
Early results of ultra-low-dose CT-scan for extremity traumas in emergency room
Introduction
Traumatic injuries of extremities are common in the emergency physicians daily practice (1-5). Conventional digital radiography is the first modality used for diagnosis of these extremities traumatic lesions and used for the management of these injured patients. However, this examination has several limitations among which poor patient cooperation regarding joint mobility if pain is too important, which may limit the number of radiographic incidence and superposition of anatomical structures causing suboptimal quality of images. All these factors limit digital radiography diagnostic performance and induce mistreatment, especially with false negative results. Indeed, for example, for ankles fractures, radiography sensitivity greatly varies between 24% and 94%, depending on the localization of the fracture (6-9). For wrist fractures, its sensitivity varies from 59% to 79%; it was shown that patients with negative digital radiographs present a fracture of the scaphoid in 18.7% cases (6). An untreated fracture of the scaphoid can lead to multiple complications such as osteonecrosis, or pseudarthrosis and scaphoid nonunion advanced collapse (SNAC) up to wrist osteoarthritis, which may induce significant consequences. Exact diagnosis of the fracture is required and lead to several and relevant treatment strategies (10).
The use of computed tomography (CT) in addition to digital radiography to assess the extent of bone and soft tissue injuries, precise injury classification, guide therapeutic management, and check the healing process, has significantly increased. This imaging modality has shown a higher sensitivity than digital radiography (6,11). Ottenin et al. showed with full-dose CT a 93–95% sensitivity and a specificity of 86 to 95% (12). However, the radiation dose delivered to the patients is higher using full-dose CT than that of digital radiography (13). As frequency of use of this technique is increasing, and regarding the useful character of CT scan for injury diagnostic and management compared to digital radiography, the question of dose reduction has become an important and relevant issue.
Several methods have been used to optimize the dose delivered among which the automatic tube current modulation (14) or X-ray filtrations (such as tin filter) (15), but the dose reduction is limited by an increase of image noise and lower overall and diagnostic image quality (16-20). These last years, the use of iterative reconstruction algorithms has allowed a substantial dose reduction and the ultra-low dose (ULD) concept has emerged: CT with doses close to that of radiography, with 3D analysis. ULD acquisitions give images of although degraded quality, but sufficient and adapted for diagnosis (18,20,21). Hamard et al. (21) have showed a higher sensitivity and specificity for detection of minor spinal, pelvic or hip traumas with ULD-CT than with radiography, using the same approaches. For peripheral skeleton imaging, Alagic et al. (22) have demonstrated that ULD-CT was a useful alternative to digital radiography, but no study has yet studied the diagnostic performance of these ULD-CT acquisitions in extremity traumas.
The objective of this study was to assess the sensitivity and specificity with ULD-CT compared to that of digital radiography in patients consulting for extremity traumas and compared to clinical management of patients in an emergency context. Secondary objectives were to evaluate the concordance of the two techniques for the following subjective evaluation criteria: image quality, quality of diagnosis and physician’s confidence level and to assess the doses delivered to the patients with each of the two techniques. We present the following article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-848/rc).
Methods
Study design and setting
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was conducted at the Nîmes University Hospital (France). It was approved by the Institutional Review Board of the Nîmes University Hospital (No. 18.10.04) and registered in clinicaltrials.gov (No. NCT04832490). All included patients signed a consent form. Our monocentric pilot study included participants between February and August 2018.
Selection of study participants
The study included all consecutive adult patients consulting within working hours (9 am–6 pm, no week-ends and public holidays) for trauma of the extremities, i.e., hands and wrists and feet and ankles, at the emergency department. Patients included had underwent, on the same day, both digital radiography (with at least 3 incidences) and ULD-CT for trauma diagnosis. Patients with severe polytraumas requiring a whole body-scan and patients for whom digital radiography was not the first imaging modality performed were not included in the study.
Interventions and measurements
Imaging technique
In case of fracture suspicion, digital radiography was performed as first imaging modality, followed with ULD-CT. Both were performed on the same day.
