Quantitative 3D breast magnetic resonance imaging fibroglandular tissue analysis and correlation with qualitative assessments: a feasibility study
Introduction
The breast is composed of fat and fibroglandular tissue (FGT), which includes epithelial and stromal elements. The amount of FGT is used to classify breast density into four different categories determined by the Breast-Imaging Reporting and Data System (BI-RADS) lexicon, which include almost entirely fatty, scattered areas of fibroglandular density, heterogeneously dense, and extremely dense for mammography and almost entirely fat, scattered FGT, heterogeneous FGT and extreme FGT for magnetic resonance imaging (MRI) (1).
High breast density is known to correlate with breast cancer risk (2-5). Patients with heterogeneously dense or extremely dense breasts have a four-fold increased risk of developing breast cancer compared to patients with fatty breasts (2-5). Many studies have demonstrated this relationship using two-dimensional (2D) qualitative evaluation of FGT on mammography. The attempt to measure a three-dimensional (3D) volume of FGT using a qualitative assessment based on a 2D mammogram (MG) is a major limiting factor of these studies (6).
Currently, FGT is qualitatively assessed by the interpreting radiologist. Such assessment can be prone to inter- and intra-observer variability due to the inherent subjectivity of the interpretation (7,8). In addition, categorizing FGT into only four qualitative groups limits statistical analysis assessing for small but potentially significant differences. The ideal evaluation of FGT requires 3D assessment to yield the highest degree of accuracy and reproducibility.
The purpose of this study is to develop a 3D quantitative technique that can provide an objective and highly accurate measurement of FGT (%) on MRI. If confirmed, this 3D methodology may be an invaluable clinical tool to more accurately assess the relationship between FGT amount and breast cancer risk. This could also provide a potential measurement tool to determine if specific breast cancer risk reduction strategies are effective.
Materials and methods
A HIPAA compliant, IRB approved study was performed of 60 randomly selected breast MRIs from 1,231 routine breast MRIs performed at our institution from 1/2013 to 12/2014. The average age of the patients was 54.2 years. All of the patient information was de-identified from the MRI images prior to computer imaging analysis.
Breast MRI technique
Each breast MRI was performed on a 1.5-Tesla or 3-Tesla system (Signa Excite, GE Healthcare) using an 8-channel Breast Array Coil (GE Healthcare). The imaging sequences utilized for computer imaging analysis include a T1-weighted fat-suppressed fast spoiled gradient-echo sequence (17/2.4; flip angle, 35°; bandwidth, 31–25 Hz) performed before and after a rapid bolus injection of contrast (gadopentate dimeglumine/Magnevist, Berlex; 0.1 mmol/L/kg of body weight), delivered through an IV catheter. Section thickness was 2 mm using a matrix of 256×192 and a field of view of 18–22 cm. Frequency was in the antero-posterior direction.
Qualitative FGT assessment
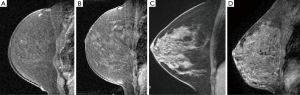
Three breast fellowship trained radiologists by consensus classified the mammographic density and the amount of MRI FGT in accordance with BI-RADS categories (1). The mammographic breast density was recorded on a 4-point scale (1–fatty, 2–scattered, 3–heterogeneously dense, 4–extremely dense). The MRI FGT was assessed by using a combination of T1-weighted non–fat-suppressed and fat-suppressed imaging. FGT was defined as any non-fatty non-cystic breast parenchyma. The amount of FGT on MRI was recorded on a 4-point scale (1–almost entirely fat, 2–scattered FGT, 3–heterogeneous FGT, 4–extreme FGT) (Figure 1).
Computer imaging analysis
The quantitative computer imaging analysis of FGT on breast MRI was performed blinded to the qualitative assessment. The proprietary semi-automated segmentation algorithm combines the region-based active contours and a level set approach which has been validated for use in other diseases and further modified for this particular application (9-14). This segmentation algorithm and a number of manual interaction functions, such as selection of a region of interest and modification of suboptimal contour results, have been integrated into a viewing system developed by customized software (Matlab, Mathworks, Natick, MA, USA).
