microvascular perfusion in athletes wearing compression garments and/or after muscle exercises.
We hypothesized that the regeneration phase after EIMD that induces DOMS is associated with increased arterial microvascular muscle perfusion. Further, we expected that wearing compression garments would alter muscle perfusion at rest and during regeneration phase of DOMS. Therefore, the aim of this study was to non-invasively examine the microvascular muscle perfusion and the effect of wearing compression garments at resting conditions and after the induction of DOMS using PCASL. Our results could help improve our understanding of the physiological aspects of ultrastructural muscle lesions and the impact of compression garments during regeneration. We present the following article in accordance with the CONSORT reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1104/rc).
Methods
Study population
Fourteen healthy volunteers from medical and sports facilities were enrolled from 03/2021 to 04/2021 (Table 1). The average training frequency of the healthy volunteers corresponded to grade 3 of the Valderrabano Sport Scale (2.7±0.3) with more than 5 training h per week. Inclusion criteria were no history of chronic diseases (such as hypertension, diabetes, renal impairment), no history of muscle injury, overuse or current acute injuries of the lower limbs. All volunteers were asked to forego muscle exercise 72 h before and 48 h after the first MRI acquisition. Volunteers with regular training habits that included plyometric or eccentric exercises and with any symptoms of lower limb muscle soreness within three months prior to the study were excluded (20). The study was performed in accordance with the Declaration of Helsinki (as revised in 2013). The measurements were carried out in accordance with institutional guidelines and with the approval of the institutional ethics committee of Friedrich-Alexander University Erlangen-Nürnberg, Germany (No. 33_16 B). All volunteers provided informed consent prior to study participation.
Table 1
| Characteristics | All (n=14) |
|---|---|
| Gender (female/male) | 6/8 |
| Age (years) | 24.2±2.8 |
| Height (cm) | 1.80±0.07 |
| Weight (kg) | 75.1±13.8 |
| BMI (kg/m2) | 22.4±3.1 |
| CK (U/L) | 143.5±123.2 |
Continuous variables expressed as mean ± standard deviation (SD). BMI, body mass index; CK, creatine kinase.
Study design
The volunteers underwent a total of five PCASL MRI scans of both lower legs (Figure 1). The baseline MRI scan of the left and right lower leg was conducted without any compression garments after a resting period of 20 min. Directly after the first ASL measurement, a compression garment (Bauerfeind AG, Zeulenroda-Triebes, Germany, Compression Sock Performance, 21–22 mmHg, 75% polyamide, 25% elastane) was placed on a randomly selected lower leg on the table of the MR scanner and a second measurement was conducted to examine the effect of compression at rest. These acquisitions were followed by a standardized exhausting exercise intervention, as described below. The exercise intervention was performed in a room directly adjacent to the MR scanner to minimize the delay between finishing exercise and MR acquisition to approximately less than one minute. So, directly after finishing the exercise protocol, the third ASL measurements were carried out. After wearing the compression garment for 6 h, the volunteers took off the garment right before the fourth scan. The last measurement was conducted 48 h after completing the exercise intervention.
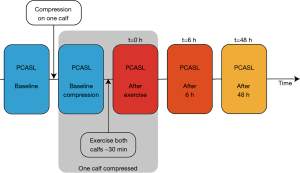
Exercise intervention
The volunteers performed a standardized training with plyometric and eccentric exercises for both lower legs to induce DOMS (20-22). For warm-up, heel raises (two sets of 15 repetitions, 20 s break in between) were performed. The plyometric exercise was carried out immediately after the warm-up. The volunteers were instructed to perform drop jumps from a 25 cm box. For this, the participants had to hop off the box, land with both feet, and jump vertically with the shortest possible contact time with the ground. This exercise was conducted in 5 sets of 10 repetitions each, with 5 s breaks between the jumps and 1 min between the sets. Next, an intense eccentric exercise was performed on a manufactured slant plate with a tilt of −35° with the volunteer wearing a weight vest (approximately 40% of their body weight) as described previously (13,20-22) (Figure 2): maximal contraction of the calf muscle for 1 s by raising the heels, with subsequent lowering of the heels within 3 s until the soles were below 0°. To return to the initial position (heels raised), the participants had to pull themselves up on a pull-up bar installed above their heads to focus on eccentric contraction. All volunteers performed 4 sets, 50 repetitions each, and rested 60 s between each set, followed by 1 additional set until muscle fatigue, so that no further repetition of eccentric exercise was possible.

