
Liver involvement in a hereditary hemorrhagic telangiectasia patient with Gd-EOB-DTPA enhanced MRI: a case description
Introduction
Hereditary hemorrhagic telangiectasia (HHT), or Rendu–Osler–Weber disease, is an autosomal dominant disease caused by a mutation in one of several genes. It has an estimated prevalence of approximately 1 in 5,000 (1). At a minimum, gene mutations described in HHT include ACVRL1, ENG, SMAD4, and GDF2 (2). The disease of HHT is characterized by vascular lesions, including arteriovenous malformations (AVMs) and telangiectasia (3). The recognized manifestations of HHT are all due to abnormalities of the vascular structure (4). They can occur anywhere in the body, such as in the central nervous system, lungs, liver, or spine. Liver involvement occurs in 41–84% of patients with HHT (5). Widespread diffuse liver vascular malformations characterize liver involvement in HHT. When symptomatic, patients present with high output heart failure, portal hypertension, and biliary disease (6). Imaging can determine whether the shunting is principally due to the hepatic or the portal vein; however, no strong correlation has been observed between CT findings and the clinical subtype of HHT liver disease (7). An abnormal liver vascular supply due to intrahepatic shunts causes nodular hyperplasia of hepatocytes, including diffuse [leading to nodular regenerative hyperplasia (NRH)] and focal [leading to focal nodular hyperplasia (FNH)] hyperplasia (6). Reports show that the prevalence of FNH in patients with HHT is 2.9% (100-fold greater than in the general population) (8). Although it is considered a benign liver lesion, its presence can lead to a misdiagnosis of hepatocellular carcinoma when a mass appears in a seemingly cirrhotic liver. A multicenter study showed that hepatobiliary phase imaging with gadobenate dimeglumine can accurately distinguish benign lesions from malignant or high-risk lesions (9).
Imaging examinations play an important role in the diagnosis of HHT. Only Shimoyama et al. (10) have reported the imaging features of gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging (MRI) in patients with liver involvement in HHT. Herein, we report a patient with liver involvement in HHT to demonstrate the typical imaging features of liver involvement in HHT on Gd-EOB-DTPA-enhanced MRI.
Case presentation
A 40-year-old female visited the obstetrics and gynecology clinic at the local hospital for a post-induced labor review in October 2019. At that time, as a routine laboratory test, her liver function showed alanine aminotransferase (ALT) 87 U/L, aspartate aminotransferase (AST) 58 U/L, alkaline phosphatase (ALP) 149.9 U/L, and gamma-glutamyl transferase (GGT) 216.2 U/L. The patient did not seek further treatment at that time. In November 2019, she presented with right lower abdominal pain without obvious cause. The pain self-resolved and was accompanied by mild dizziness and weakness. Liver function tests were performed to determine the cause of the abdominal pain, with the following results: ALT 43 U/L, AST 43 U/L, ALP 145 U/L, and GGT 270.2 U/L.
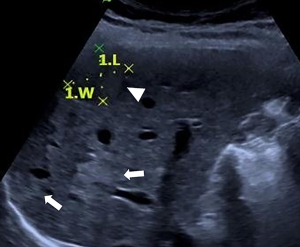
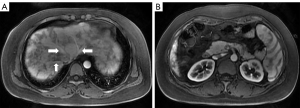
After that, in November 2019, the patient came to the Beijing Friendship Hospital, Capital Medical University, Beijing for further diagnosis. We performed repeat liver function tests to determine whether there had been any changes in the liver function. The results showed the following: ALT 42 U/L, AST 41.5 U/L, ALP 169 U/L, and GGT 210 U/L. Other laboratory results showed the following: immunoglobulin M (IgM) 264 mg/dL and antimitochondrial antibody (AMA)-M2(−). We arranged a hepatic ultrasound examination (Figure 1), which revealed heterogeneous liver parenchymal echo, multiple hepatic nodules, and splenomegaly. The patient underwent a gadopentetate dimeglumine (Gd-DTPA)-enhanced MRI for further diagnosis. The contrast-enhanced MRI revealed dilated and tortuous hepatic arterial branches, early filling of portal or hepatic venous trunks, heterogeneous enhancement of parenchyma in the arterial phase, multiple abnormal hepatic nodules, and splenomegaly. We instructed the patient to take ursodeoxycholic acid as symptomatic treatment of cholestasis. After one month of treatment, in December 2019, the patient came to the outpatient department for review, which revealed that her liver function had slightly improved. We instructed her to take ursodeoxycholic acid regularly; however, she failed to adhere to those instructions. In June 2020, the patient returned to the outpatient department for review. The subsequent liver function test showed the following: ALT 15 U/L, AST 19.9 U/L, ALP 118 U/L, and GGT 50 U/L; she then discontinued taking ursodeoxycholic acid. In September 2020, the patient was still experiencing occasional right lower abdominal pain with weakness. She was admitted to hospital for further diagnosis and treatment.