Digital radiographs were performed on an X-ray room Digital Diagnost® (Philips Healthcare, Amsterdam, The Netherlands), with X-ray projections limited to an anteroposterior and lateral projection for hands/wrists and feet/ankles examinations.
ULD-CT images were acquired using a Somatom Definition AS+ CT-scan (Siemens Healthineers, Forchheim, Germany). Acquisition parameters were as follows: beam collimation of 64×0.6 mm; pitch factor of 0.85 (as recommended by the manufacturer); rotation time of 1.0 s. A field of view of 250 mm was defined for the two protocols, adjusted by technologists depending on the patient’s anatomy. The tube voltage was fixed at 80 kVp, and the tube current was set at 12 mAs for hands and wrists and at 15 mAs for feet and ankles. Both the automatic adjustment of tube voltage (CARE kV) and the automatic tube current modulation (CARE Dose4D) were disabled. The images were reconstructed in transverse/axial plane of 1-mm thickness and 0.7 mm overlapping, and raw data were reconstructed using the level 4 of the SAFIRE® (Sinogram Affirmed Iterative Reconstruction) algorithm. The reconstruction kernels used were “moderately smooth” (I30f) for soft tissue exploration and “strongly sharp” (I70f) for bone exploration for all locations.
Imaging interpretation
Image analysis and interpretation were performed retrospectively in a blinded manner by two radiologists, a junior reader with a 3-year experience (TA, reader 1) and a senior reader with an 11-year experience (FS, reader 2). The two radiologists separately analyzed all radiographs after image anonymization and randomization of the reading rank, blinded to the physical and clinical examination. To limit interpretation biases, a period of 4 weeks was observed before interpretation of the ULD-CT images. They were analyzed separately by the two same radiologists, in the same blinded manner than radiographs, also after randomization of the reading rank. The images were visualized on GE Healthcare® Centricity Picture Archiving and Communication System (PACS); all ULD-CT images were read in multiplanar 3-mm reconstructed slices.
Study outcomes
The primary endpoint was to assess the sensitivity and specificity with ULD-CT compared to that of digital radiography, to diagnose post-traumatic extremity fractures. The “fracture” status corresponded to the detection of at least one fracture. In the absence of conventional CT or MRI performed as gold-standard test, the sensitivity and specificity were computed with two different reference tests, as previously published (21). First, the best value comparator (BVC) based on a systematic review of both imaging tests by the two readers, who determined in consensus the final fracture status for each patient. Second, a latent class model (LCM) using Bayesian inference was also performed using the reader 2’s values. This method has been previously described in the literature in diagnostic studies when a gold standard is not available (23,24). LCMs considered the target disease condition as an unmeasured (“latent”) variable and the observed diagnostic tests are considered as imperfect classifiers of the disease status. The basis of the Bayesian inference is to combine the information drawn from the study with the prior information for the given parameters (i.e., the diagnostic performance of ULD-CT and radiographs for fracture diagnosis) in order to provide posterior estimation for these parameters with less bias (25).
Results were compared to clinical follow-up: fracture treatment (surgery, immobilization, return home without immobilization with medical treatment) or not was recorded in routine practice and retrospectively analyzed on the medical record. Follow-up was performed by reader 1, using the patients’ medical files, until the patient’s discharge. To ensure taking into account late or delayed diagnoses, data were collected at least 6 months after patient examination at the emergency department. In case the patients did not return, initial diagnosis was considered.
Secondary endpoints were inter-observer agreement between the two readers, overall image quality, confidence level and quality of diagnosis assessed using a scale previously published (24). Overall image quality (i.e., overall impression, ability to see structures accounting with artefacts and image noise) was rated from 1 to 4: 1= unevaluable; 2= interpretable despite moderate technical problems; 3= fully interpretable with mild technical problem; 4= fully interpretable with no technical problem. Technical problems included failed centering or movement artifact on digitals radiographs and artifact or noise on ULD-CT images. Diagnostic image quality (i.e., is the image quality sufficient to provide diagnosis, rated as 1= unacceptable; 2= suboptimal; 3= acceptable; 4= above average; 5= excellent) and diagnostic confidence level (i.e., confidence of the radiologist in the diagnosis given, rated as 1= very poor; 2= poor; 3= average; 4= high; 5= excellent) were also assessed.