MRI
All measurements were performed on a 3T MR system (MAGNETOM Prisma, Siemens Healthcare GmbH, Erlangen, Germany) using an 18-channel body array coil. Perfusion measurements of both lower legs were conducted using a PCASL sequence with a 2D gradient-echo echo-planar (EPI) readout. The PCASL labeling and control scheme consists of an unbalanced gradient design and Hanning window shaped RF pulses as recommended by Alsop et al. (18). The following parameters were used: average B1 =1.7 µT, maximum gradient Gmax =9 mT/m, average gradient Gave =1 mT/m with a labeling duration of 2,000 ms and a post-labeling delay of 1,900 ms as described by Englund et al. for lower leg perfusion measurements (16). Labeling/control positioning was done with the help of a fast time-of-flight angiography protocol [repetition time (TR) =11 ms, echo time (TE) =3.7 ms, 0.9×0.9×1 mm3, flip angle =15°, GRAPPA PAT 3, scan time (TA) =1:16 min] and located approx. 60 mm superior to the imaging layers.
For the EPI readout, a TE of 10 ms, flip angle of 90°, phase partial Fourier of 6/8, and bandwidth of 2,435 Hz/px were applied. Ten slices with a slice thickness of 10 mm and an in-plane resolution of 3×3 mm2 were acquired. To suppress fat signal, a frequency-selective fat saturation pulse was used prior to every readout RF pulse for every slice, with a flip angle of 110°. In total, 24 labeling/control scans and one additional M0-Scan (same parameters, but without preparation pulses) were performed with a TR of 4,500 ms, resulting in a scan time of 1:54 min.
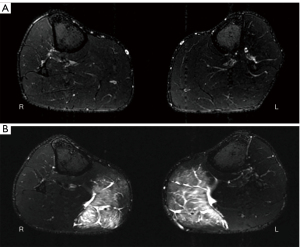
Additionally, a transversal T2-weighted TIRM (turbo inversion recovery magnitude) sequence (TR =6,140 ms, TE =68 ms, 0.9×0.9×4 mm3, TA =4:11 min) of both lower legs was conducted at baseline and after 48 h.
Qualitative imaging analysis
A 3-grade modified Peetrons classification was used to assess EIMD (23): grade 0 indicated a negative MRI without any visible pathology, grade I indicated edema but no architectural disorganization, grade II indicated architectural disruption with partial tear, and grade III indicated total muscle rupture (23). All subjects with grade I lesions in the acquired TIRM images were considered to have induced DOMS (3).
Perfusion quantification (PCASL)
All preprocessing steps and quantification calculations were performed on MATLAB 2017b (MathWorks, Massachusetts, USA) with SPM12 (Wellcome Trust Centre for Human Neuroimaging, London, UK) (24).
First, the PCASL data were motion corrected, smoothed with a Gaussian kernel of 3 mm, and prepared via a global threshold to eliminate background signal. To compute the perfusion, labeling and control pairs were subtracted from each other ΔS=Scontrol-Slabeling and quantified using the model described in (18):
with λ being the blood–tissue partition coefficient =0.9 mL/g (14), T1,blood =1,664 ms (25), α the labeling efficiency =0.85 (18), τ the labeling duration, and S0 the signal of the M0-Scan. The perfusion f therefore has the dimension (mL/100 g/min).
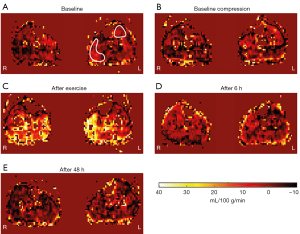
For the evaluation of the data, one slice was selected in every time point to evaluate the same position in head-feet direction. Two regions of interest were drawn, one in the gastrocnemius medialis (GM) and one in the tibialis anterior muscle (TA; Figure 3). Large vessels were excluded by a threshold (>40 mL/100 g/min) to examine muscle perfusion only.
Kinase levels of creatine
At baseline and 48 h after the exercises, blood creatine kinase (CK) levels were measured to infer an induction of DOMS. Therefore, blood was collected (5 mL) into serum tubes by vein puncture. CK measurement was performed using the UV test in accordance to the IFCC method (37 ℃).