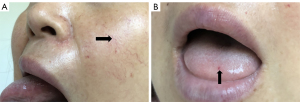
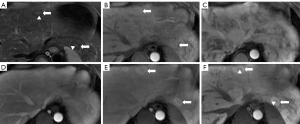
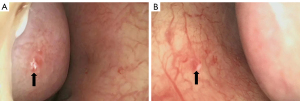
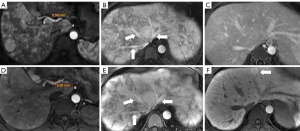
The patient had undergone labor induction a year earlier and curettage six months earlier. There were bilateral telangiectasias on the tongue and her cheeks (Figure 2A,2B). The results of an abdominal physical examination were negative for giving some clues to the cause of abdominal pain. After hospitalization, blood analysis revealed mild anemia with hemoglobin 91 g/L. The liver function examination showed ALT 24 U/L, AST 22.6 U/L, ALP 115 U/L, and GGT 89 U/L. The prothrombin and partial thromboplastin times were normal. Alpha-fetoprotein, ceruloplasmin, antibody to liver/kidney microsomal type 1, and soluble liber antigen were negative. For further diagnosis of multiple hepatic nodules, Gd-EOB-DTPA-enhanced MRI was performed, which revealed a dilated and tortuous hepatic artery (HA) (Figure 3) and multiple hepatic nodules (Figure 4A-4F). In the arterial phase, the liver enhancement was heterogeneous, with a mosaic pattern of perfusion, and hepatic venous trunks exhibited early enhancement when they were supposed to be enhanced in the portal phase (Figure 5A,5B). After carefully analysing the images, enhanced MRI indicated hepatic vascular lesions, and HHT was considered. Multiple hepatic nodules were diagnosed as focal nodular hyperplasia. In addition to the imaging examination results, we learned that she had experienced episodes of recurrent epistaxis since childhood, but no one else in the family had similar symptoms.
Nasopharyngolaryngoscopy showed multiple telangiectasias in the nasal mucosa (Figure 6). Upper gastrointestinal endoscopy showed no telangiectasias in the stomach, and there were no findings of either esophageal varices or portal hypertensive gastropathy. There were no abnormal findings of high output heart failure on the echocardiography. The patient’s genetic testing identified an ACVRL1 variant, NM 000020.2 exon 3: c.263A>G (chr12-52307084 p.Y88C). Due to old age, her parents did not undergo genetic testing.
The middle-aged patient presented with recurrent epistaxis, telangiectasias at characteristic sites (the tongue, face, lip and nasal mucosa), and liver AVMs that were confirmed by enhanced MRI. The fulfillment of three of the four criteria supported the diagnosis of HHT type I according to the Curacao criteria (11) combined with genetic testing, which identified an ACVRL1 variant. On account of the mild symptoms without serious complications and vascular malformations requiring interventional treatment, we recommended that the patient continue to take ursodeoxycholic acid and be followed up for observation every six months without additional treatment. Throughout telephone follow-up, the patient took the medicine regularly and experienced no discomfort.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
This was a case of liver involvement in HHT with Gd-EOB-DTPA-enhanced MRI. The characteristic imaging features of liver involvement in HHT include dilated and tortuous hepatic arterial branches, early filling of portal or hepatic venous trunks, heterogeneous enhancement of parenchyma in the arterial phase (12), and multiple hepatic nodules (6).
Dilated and tortuous hepatic arterial branches
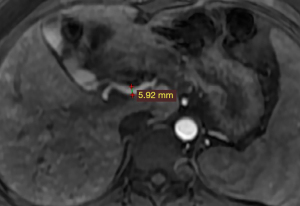
The vessels can be better depicted in contrast-enhanced MRI examinations, and we were able to accurately measure the diameter of the blood vessels and observe the course and origin of the blood vessels. Generally, the common HA originates from the celiac trunk. In patients with HHT, the common HA is dilated. The size of the common HA is considered enlarged if the diameter is greater than 4.5 mm, and it is measured before the origin of the gastroduodenal artery (7).
In this case, the diameter of the common HA was measured at 5.9 mm (Figure 3). Therefore, we could diagnose that the patient had a dilated HA.