Dosimetry
The dose area product (DAP) was obtained from the DICOM file of each X-ray projection for each patient, and the dose length product (DLP) from the dose report available for each patient. Both dosimetric indicators were retrieved from the PACS.
The effective dose was calculated for each radiographic (ERX) and ULD-CT (EULD) examination. For radiography, it was calculated by multiplying the DAP by region-specific conversion coefficient (eDAP): 0.01 mSv·Gy−1·cm−2, and by multiplying the DLP by the region-specific conversion coefficient (eDLP): 0.0002 mSv·Gy−1·cm−1 for ULD-CT.
Statistical analyses
Statistical analyses in frequentist inference were performed using the Statistical Analysis Software SAS® University Edition version 2.5 9.4 M4 and those in Bayesian inference using R (Version R 3.3.3 GUI 1.69 Mavericks build 7328®Copyright 2004® 2016 R Foundation for Statistical Computing).
Quantitative variables were described using medians and interquartile ranges. Performance of ULD-CT and radiographs was first evaluated comparing to clinical follow-up and BVC. Specificities and sensitivities were presented with their 95% CI. True positive, true negative, false positive and false negative cases for radiography and ULD-CT were reported from reader 2 interpretation.
The Bayesian approach is based on the contribution of information published in the literature. An LCM with conditional independence was performed, from reader 2 analysis. Model details and program are presented in Supplementary material (Appendix 1). An a priori distribution of five estimated parameters (fracture prevalence, sensitivity and specificity ULD-CT and radiographs) was determined using beta laws hyper parameters based on literature data (6,8,12,26), “hyperparameters” details are presented in Supplementary material (Table S1). A posteriori distributions of these five parameters were calculated by numerical simulations using a Hamiltonian Monte Carlo sampling algorithm after model convergence (27). Statistical inferences were based on three chains with 10,000 iterations each after the burn-in period. Adequate convergence for the MCMC simulations was assessed visually using traceplots, Gelman-Rubin plots, and Gelman tests. Model goodness-of-fit was assessed by comparing the observed data of the cross-tabulation and the predictions under the model. The probability of recovery, graphic comparison of the marginal distributions of both sensitivity and specificity, was used to compare diagnostic performance. The threshold was set-up at <0.025 or >0.975 to conclude to a difference between the two techniques (ULD-CT and radiographs). A sensitivity analysis was performed to test the robustness of the estimates obtained according to the a priori distributions.
The inter-observer agreement between the two readers both for fracture detection and image quality was assessed for ULD-CT and radiographs. It was calculated using the weighted Cohen kappa test and presented with its 95% confidence interval (95% CI). The effective doses were compared by a paired student test. A P value lower than 0.05 was considered significant.
Results
Patients
One hundred and seven patients were eligible to participate in the study. Thirty-one patients were excluded from analysis because of missing radiographic incidences (only face and profile were available) necessary for optimal interpretation. All remaining patients, 76 patients, were included in the analysis. They were 41 men (53.9%) and 35 women (46.1%) of median age 38.5 years (range, 18–89 years) (Table 1). Forty-five patients (59.2%) underwent a foot/ankle examination and 31 patients (40.8%) a hand/wrist examination; 84.3% of patients received conservative treatment (analgesics alone or immobilization) and 15.7% underwent surgery. Only 6.6% were hospitalized (median duration 2 days, range, 2–7 days). The overall fracture prevalence, estimated using the Bayesian approach, was 38% (95% CI, 24–53%).