Analysis of statistics
Perfusion values were checked for normality with the Kolmogorov-Smirnov test. Further the values for every time point and volunteer were compared to each other by performing a two-way ANOVA test followed by a Bonferroni corrected multiple comparison test (MATLAB 2017b, MathWorks, Massachusetts, USA).
Normality of the CK values was checked via the Shapiro-Wilk test. If the data were not normally distributed, the Wilcoxon signed-rank test was used. Otherwise a paired t-test was used to compare data from baseline to follow-up after 48 h. A significance level of 0.05 was used.
Results
No adverse events occurred during the study. Grade I lesions in the GM were detectable in all 14 volunteers according to the modified Peetrons graduation 48 h after the exercise intervention (Figure 4). Moreover, the mean CK value increased from 143.5±123.2 U/L (mean ± standard deviation) at baseline to 4,259.8±6,841.2 U/L after exercise (P=0.003). Consequently, all 14 volunteers were included for further assessments, as an induction of DOMS was confirmed.
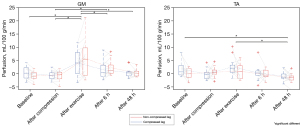
Perfusion values did not show significant differences between the compressed and non-compressed lower leg at any time point for the GM (P=0.65). The PCASL analysis revealed significantly increased perfusion in the GM directly after exercise (t=0 h) for both lower legs (P<0.001). The mean perfusion values directly after the exercise were 4.97±5.59 mL/100 g/min. No significant changes compared with baseline were observed for the GM at 6 h (P=0.16) and 48 h (P=1.0) after exercise. Perfusion was significantly reduced after 48 h for both non-compressed and compressed GM compared to the measurements directly after exercise (P<0.001).
No signs of EIMD were present in the TA based on the evaluation of the TIRM images 48 h after exercise. Perfusion values did not present significant differences between the compressed and non-compressed lower leg at any time point for the TA (P=0.05). No significant change in perfusion appeared in the TA directly after exercise (t=0 h) compared to baseline (P=1.0), whereas a decrease in tissue perfusion was present after 48 h compared to the measurements directly after the exercise intervention (P≤0.001; Figure 5).
Discussion
This study evaluated arterial muscle perfusion of the lower leg after exhausting exercise inducing DOMS and assessed the impact of compression garments during rest and recovery. Our results indicate that external pressure caused by sports compression socks (21–22 mmHg) does not modify local tissue perfusion at rest. Moreover, application of compression during intensive exercise and for the first 6 h after training did not affect arterial muscle perfusion after DOMS had been induced. Further, after the induction of DOMS, blood supply showed a normalization independent of compression at 6 and 48 h after exercise.
Skeletal muscle perfusion is of great importance for muscle recovery by promoting the supply of energy substrates and oxygen during both training and regeneration (11). However, non-invasive assessment of muscle perfusion is challenging and requires high-end technical approaches (11). A reliable and non-invasive approach to investigate muscle perfusion in different body parts, including the lower leg, is MR-based ASL, which has already been successfully applied in several other studies (14-17,19). Pollak et al. demonstrated high reproducibility of ASL perfusion measurements at 3T in healthy volunteers and in patients with peripheral arterial disease (17). Further, ASL enabled differentiation between these groups by capturing a reduced blood flow in the lower legs of patients with peripheral arterial disease (17). A short-term exercise-induced increase in perfusion in the calf muscles assessed with ASL was first described in 1999 and was observed in several other studies with different exercise regimens and at different field strengths (15,17,26); however, follow-up examinations during the regeneration phase after exercise are lacking. In the context of EIMD and DOMS, a lot of underlying pathophysiological pathways stay unknown; especially, alterations of tissue perfusion during recovery and the impact of compression have not yet been sufficiently understood (27). As expected, arterial perfusion of the GM increased significantly in the comparison between rest and directly after the exercise in our study. Increased tissue perfusion after exercise may be favored by an lack of oxygen and by the concentration of metabolic substrates (28). The mean muscle perfusion of the non-compressed GM peaked at 5.82±6.56 mL/100 g/min directly after exercise, which is less than the values measured in other studies investigating the perfusion of the lower leg after exercise, highlighting feasibility and reproducibility issues (15,17,19,26). The cited studies achieved perfusion values of 27±16 mL/100 g/min (15) up to 80±23 mL/100 g/min (17) with exercise interventions of low or medium intensity, which are not suitable to induce DOMS. In contrast to our study, the exercise interventions in these studies took place within the MR scanner to reduce time delays between exercise and ASL measurements. Eccentric or even plyometric exercises like those performed in our study, which are able to induce DOMS, cannot be performed in a lying position within the small bore of a MR scanner. The time delay between the end of the exercise intervention and the MR scan performed immediately after the exercise intervention in our investigation explains the comparatively low peak values. However, our aim to investigate muscle perfusion during regeneration in EIMD and the impact of compression garments is not affected by the performance of high-intensity exercises outside the scanner.