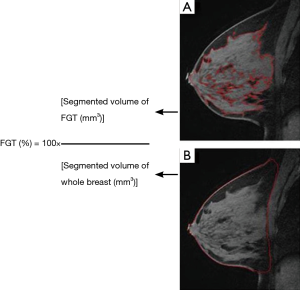
FGT quantification was performed by a radiologist using pre-contrast T1-weighted fat-suppressed images, manually selecting a region of interest that outlines the whole breast contour defined by antero-posterior border (nipple/skin to the pre-pectoral region), medial-lateral border (medial and lateral most breast tissue), and inferior-superior border (lower most breast tissue to the low axilla) (Figure 2A). Next, FGT was outlined by the boundary localization of the contrasting glandular tissue and exclusion of the adjacent fat (Figure 2B). Once the segmentation was completed on an image, the whole breast contour and FGT contour were propagated to neighboring images, serving as an initial region of interest for subsequent segmentations on the neighboring images and manual adjustments were made as needed. Once the segmentation was finalized, the computer program generated the whole breast volume and FGT volume. The process for each patient on average was performed in less than 5 minutes. The percent FGT was calculated using these values (Figure 2).
Statistical analysis was performed including Pearson product-moment correlation coefficient (r) calculation using the IBM SPSS software (version 18).
Results
The distribution of mammographic density qualitative assessments were fatty (3.3%, 2/60), scattered (43.3%, 26/60), heterogeneous (41.7%, 25/60) and extremely dense (11.7%, 7/60). The distribution of MRI FGT qualitative assessments were almost entirely fat (3.3%, 2/60), scattered FGT (51.7%, 31/60), heterogeneous FGT (38.3%, 23/60) and extreme FGT (6.7%, 4/60). The range of quantified FGT (%) was 3.4% to 46.6% with the mean of 15.3% (SE 1.3%).
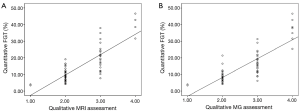
There was a significant positive correlation between quantitative MRI FGT assessment and qualitative MRI FGT (r=0.809, n=60, P<0.001) (Figure 3A) and mammographic density assessment (r=0.805, n=60, P<0.001) (Figure 3B). There was a significant correlation between qualitative MRI FGT assessment and mammographic density assessment (r=0.725, n=60, P<0.001) (Table 1). The four qualitative assessment categories of MRI FGT correlated with the calculated mean quantitative FGT (%) of 4.61% (95% CI, 0–12.3%), 8.74% (7.3–10.2%), 18.1% (15.1–21.1%), 37.4% (29.5–45.3%). The four qualitative assessment categories of mammographic density correlated with the calculated mean quantitative FGT (%) of 4.61% (95% CI, 0–12.3%), 8.11% (7.1–9.5%), 16.4% (13.1–19.4%), 34.4% (26.5–41.1%).

Full table
Discussion
There is strong evidence that breast density is an independent risk factor for breast cancer, likely due to a greater amount of epithelial cells that can potentially become cancerous. Breast density is reportedly associated with four-fold increase in the risk for breast cancer (2-5). However, the exact increase in risk remains unclear partly due to variable classification systems used for assessing breast density including the BI-RADS classification, percentage classification and the Wolfe classification (1-5). Some argue that the relative breast cancer risk due to breast density is much smaller than that of other major risk factors, such as age, family history, hormonal factors and genetic mutations. However, given that approximately 50% of the screening population has mammographically dense breasts, the risk factor of density alone to the population is likely more significant than other stronger but less common risk factors (3-5). In addition, breast cancer risk associated with FGT amount may be underestimated in the reported studies due to categorizing breast density into a few qualitative groups, which can significantly limit statistical analysis.
In addition, using these qualitative density categories, considerable inter- and intra-observer variability is present. In a study by Nicholson et al., inter-reader agreement regarding breast density was low (49%) with most of the agreement occurring at the two extremes (fatty vs. extremely dense breasts) (7). In another study Kerlikowske et al. showed that the inter-reader and intra-reader agreements regarding density assessments were κ=0.59 and κ=0.72 respectively, indicating imperfect reliability of density interpretation even by the same reader (8).