Table 1
| Variables | Wrists/hands (n=31) | Ankles/feet (n=45) | Total (n=76) |
|---|---|---|---|
| Gender, n (%) | |||
| Men | 16 (51.6) | 25 (55.5) | 41 (53.9) |
| Women | 15 (48.4) | 20 (44.5) | 35 (46.1) |
| Age (years), median [range] | 41 [18–89] | 37 [19–82] | 38.5 [18–89] |
| Cause of trauma, n (%) | |||
| Road accident | 3 (9.7) | 4 (8.9) | 7 (9.2) |
| Household accident | 16 (51.6) | 22 (48.9) | 38 (50.0) |
| Sports accident | 6 (19.4) | 7 (15.6) | 13 (17.1) |
| Workplace accident | 6 (19.4) | 12 (26.7) | 18 (23.7) |
| Treatments, n (%) | |||
| Analgesics alone | 18 (58.0) | 30 (66.6) | 48 (63.1) |
| Immobilization | 6 (19.4) | 10 (22.2) | 16 (21.2) |
| Surgery | 7 (22.5) | 5 (11.1) | 12 (15.7) |
| Emergency surgery | 2 (28.6) | 3 (60.0) | 5 (41.7) |
| Differed surgery | 5 (71.4) | 2 (40.0) | 7 (58.3) |
| Patient care, n (%) | |||
| Day care | 29 (93.5) | 42 (93.3) | 71 (93.4) |
| Hospitalisation | 2 (6.5) | 3 (6.7) | 5 (6.6) |
| Duration of hospital stay (days), median [range] | 2 [2–2] | 5 [2–7] | 2 [2–7] |
Fracture detection
Reader 1 detected 23 fractures (30.3%) on digital radiographs versus 28 on ULD-CT images (36.8%), and reader 2, 24 fractures (31.6%) on radiographs versus 31 on ULD-CT images (40.8%) (Table 2). The inter-observer agreement for fracture detection for all locations was excellent for radiographic fractures (k=0.94, 95% CI: 0.73–0.98) and for ULD-CT (k=0.91, 95% CI: 0.69–0.99).
Table 2
| Localizations | Radiography | ULD-CT | P values^ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reader 1, n (%) | Reader 2, n (%) | Agreement coefficient, k [95% CI] | Reader 1, n (%) | Reader 2, n (%) | Agreement coefficient, k [95% CI] | Reader 1 | Reader 2 | |||
| Wrists/hands (n=31) | 12 (38.7) | 13 (41.9) | 0.93 [0.72–0.98] | 14 (45.1) | 15 (48.3) | 0.93 [0.71–0.99] | 0.34 | 0.42 | ||
| Ankles/feet (n=45) | 11 (24.4) | 11 (24.4) | 0.91 [0.70–0.98] | 14 (31.1) | 16 (35.5) | 0.90 [0.72–0.98] | 0.14 | 0.10 | ||
| Total (n=76) | 23 (30.3) | 24 (31.6) | 0.93 [0.73–0.98] | 28 (36.8) | 31 (40.8) | 0.91 [0.69–0.99] | 0.20 | 0.15 | ||
A P value lower than 0.05 was considered significant. ^, radiographs vs. ULD-CT for each reader. ULD-CT, ultra-low dose computed tomography scan.
On the 24 abnormalities reported by reader 2 on digital radiography, according to clinical follow-up, 3 were false positive and 21 true positive examinations. Using ULD-CT, 10 false negative results of digital radiography were identified. Two cases of fractures not detected (case 1) or suspected (case 2) on digital radiography, and visible and confirmed using ULD-CT, are presented in Figure 1. Among these 10 false negative examinations, patients were treated with plaster casting (n=6) or with buddy taping (n=4). Regarding ULD-CT, 2 false positive and 31 true positive were reported. Two false negative examinations were corrected by consensus analysis of the two readers. The remaining 41 patients (true negative) were medically treated for pain and hematoma, without immobilization. No adverse events were reported during or after radiography or ULD-CT.
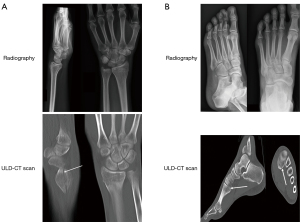
Diagnostic performance
Specificity was almost similar for digital radiography and ULD-CT whatever the statistical approach used, respectively: 94% and 95% with the BVC approach, 93% and 96% with the clinical-based and Bayesian approach (Table 3), without significant statistical difference (P of recovery =0.848 with the Bayesian approach, Figure 2).