At 6 and 48 h after the exercise intervention, arterial muscle perfusion of the GM did not show significant changes compared to baseline measurements, which is contrary to our assumptions. We expected increased muscle perfusion given the known inflammatory and immune responses in the context of DOMS, which start immediately after training and occur over several days (6). The inflammation ensures the removal of tissue debris from the injured area and promotes muscle repair by activating muscle cells (6). The inflammatory and immune responses are mediated by various cells infiltrating the muscle tissue and by the upregulation of circulating pro-inflammatory cytokines and growth factors (3,6). That the intensity of our exercise intervention was sufficient to induce the crucial inflammatory stages for functional recovery in muscle trauma can be assumed, as we could verify a successful induction of DOMS in all volunteers after 48 h by observed intramuscular edema in the GM and by significant rise of CK levels (23). Furthermore, the exercise intervention used in the current study has been defined in precedent evaluations and consists of eccentric and plyometric exercises addressing the stretch-shortening cycle that have been known to create ultrastructural muscle damage (20-22). However, the normalization of muscle perfusion 6 h after exercise may be relevant for therapeutic strategies (e.g., cold therapy) with the aim to attenuate the medical condition of DOMS, which usually appear 48–72 h after exercise (3,29). In contrast, the TA, which was not affected by the exercise intervention and served as a kind of internal reference, showed no signs of trauma in the follow-up MR after 48 h in any volunteer, nor were any changes in perfusion observed compared to baseline. These findings are in line with our experiences in previous studies and with the results of other publications (13,15).
In addition to compression therapy a wide variety of interventions (e.g., cold water immersion therapy, whole body cryotherapy, heat therapy, active forms of regeneration, physical therapy, oral medications and nutrition) aiming to prevent and overcome the traumatic intramuscular changes during DOMS (29). The application of compression clothing is widely-used among recreational and professional sportsmen with the goal to enhance regeneration and performance (29). Anyhow, ideal wearing regimens and the effect of wearing compression clothing for the protection of EIMDare topics of controversial debates (10). The broad variability in investigational designs, accompanied with variance in the length and timing of the used of compression regime, types of training interventions, and sports habits of the cohorts analyzed, have led to incongruous findings and recommendations (9). The current study attempts to reflect an ordinary training session with high intensity or a day of competition with different manners of muscle contractions and with a realistic wearing regimen of compression socks. Compression clothing is regularly used during exercise and during regeneration phase multiple h afterwards (e.g., during the drive home or until going to sleep in the evening). The impact of wearing compression garments on alterations in intramuscular perfusion at rest and during recovery from high-intensity exercises remains to be sufficiently clarified (27). Wearing compression garments (21–22 mmHg) during and for 6 h after exercise had no effect on arterial muscle perfusion in either the stressed GM or the TA at any time point assessed in our study. The values obtained in the measurements performed at rest and at 48 h after exercise are consistent with our previously reported results evaluating the effect of compression on muscle perfusion at rest and in DOMS using contrast-enhanced ultrasound (CEUS) and IVIM MR perfusion imaging (13,20). Sperlich et al. also reported no change in muscle perfusion at rest applying compression of 37 mmHg to the thigh muscles using positron emission tomography, whereas reduced muscle perfusion occurred 15 min after a high-intensity cycling exercise was performed (11). In contrast, Bochmann et al. described a rise of arterial inflow at the forearm when using 13 to 23 mmHg of external compression assessed with venous occlusion plethysmography (12). Anyhow, measurements with venous occlusion plethysmography for the assessment of tissue perfusion cannot directly perceive alterations in muscle perfusion and might be impaired by a relevant portion of arteriovenous shunts within the hands (30). CEUS can be used for a quantitative assessment of muscle perfusion, but is limited by the need of the injection of a contrast agent. Broatch et al. reported in a recently published study using CEUS, that compression reduced muscle microvascular blood volume and attenuated the exercise-induced increase in microvascular velocity and flow immediately after exercise and 1 hour after repeated sprint exercise on a cycle ergometer (31). Against this background, using PCASL for the assessment of muscle perfusion in the context of compression therapy seems to have advantages due to its non-invasiveness and the possibility to assess perfusion during wearing of intact compression garments.