Early filling of hepatic venous trunks
On normal Gd-EOB-DTPA-enhanced MRI, the arterial phase refers to the HA and branches being fully enhanced and the hepatic veins not being enhanced by antegrade flow (13). Hepatic venous trunks have no enhancement in the arterial phase and then have significant enhancement in the portal phase. In patients with HHT, early filling of hepatic venous trunks occurs in the arterial phase, which should not appear in this phase. Such an abnormal finding may indicate the existence of an arteriovenous fistula. In this case, the hepatic venous trunks were enhanced in the arterial phase (Figure 5A). Only by understanding the normal pattern of enhancement of the abdominal organs in the arterial phase can we dustinguish these abnormal imaging features.
Heterogeneous enhancement in the arterial phase
The normal liver parenchyma showed homogeneous enhancement in both the arterial phase and portal phase, with peak enhancement appearing on the portal phase on multiphase enhanced MRI. Heterogeneous enhancement in the arterial phase may indicate the presence of hepatic vascular abnormalities. When the liver is involved in HHT, the liver enhancement is a heterogeneous enhancement in the arterial phase, with a mosaic pattern of perfusion characterized by multiple areas of transient hepatic attenuation difference indicative of an arterioportal shunt (65% of cases), telangiectasias (63% of cases), and confluent vascular masses (25% of cases) (12). The Gd-EOB-DTPA-enhanced MRI of this case showed the heterogeneous enhancement of liver parenchyma in the arterial phase (Figure 5A).
Multiple hepatic nodules
Typically, FNH is iso- or hypo-intense on T1-weighted images, is slightly hyper or isointense on T2-weighted images, and has a hyperintense central scar on T2-weighted images (14). In FNH with both extracellular contrast agent MRI (ECA-MRI) and hepatobiliary contrast agent MRI (HBA-MRI), intense enhancement of the lesions was present in the early dynamic phase (arterial and portal phase) (15,16). With hepatobiliary-specific agents, in the hepatocyte phase after 10 and 20 min, enhancement was seen in 88% and 90% of lesions, respectively, and all identified central scars were hypointense after 20 min (15). The imaging features of multiple hepatic nodules, in this case, were in accordance with the typical FNH mentioned above. Finally, we diagnosed the multiple hepatic nodules of this case as FNH.
All of the characteristic imaging features, including dilated and tortuous HA branches, early filling of portal or hepatic venous trunks, heterogeneous enhancement of parenchyma in the arterial phase, and multiple hepatic nodules, were present in this case. The case was diagnosed as HHT, and imaging played an important role in the diagnosis of this patient. The imaging can identify not only an abnormal function of the liver but also some severe complications, such as portal hypertension and cardiomegaly.
In this case, the patient successfully underwent ECA-MRI and HBA-MRI. The imaging features of dilated and tortuous HA branches, early filling of portal or hepatic venous trunks and heterogeneous enhancement of parenchyma in the arterial phase showed no difference in the two different contrast agents MRIs. The two MRIs showed intense enhancement of the multiple hepatic nodules in the arterial and portal phases. However, multiple hepatic nodules showed high signal intensity in the liver-specific phase of HBA-MRI, which were shown more clearly than in the delayed phase of ECA-MRI (Figure 7) (17). This finding contributed to the diagnosis of FNH.
Once diagnosed with HHT, a liver biopsy is unnecessary because the risk of bleeding with the percutaneous transcapsular route increases. Therefore, radiologists must find and analyze abnormal findings of the liver based on the correct recognition of normal liver dynamic contrast enhancement imaging.
Currently, the therapy of liver involvement in HHT is mainly symptomatic treatment (6). The clinical guideline recommends intensive first-line management only for patients with complicated and/or symptomatic liver venous malformations (VMs), tailored to the type of liver VM complication (1). Fortunately, the relevant imaging examinations revealed that the patient did not have portal hypertension and cardiomegaly complications; there were no AVMs in the brain, lung, and upper gastrointestinal tract. Therefore, no additional interventional treatment was recommended for the patient, and we instructed her to take oral iron and ursa as symptomatic treatment of anemia and cholestasis. As the disease may progress and the patient may develop associated complications, we recommend the patient is followed up every six months or annually.