The purpose of this study was to develop a 3D quantification technique to reliably measure FGT. Quantitative measures of FGT were computed with data derived from breast MRI using a computerized technique and correlated significantly with conventional qualitative assessments reached by consensus of three experienced breast imagers. The computer algorithm used for quantifying the amount of breast FGT combines the region-based active contours and a level set approach, which was originally developed for hepatic lesions and has since been successfully adapted and validated for use in brain tumors, renal cell carcinomas, lymphoma and peritoneal mesothelioma (9-13). Other computer algorithms have also been proposed to quantify FGT including a method using a Fuzzy c-means (FCM) data clustering technique in which a dataset is grouped into n clusters with every data point in the data set belonging to every cluster to a certain degree (14). The advantages of the program used in this study are robust accurate segmentation with easy initialization and efficient modification. Once the segmentation is completed on an image, the whole breast contour and FGT contour are automatically propagated to the neighboring images and repeated until all the images are segmented for 3D analysis. This enables complete evaluation of the whole breast, a clear advantage of our method compared to evaluation of few selected images in previously published methods.
The range of quantified FGT (%) (3.4–46.6%) for the four qualitative categories was lower than expected based on the BI-RADS 4th Edition breast density percentage definition (0 to 100%) (15). This is likely due to better estimation of the amount of fat by the computer algorithm compared to the visual assessment and is consistent with the commercially available automated breast density assessment program for mammography (16-18). In addition, the 3D MRI likely includes larger area of fatty breast located in the retro-glandular and lower axillary regions yielding lower overall percentage of the quantified FGT. Of note, the percentage definition of the breast density is no longer part of the most current BI-RADS (5th edition) (1).
To minimize the subjectivity of these assessments, computer assisted quantitative methods of assessing breast density have been developed focusing on mammography. However, there is an inherent inaccuracy of measuring 3D FGT utilizing a 2D image as evidenced by significant changes in density measurements related to variable positioning and variable inclusion of the retro-glandular fat. Ultimately, a 3D quantitative method for measuring the volume of FGT, such as our method, is needed for accurate assessment of this important measurement.
Recently, studies have shown that women undergoing tamoxifen therapy who had reduction in breast density had a greater reduction in breast cancer risk and improved survival compared to women who did not have similar decrease in breast density (19,20). The need for accurate 3D quantification of FGT is important not only for accurately assessing breast cancer risk but also for testing effectiveness of chemoprevention strategies.
There are a few limitations with this study. Inter-reader reproducibility and intra-reader reproducibility were not investigated as the primary aim was to look at the feasibility of our setup. The current approach was semi-automated, which requires initial selection of the region of interest with potential subjectivity bias. Currently, the technique requires quality control check of all the images to ensure optimal segmentation. While this technique is not yet fully automated, it is still more objective and effective in assessing FGT than the conventional qualitative assessment. There is active research currently underway to fully automate the segmentation process.
Conclusions
Quantitative measures of FGT were computed with data derived from breast MRI using a semi-automated computerized method which correlated significantly with conventional qualitative assessments. This quantitative technique may prove to be a valuable tool in clinical use by providing computer generated standardized measurements with limited intra or inter-observer variability. Given its potential importance as a biomarker of breast cancer risk, a reliable and objective quantitative measurement of FGT will be invaluable. This study offers a reliable method for 3D quantitative analysis of FGT on breast MRI, which can be used to both validate and further explore the relationship between FGT amount and breast cancer.