Our study has some limitations. First, our data are representative of the applied study setting and should be considered cautiously if different compression levels or different wearing times are applied. The evaluation of the muscle perfusion in the lower leg was only carried out by PCASL measurements. Although this is a well-established non-invasive imaging method for the quantification of soft tissue perfusion, this could have been compared with, for example, contrast-enhanced ultrasound (32) or spectral Doppler (33). However, unlike most other measurement techniques, PCASL allows the assessment of perfusion while wearing compression garments (17). Nevertheless, PCASL perfusion signals showed to be very low without sports load, therefore only perfusion changes were considered for these time points. By using PCASL we only evaluated arterial muscle perfusion and did not assess venous return, which may also be altered by the applied compression garments. We probably missed changes in peak perfusion as we have a time delay between finishing the exercise protocol and the MR acquisition, which was kept as short as possible by performing the exercise intervention in an adjacent room next to the MR scanner. Moreover, the number of participants included in our study (n=14) is small, although it is bigger as the median size of cohorts of recent methodological MR studies (34). Considering that there can be interindividual variations in the expression of DOMS there might have been non-responders (volunteers without the evidence for induction of DOMS) (6). Notably, we also acquired T2-weighted high-resolution MR images at follow-up, which confirmed the successful induction of DOMS, as evidenced by the presence of intramuscular edema, in every participant. Further, each volunteer was used as their own reference, and one lower leg might affect the results of the contralateral leg caused by systemic inflammatory processes. Nonetheless, we used every participant as its own control, as relevant interindividual changes in DOMS and CK expression have been noticed in several studies and also appeared in our preliminary tests (6,35).
Conclusions
Our study provides new insights into arterial muscle perfusion in the context of exhausting exercise during the regeneration phase, showing normalized muscle perfusion 6 and 48 h after exercise despite induced DOMS. The findings of our study reveal that wearing compression socks (21–22 mmHg) does not alter arterial muscle perfusion at rest, nor does it cause any relevant changes in the recovery phase of DOMS. This may have implications for future therapeutic strategies and for the understanding of pathophysiological pathways in DOMS.
Acknowledgments
Funding: This work was funded by Bauerfeind AG, Zeulenroda-Triebes, Germany (No. 3007394). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-21-1104/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1104/coif). The authors report that this work was funded by Bauerfeind AG, Zeulenroda-Triebes, Germany (No. 3007394). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of Friedrich-Alexander University Erlangen-Nürnberg, Germany (No. 33_16 B) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Järvinen TA, Järvinen M, Kalimo H. Regeneration of injured skeletal muscle after the injury. Muscles Ligaments Tendons J 2014;3:337-45. [Crossref] [PubMed]
- Ekstrand J, Hägglund M, Waldén M. Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med 2011;39:1226-32. [Crossref] [PubMed]
- Hotfiel T, Freiwald J, Hoppe MW, Lutter C, Forst R, Grim C, Bloch W, Hüttel M, Heiss R. Advances in Delayed-Onset Muscle Soreness (DOMS): Part I: Pathogenesis and Diagnostics. Sportverletz Sportschaden 2018;32:243-50. [Crossref] [PubMed]