Table 3
| Modalities | Best value comparator | Clinical follow-up based approach | Bayesian interference approach | |||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | |||
| Radiography, % [95% CI] | 70 [54–84] | 94 [86–100] | 68 [57–78] | 93 [87–99] | 76 [71–81] | 93 [87–97] | ||
| ULD-CT, % [95% CI] | 93 [87–96] | 95 [90–100] | 94 [88–99] | 96 [90–100] | 90 [87–93] | 96 [93–98] | ||
ULD-CT, ultra-low dose computed-tomography; 95% CI, 95% confidence interval.
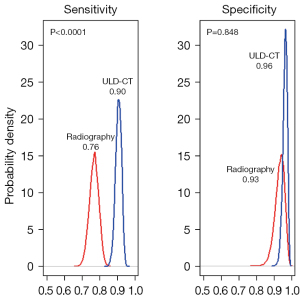
Sensitivity to detect fractures was significantly higher for the ULD-CT scan than for digital radiography (P of recovery <0.001, Figure 2): 93% (95% CI: 87–96%) for ULD-CT versus 70% (95% CI: 54–84%) for digital radiography using the BVC method, 94% (95% CI: 88–99%) and 68% (95% CI: 57–78%) with the clinical-based approach, and 90% (95% CI: 87–93%) and 76% (95% CI: 71–81%), with the Bayesian approach (Table 3).
A sensitivity analysis was performed to check overfitting of the Bayesian model. In the non-informative model (i.e., no a priori literature data included in the model), both sensitivity and specificity of ULD-CT decreased slightly but the two parameters remained superior to that of radiography (Table S2).
Subjective image quality assessment
For all three criteria, image quality, diagnostic image quality and diagnostic confidence level, the inter-observer weighted agreement coefficients were higher for the ULD-CT images than for the digital radiographs (Table 4). A significant difference between the two imaging techniques was reported for the 3 quality criteria by reader 1, whereas only the confidence level was significantly better using ULD-CT than using digital radiography according to reader 2. Concordance between the two readers was higher for ULD-CT (Table 4).
Table 4
| Items | Scores | Radiography | ULD-CT | P values^ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reader 1, n (%) | Reader 2, n (%) | Agreement coefficient, k [95% CI] | Reader 1, n (%) | Reader 2, n (%) | Agreement coefficient, k [95% CI] | Reader 1 | Reader 2 | ||||
| Overall image quality* | Unevaluable | 1 (1.4) | 0 | 0.24 [0.08–0.37] | 0 | 0 | 0.88 [0.66–0.98] | 0.0001 | 0.23 | ||
| Interpretable in spite of moderate technical problem | 8 (10.5) | 1 (1.4) | 1 (1.4) | 0 | |||||||
| Fully interpretable with mild technical problem | 38 (50.0) | 14 (18.4) | 6 (7.9) | 8 (10.5) | |||||||
| No technical problem | 29 (38.0) | 61 (80.2) | 69 (90.7) | 68 (89.4) | |||||||
| Diagnostic image quality** | Unacceptable | 0 | 0 | 0.13 [0.0–0.31] | 0 | 0 | 0.99 [0.70–0.99] | 0.0001 | 0.15 | ||
| Suboptimal | 12 (15.7) | 0 | 0 | 0 | |||||||
| Acceptable | 53 (69.7) | 2 (2.6) | 2 (2.6) | 1 (1.4) | |||||||
| Above average | 11 (14.5) | 24 (31.5) | 12 (17.7) | 12 (17.7) | |||||||
| Excellent | 0 | 50 (65.7) | 62 (81.5) | 63 (82.8) | |||||||
| Confidence level | Very poor | 0 | 0 | 0.10 [0.0–0.28] | 0 | 0 | 0.90 [0.72–0.99] | 0.0001 | 0.01 | ||
| Poor | 18 (23.6) | 1 (1.4) | 0 | 0 | |||||||
| Average | 45 (59.2) | 3 (3.9) | 2 (2.6) | 1 (1.4) | |||||||
| High | 13 (17.1) | 35 (46.0) | 14 (18.4) | 16 (21.0) | |||||||
| Excellent | 0 | 37 (48.6) | 60 (78.9) | 59 (77.6) | |||||||
A P value lower than 0.05 was considered significant. ^, radiographs vs. ULD-CT for each reader; *, overall image quality, overall impression, ability to see structures accounting with the artefacts and image noise; **, diagnostic image quality, image quality sufficient to provide diagnosis. ULD-CT, ultra-low dose computed-tomography scan; 95% CI, 95% confidence interval.