Acknowledgements
We would like to thank Madeleine Esme for administrative support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American College of Radiology. Breast imaging reporting and data system (BI-RADS), 5th edition. Available online: http://www.acr.org/Quality-Safety/Resources/BIRADS
- Byrne C, Schairer C, Brinton LA, Wolfe J, Parekh N, Salane M, Carter C, Hoover R. Effects of mammographic density and benign breast disease on breast cancer risk (United States). Cancer Causes Control 2001;12:103-10. [Crossref] [PubMed]
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2006;15:1159-69. [Crossref] [PubMed]
- Boyd NF, Martin LJ, Yaffe MJ, Minkin S. Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res 2011;13:223. [Crossref] [PubMed]
- Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ. Mammographic density and the risk and detection of breast cancer. N Engl J Med 2007;356:227-36. [Crossref] [PubMed]
- Kopans DB. Basic physics and doubts about relationship between mammographically determined tissue density and breast cancer risk. Radiology 2008;246:348-53. [Crossref] [PubMed]
- Nicholson BT, LoRusso AP, Smolkin M, Bovbjerg VE, Petroni GR, Harvey JA. Accuracy of assigned BI-RADS breast density category definitions. Acad Radiol 2006;13:1143-9. [Crossref] [PubMed]
- Kerlikowske K, Grady D, Barclay J, Frankel SD, Ominsky SH, Sickles EA, Ernster V. Variability and accuracy in mammographic interpretation using the American College of Radiology Breast Imaging Reporting and Data System. J Natl Cancer Inst 1998;90:1801-9. [Crossref] [PubMed]
- Methods and Systems for Segmentation of Organs and Tumors and Objects. Patent (pending) PCT/US13/36166, April, 2013. Available online: http://www.google.com/patents/WO2013155300A1?cl=en
- Zaretsky J, Guo X, Fu J, Zhao B. Novel radiologic features to predict hepatocellular carcinoma recurrence after liver transplantation: a pilot study. 62nd Annual Meeting of the American Association for the Study of Liver diseases (AASLD), San Francisco, California, Nov 3-8, 2011. Available online: http://liverlearning.aasld.org/aasld/2011/thelivermeeting/13496/jonah.zaretsky.novel.radiologic.features.to.predict.hepatocellular.carcinoma.html?history_id=1012976
- Abou-Alfa GK, Zhao B, Chou JF, Ma J, Capanu M, Koga M, Lee R, Othomo T, Germino J, Schwartz L. 318 Effects of Radiologic Tumor Response of Anti-Glypican-3 GC33 and Multi Tyrosine Kinases Inhibitor Sorafenib in Hepatocellular Carcinoma. EJC 2012;48:97. Available online: http://www.ejcancer.com/article/S0959-8049(12)72116-0/pdf
- Leinwand JC, Zhao B, Guo X, Krishnamoorthy S, Qi J, Graziano JH, Slavkovic VN, Bates GE, Lewin SN, Allendorf JD, Chabot JA, Schwartz LH, Taub RN. Quantitative X-ray computed tomography peritoneography in malignant peritoneal mesothelioma patients receiving intraperitoneal chemotherapy. Ann Surg Oncol 2013;20 Suppl 3:S553-9. [Crossref] [PubMed]
- Chow DS, Qi J, Guo X, Miloushev VZ, Iwamoto FM, Bruce JN, Lassman AB, Schwartz LH, Lignelli A, Zhao B, Filippi CG. Semiautomated volumetric measurement on postcontrast MR imaging for analysis of recurrent and residual disease in glioblastoma multiforme. AJNR Am J Neuroradiol 2014;35:498-503. [Crossref] [PubMed]
- Wu S, Weinstein SP, Conant EF, Kontos D. Automated fibroglandular tissue segmentation and volumetric density estimation in breast MRI using an atlas-aided fuzzy C-means method. Med Phys 2013;40:122302. [Crossref] [PubMed]
- American College of Radiology. Breast imaging reporting and data system (BI-RADS), 4th edition. Available online: http://www.acr.org/Quality-Safety/Resources/BIRADS/About-BIRADS/How-to-Cite-BIRADS
- Gubern-Mérida A, Kallenberg M, Platel B, Mann RM, Martí R, Karssemeijer N. Volumetric breast density estimation from full-field digital mammograms: a validation study. PLoS One 2014;9:e85952. [Crossref] [PubMed]
- Wang J, Azziz A, Fan B, Malkov S, Klifa C, Newitt D, Yitta S, Hylton N, Kerlikowske K, Shepherd JA. Agreement of mammographic measures of volumetric breast density to MRI. PLoS One 2013;8:e81653. [Crossref] [PubMed]
- Highnam R, Brady M, Yaffe M, Karssemeijer N, Harvey J. Robust Breast Composition Measurement – Volpara™. IWDM 2010, LNCS 2010;6136:342-49. Available online: http://volparasolutions.com/solutions/volparadensity/validation-of-volparadensity-using-breast-mri/
- Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, Forbes JF, Warren RM. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst 2011;103:744-52. [Crossref] [PubMed]
- Li J, Humphreys K, Eriksson L, Edgren G, Czene K, Hall P. Mammographic density reduction is a prognostic marker of response to adjuvant tamoxifen therapy in postmenopausal patients with breast cancer. J Clin Oncol 2013;31:2249-56. [Crossref] [PubMed]