- Ulbricht A, Gehlert S, Leciejewski B, Schiffer T, Bloch W, Höhfeld J. Induction and adaptation of chaperone-assisted selective autophagy CASA in response to resistance exercise in human skeletal muscle. Autophagy 2015;11:538-46. [Crossref] [PubMed]
- Yanagisawa O, Sakuma J, Kawakami Y, Suzuki K, Fukubayashi T. Effect of exercise-induced muscle damage on muscle hardness evaluated by ultrasound real-time tissue elastography. Springerplus 2015;4:308. [Crossref] [PubMed]
- Hody S, Croisier JL, Bury T, Rogister B, Leprince P. Eccentric Muscle Contractions: Risks and Benefits. Front Physiol 2019;10:536. [Crossref] [PubMed]
- Peake J, Nosaka K, Suzuki K. Characterization of inflammatory responses to eccentric exercise in humans. Exerc Immunol Rev 2005;11:64-85. [PubMed]
- Beliard S, Chauveau M, Moscatiello T, Cros F, Ecarnot F, Becker F. Compression garments and exercise: no influence of pressure applied. J Sports Sci Med 2015;14:75-83. [PubMed]
- Hill J, Howatson G, van Someren K, Leeder J, Pedlar C. Compression garments and recovery from exercise-induced muscle damage: a meta-analysis. Br J Sports Med 2014;48:1340-6. [Crossref] [PubMed]
- Born DP, Sperlich B, Holmberg HC. Bringing light into the dark: effects of compression clothing on performance and recovery. Int J Sports Physiol Perform 2013;8:4-18. [Crossref] [PubMed]
- Sperlich B, Born DP, Kaskinoro K, Kalliokoski KK, Laaksonen MS. Squeezing the muscle: compression clothing and muscle metabolism during recovery from high intensity exercise. PLoS One 2013;8:e60923. [Crossref] [PubMed]
- Bochmann RP, Seibel W, Haase E, Hietschold V, Rödel H, Deussen A. External compression increases forearm perfusion. J Appl Physiol (1985) 2005;99:2337-44. [PubMed]
- Riexinger A, Laun FB, Höger SA, Wiesmueller M, Uder M, Hensel B, Forst R, Hotfiel T, Heiss R. Effect of compression garments on muscle perfusion in delayed-onset muscle soreness: A quantitative analysis using intravoxel incoherent motion MR perfusion imaging. NMR Biomed 2021;34:e4487. [Crossref] [PubMed]
- Wu WC, Mohler E 3rd, Ratcliffe SJ, Wehrli FW, Detre JA, Floyd TF. Skeletal muscle microvascular flow in progressive peripheral artery disease: assessment with continuous arterial spin-labeling perfusion magnetic resonance imaging. J Am Coll Cardiol 2009;53:2372-7. [Crossref] [PubMed]
- Schewzow K, Fiedler GB, Meyerspeer M, Goluch S, Laistler E, Wolzt M, Moser E, Schmid AI. Dynamic ASL and T2-weighted MRI in exercising calf muscle at 7 T: a feasibility study. Magn Reson Med 2015;73:1190-5. [Crossref] [PubMed]
- Englund EK, Rodgers ZB, Langham MC, Mohler ER 3rd, Floyd TF, Wehrli FW. Measurement of skeletal muscle perfusion dynamics with pseudo-continuous arterial spin labeling (pCASL): Assessment of relative labeling efficiency at rest and during hyperemia, and comparison to pulsed arterial spin labeling (PASL). J Magn Reson Imaging 2016;44:929-39. [Crossref] [PubMed]
- Pollak AW, Meyer CH, Epstein FH, Jiji RS, Hunter JR, Dimaria JM, Christopher JM, Kramer CM. Arterial spin labeling MR imaging reproducibly measures peak-exercise calf muscle perfusion: a study in patients with peripheral arterial disease and healthy volunteers. JACC Cardiovasc Imaging 2012;5:1224-30. [Crossref] [PubMed]
- Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJ, Wang DJ, Wong EC, Zaharchuk G. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 2015;73:102-16. [Crossref] [PubMed]
- Mahmud SZ, Gladden LB, Kavazis AN, Motl RW, Denney TS, Bashir A. Simultaneous Measurement of Perfusion and T2* in Calf Muscle at 7T with Submaximal Exercise using Radial Acquisition. Sci Rep 2020;10:6342. [Crossref] [PubMed]