Dosimetry
Dosimetry data are presented in Table 5 for both digital radiography and ULD-CT. The effective dose for wrists and hands was significantly higher for ULD-CT than for radiography (0.84±0.14 for ULD-CT and 0.58±0.27 for radiography, P=0.0002). It was not significantly different between the two modalities for ankles and feet.
Table 5
| Localizations | Digital radiography | ULD-CT | P value [E (ULD-CT) vs. E (radiography)] | |||
|---|---|---|---|---|---|---|
| DAP (mGy·cm2) | E (µSv) | DLP (mGy·cm2) | E (µSv) | |||
| Wrists/hands | 57.6±26.5 | 0.58±0.27 | 4.2±0.7 | 0.84±0.14 | 0.0002 | |
| Ankles/feet | 144.5±77.9 | 1.44±0.78 | 7.5±1.6 | 1.50±0.32 | 0.09 | |
Values are expressed as mean ± standard deviation. A P value lower than 0.05 was considered significant. ULD-CT, ultra-low dose computed-tomography scan; DAP, dose area product; DLP, dose length product; E, effective dose.
Discussion
For the first time, this study showed that the sensitivity of ULD-CT scan, a CT-scan close to digital radiography in terms of dose, was significantly better than that of digital radiography for the detection of fractures of the extremities. More fractures were detected with ULD-CT than with digital radiography by two blinded readers of different radiological experience. Regarding the overall image quality, the diagnostic confidence level was found significantly better for ULD-CT than for digital radiography by both readers.
Many studies, mostly retrospective, have compared diagnostic performance of digital radiography and full-dose CT and concluded to a moderate to poor sensitivity of digital radiography for fracture detection, especially in complex locations (26), and to a better sensitivity and specificity of full-dose CT (12). Regarding ULD-CT protocols in fracture detection, a study in 398 patients did not show any difference in sensitivity or specificity of fracture detection between standard-dose and low-dose CT, with no impact on image quality (28). Further prospective studies are now needed to compare the diagnostic performances of full-dose conventional CT and ULD-CT for fracture detection.
In this study, we compared the diagnostic performance, image quality and dosimetry data of ULD-CT and digital radiography. Fracture detection was higher with ULD-CT than with radiographs although it was not found significantly different. These fracture detection rates are concordant or lower with that published in the literature (6,12). This may be due to the relatively small sample size in our study. One recent study assessed fracture detection using ULD-CT in 203 patients and reported an improved detection on ULD-CT as compared to that of digital radiography (diagnostic OR =2.0, 95% CI: 1.4–3.0), but sensitivity and specificity were not estimated (22). We reported sensitivity and specificity (90–93% and 95–96%) for ULD-CT comparable to that shown for full-dose CT (12). We showed a higher sensitivity of ULD-CT compared to radiographs for fracture detection, and specificity was comparable using the two techniques. Clinical outcomes showed fractures not detected using digital radiography, but reported using ULD-CT in 10 patients, 6 of whom required immobilization.