Acknowledgments
Funding: This work was supported by the Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (No. ZYLX202101).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-45/coif). All authors report that this work was supported by the Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (No. ZYLX202101). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Faughnan ME, Mager JJ, Hetts SW, Palda VA, Lang-Robertson K, Buscarini E, et al. Second International Guidelines for the Diagnosis and Management of Hereditary Hemorrhagic Telangiectasia. Ann Intern Med 2020;173:989-1001. [Crossref] [PubMed]
- Hetts SW, Shieh JT, Ohliger MA, Conrad MB. Hereditary Hemorrhagic Telangiectasia: The Convergence of Genotype, Phenotype, and Imaging in Modern Diagnosis and Management of a Multisystem Disease. Radiology 2021;300:17-30. [Crossref] [PubMed]
- Kritharis A, Al-Samkari H, Kuter DJ. Hereditary hemorrhagic telangiectasia: diagnosis and management from the hematologist's perspective. Haematologica 2018;103:1433-43. [Crossref] [PubMed]
- Guttmacher AE, Marchuk DA, White RI Jr. Hereditary hemorrhagic telangiectasia. N Engl J Med 1995;333:918-24. [Crossref] [PubMed]
- Khalid SK, Garcia-Tsao G. Hepatic vascular malformations in hereditary hemorrhagic telangiectasia. Semin Liver Dis 2008;28:247-58. [Crossref] [PubMed]
- Garcia-Tsao G. Liver involvement in hereditary hemorrhagic telangiectasia (HHT). J Hepatol 2007;46:499-507. [Crossref] [PubMed]
- Wu JS, Saluja S, Garcia-Tsao G, Chong A, Henderson KJ, White RI Jr. Liver involvement in hereditary hemorrhagic telangiectasia: CT and clinical findings do not correlate in symptomatic patients. AJR Am J Roentgenol 2006;187:W399-405. [Crossref] [PubMed]
- Buscarini E, Danesino C, Plauchu H, de Fazio C, Olivieri C, Brambilla G, Menozzi F, Reduzzi L, Blotta P, Gazzaniga P, Pagella F, Grosso M, Pongiglione G, Cappiello J, Zambelli A. High prevalence of hepatic focal nodular hyperplasia in subjects with hereditary hemorrhagic telangiectasia. Ultrasound Med Biol 2004;30:1089-97. [Crossref] [PubMed]
- Morana G, Grazioli L, Kirchin MA, Bondioni MP, Faccioli N, Guarise A, Schneider G. Solid hypervascular liver lesions: accurate identification of true benign lesions on enhanced dynamic and hepatobiliary phase magnetic resonance imaging after gadobenate dimeglumine administration. Invest Radiol 2011;46:225-39. [Crossref] [PubMed]
- Shimoyama Y, Kakizaki S, Katano A, Takakusaki S, Mizuide M, Ichikawa T, Sato K, Takagi H, Mori M. Hereditary hemorrhagic telangiectasia with multiple hepatic and pulmonary nodular lesions. Clin J Gastroenterol 2009;2:131-6. [Crossref] [PubMed]
- Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ, Kjeldsen AD, Plauchu H. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet 2000;91:66-7. [Crossref] [PubMed]
- Torabi M, Hosseinzadeh K, Federle MP. CT of nonneoplastic hepatic vascular and perfusion disorders. Radiographics 2008;28:1967-82. [Crossref] [PubMed]
- Kambadakone AR, Fung A, Gupta RT, Hope TA, Fowler KJ, Lyshchik A, Ganesan K, Yaghmai V, Guimaraes AR, Sahani DV, Miller FH. LI-RADS technical requirements for CT, MRI, and contrast-enhanced ultrasound. Abdom Radiol (NY) 2018;43:56-74. [Crossref] [PubMed]
- Hussain SM, Terkivatan T, Zondervan PE, Lanjouw E, de Rave S, Ijzermans JN, de Man RA. Focal nodular hyperplasia: findings at state-of-the-art MR imaging, US, CT, and pathologic analysis. Radiographics 2004;24:3-17; discussion 18-9. [Crossref] [PubMed]
- Zech CJ, Grazioli L, Breuer J, Reiser MF, Schoenberg SO. Diagnostic performance and description of morphological features of focal nodular hyperplasia in Gd-EOB-DTPA-enhanced liver magnetic resonance imaging: results of a multicenter trial. Invest Radiol 2008;43:504-11. [Crossref] [PubMed]
- Campos JT, Sirlin CB, Choi JY. Focal hepatic lesions in Gd-EOB-DTPA enhanced MRI: the atlas. Insights Imaging 2012;3:451-74. [Crossref] [PubMed]
- Fowler KJ, Brown JJ, Narra VR. Magnetic resonance imaging of focal liver lesions: approach to imaging diagnosis. Hepatology 2011;54:2227-37. [Crossref] [PubMed]