- Heiss R, Kellermann M, Swoboda B, Grim C, Lutter C, May MS, Wuest W, Uder M, Nagel AM, Hotfiel T. Effect of Compression Garments on the Development of Delayed-Onset Muscle Soreness: A Multimodal Approach Using Contrast-Enhanced Ultrasound and Acoustic Radiation Force Impulse Elastography. J Orthop Sports Phys Ther 2018;48:887-94. [Crossref] [PubMed]
- Heiss R, Hotfiel T, Kellermann M, May MS, Wuest W, Janka R, Nagel AM, Uder M, Hammon M. Effect of Compression Garments on the Development of Edema and Soreness in Delayed-Onset Muscle Soreness (DOMS). J Sports Sci Med 2018;17:392-401. [PubMed]
- Hotfiel T, Kellermann M, Swoboda B, Wildner D, Golditz T, Grim C, Raithel M, Uder M, Heiss R. Application of Acoustic Radiation Force Impulse Elastography in Imaging of Delayed Onset Muscle Soreness: A Comparative Analysis With 3T MRI. J Sport Rehabil 2018;27:348-56. [Crossref] [PubMed]
- Ekstrand J, Healy JC, Waldén M, Lee JC, English B, Hägglund M. Hamstring muscle injuries in professional football: the correlation of MRI findings with return to play. Br J Sports Med 2012;46:112-7. [Crossref] [PubMed]
- Wang Z, Aguirre GK, Rao H, Wang J, Fernández-Seara MA, Childress AR, Detre JA. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging 2008;26:261-9. [Crossref] [PubMed]
- Lu H, Clingman C, Golay X, van Zijl PC. Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn Reson Med 2004;52:679-82. [Crossref] [PubMed]
- Frank LR, Wong EC, Haseler LJ, Buxton RB. Dynamic imaging of perfusion in human skeletal muscle during exercise with arterial spin labeling. Magn Reson Med 1999;42:258-67. [Crossref] [PubMed]
- Mizuno S, Arai M, Todoko F, Yamada E, Goto K. Wearing lower-body compression garment with medium pressure impaired exercise-induced performance decrement during prolonged running. PLoS One 2017;12:e0178620. [Crossref] [PubMed]
- Filli L, Boss A, Wurnig MC, Kenkel D, Andreisek G, Guggenberger R. Dynamic intravoxel incoherent motion imaging of skeletal muscle at rest and after exercise. NMR Biomed 2015;28:240-6. [Crossref] [PubMed]
- Heiss R, Lutter C, Freiwald J, Hoppe MW, Grim C, Poettgen K, Forst R, Bloch W, Hüttel M, Hotfiel T. Advances in Delayed-Onset Muscle Soreness (DOMS) - Part II: Treatment and Prevention. Sportverletz Sportschaden 2019;33:21-9. [Crossref] [PubMed]
- Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharmacol 2001;52:631-46. [Crossref] [PubMed]
- Broatch JR, O'Riordan SF, Keske MA, Betik AC, Bishop DJ, Halson SL, Parker L. Reduced post-exercise muscle microvascular perfusion with compression is offset by increased muscle oxygen extraction: Assessment by contrast-enhanced ultrasound. FASEB J 2021;35:e21499. [Crossref] [PubMed]
- Kellermann M, Heiss R, Swoboda B, Gelse K, Freiwald J, Grim C, Nagel A, Uder M, Wildner D, Hotfiel T. Intramuscular Perfusion Response in Delayed Onset Muscle Soreness (DOMS): A Quantitative Analysis with Contrast-Enhanced Ultrasound (CEUS). Int J Sports Med 2017;38:833-41. [Crossref] [PubMed]
- Hotfiel T, Swoboda B, Krinner S, Grim C, Engelhardt M, Uder M, Heiss RU. Acute Effects of Lateral Thigh Foam Rolling on Arterial Tissue Perfusion Determined by Spectral Doppler and Power Doppler Ultrasound. J Strength Cond Res 2017;31:893-900. [Crossref] [PubMed]
- Hanspach J, Nagel AM, Hensel B, Uder M, Koros L, Laun FB. Sample size estimation: Current practice and considerations for original investigations in MRI technical development studies. Magn Reson Med 2021;85:2109-16. [Crossref] [PubMed]
- Kraemer WJ, Bush JA, Wickham RB, Denegar CR, Gómez AL, Gotshalk LA, Duncan ND, Volek JS, Putukian M, Sebastianelli WJ. Influence of compression therapy on symptoms following soft tissue injury from maximal eccentric exercise. J Orthop Sports Phys Ther 2001;31:282-90. [Crossref] [PubMed]