In the absence of gold standard in our study, specificity and sensitivity were evaluated using three different methods, the BVC method, a clinical data-based method and the latent class Bayesian interference model approach. BVC, although statistically limited, is often used in clinical practice in radiology in studies with no standard of reference. We completed these results using two other methods, one based on treatment and clinical follow-up data to determine the disease status (presence or absence of fracture) and the Bayesian approach, a statistically more robust analysis based on literature data. Images were first analysed using the BVC approach, with an experienced radiologist, but using an imaging technique, ULD-CT, which may not be considered a standard of reference compared to full-dose CT or MRI. The Bayesian approach confirmed the results obtained with the two other methods and allowed statistical comparison of sensitivity and specificity of radiography and ULD-CT. The higher sensitivity of ULD-CT compared to that of radiography was found a statistically significant difference according to the Bayesian model (P recovery <0.001). Similar results were recently described for fractures of the spine and pelvis (21).
We reported high inter-observer agreement coefficients on the subjective parameters of image quality assessed by two different readers, much higher with ULD-CT than with radiography. Of note, reader 1 (junior reader) found the three indexes, overall image quality, diagnostic image quality and diagnostic confidence level, significantly better for ULD-CT whereas reader 2 (experienced reader) only found the diagnostic confidence level to be better with ULD-CT. These results suggest CT-scan interpretation seems “easier” and more reproducible especially for young or non-specialized readers, as previously shown in other studies on ULD-CT protocols (29,30).
Dosimetry data showed a similar effective dose received for ankles or feet examination with digital radiography or ULD-CT. It was statistically different, higher with ULD-CT, for wrists or hands imaging. This difference can be explained by the limitation of the number of incidences to 3 in this study, which reduces the doses delivered with digital radiography compared to usual daily practice. However, clinically speaking, this difference is, in our view, not significant; indeed, the effective dose received with ULD-CT stays extremely low, around 1 µSv. In that context, parameters used for digital radiographs allow low radiation dose received by the patients. A recent comparative dosimetry study on phantoms showed effective doses of 8.6 µSv for multidetector CT and of 1.0 µSv for digital radiography (31). The Konda et al. study showed, in patients with limb fractures, a 14-fold decreased of the mean effective dose radiation with the low-dose CT protocol compared to conventional CT (0.03 mSv for ULD-CT versus 0.43 mSv) (32). In our study, we decreased the acquisition parameters (mAs and kVp), which allowed reaching doses much lower than that previously published with full-dose CT (12). In addition, for wrists and hands, the acquisition parameters used were the lowest possible on our CT-scan.
The main limitation of our study is the absence of firm gold standard. To counter this limitation, we have used three methods, one based on imaging interpretation (BVC), the second on clinical data and the third based on literature information (Bayesian interference approach). We checked for overfitting of the Bayesian model with a sensitivity analysis; however, we could only use data extrapolated from full-dose CT results as no data with ULD-CT were yet available in the literature. Results of the sensitivity analyses showed a slight modification of the final results, which was not significant. Another limitation is that our ULD-CT protocol was set-up on a given CT-scan and is manufacturer dependent, thus it cannot be generalized as such. Furthermore, retrospective analysis of clinical evolution was performed to strengthen our data and counter the absence of firm gold standard, but with the limitations of retrospective analysis (risk of missing data). We were not able to perform subgroup analyses according to fracture location because of the relatively small number of patients in our study. This will be done in an ongoing multicentric prospective study coordinated by our Institute (NCT 04074733). Last, our study lacked prospective evaluation of the clinical impact, i.e., change in clinical strategy or care, of the better diagnostic performance of ULD-CT. Alagic et al. showed a change in the recommended treatment in 16.4% of extremities after diagnosis with ULD-CT (22). Further studies prospectively evaluating such outcomes, as well as patient information, pain management or medico-economics features (costs, work interruptions…) would definitely be of interest.
In conclusion, ULD-CT appears a reliable alternative for digital radiographs for detection of fractures of the extremities in the emergency room with a good sensitivity and specificity, a sufficient image quality and low effective radiation doses. Multicentric prospective studies are now needed to confirm our results and clinical and medico-economic impacts of such strategy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-21-848/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-848/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was conducted at the Nîmes University Hospital (France). It was approved by the Institutional Review Board of the Nîmes University Hospital (No 18.10.04). All included patients signed a consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Court-Brown CM, Caesar B. Epidemiology of adult fractures: A review. Injury 2006;37:691-7. [Crossref] [PubMed]
- Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am 2001;26:908-15. [Crossref] [PubMed]
- Shibuya N, Davis ML, Jupiter DC. Epidemiology of foot and ankle fractures in the United States: an analysis of the National Trauma Data Bank (2007 to 2011). J Foot Ankle Surg 2014;53:606-8. [Crossref] [PubMed]
- Bucholz RW, Heckman JD, Court-Brown CM, Tornetta P III. Rockwood and Green’s fractures in adults, 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2010.
- Elsoe R, Ostgaard SE, Larsen P. Population-based epidemiology of 9767 ankle fractures. Foot Ankle Surg 2018;24:34-9. [Crossref] [PubMed]
- Balci A, Basara I, Çekdemir EY, Tetik F, Aktaş G, Acarer A, Özaksoy D. Wrist fractures: sensitivity of radiography, prevalence, and patterns in MDCT. Emerg Radiol 2015;22:251-6. [Crossref] [PubMed]
- Gupta A, Batra S, Jain P, Sharma SK. Carpal alignment in distal radial fractures. BMC Musculoskelet Disord 2002;3:14. [Crossref] [PubMed]
- Basu S, Khan SH. Radiology of acute wrist injuries. Br J Hosp Med (Lond) 2010;71:M90-3. [Crossref] [PubMed]
- Mansur NS, Celestino FS, Neves C, Pereira VF, Vargas Silva PD, Matsunaga FT, Nery CA, Astur DC. Computerized Tomography Scans for Ankle Fracture: Diagnosis, Management and Surgical Plan Modifier. Foot Ankle Orthop 2022;7:2473011421S00336.
- Pellatt R, Fomin I, Pienaar C, Bindra R, Thomas M, Tan E, Mervin C, Zhang P, Keijzers G. Is Buddy Taping as Effective as Plaster Immobilization for Adults With an Uncomplicated Neck of Fifth Metacarpal Fracture? A Randomized Controlled Trial. Ann Emerg Med 2019;74:88-97. [Crossref] [PubMed]
- Welling RD, Jacobson JA, Jamadar DA, Chong S, Caoili EM, Jebson PJ. MDCT and radiography of wrist fractures: radiographic sensitivity and fracture patterns. AJR Am J Roentgenol 2008;190:10-6. [Crossref] [PubMed]
- Ottenin MA, Jacquot A, Grospretre O, Noël A, Lecocq S, Louis M, Blum A. Evaluation of the diagnostic performance of tomosynthesis in fractures of the wrist. AJR Am J Roentgenol 2012;198:180-6. [Crossref] [PubMed]
- Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med 2007;357:2277-84. [Crossref] [PubMed]
- Greffier J, Larbi A, Macri F, Beregi JP, Pereira F. Effect of patient size, anatomical location and modulation strength on dose delivered and image-quality on CT examination. Radiat Prot Dosimetry 2017;177:373-81. [Crossref] [PubMed]
- Greffier J, Pereira F, Hamard A, Addala T, Beregi JP, Frandon J. Effect of tin filter-based spectral shaping CT on image quality and radiation dose for routine use on ultralow-dose CT protocols: A phantom study. Diagn Interv Imaging 2020;101:373-81. [Crossref] [PubMed]
- Martini K, Moon JW, Revel MP, Dangeard S, Ruan C, Chassagnon G. Optimization of acquisition parameters for reduced-dose thoracic CT: A phantom study. Diagn Interv Imaging 2020;101:269-79. [Crossref] [PubMed]
- Greffier J, Dabli D, Hamard A, Belaouni A, Akessoul P, Frandon J, Beregi JP. Effect of a new deep learning image reconstruction algorithm for abdominal computed tomography imaging on image quality and dose reduction compared with two iterative reconstruction algorithms: a phantom study. Quant Imaging Med Surg 2022;12:229-43. [Crossref] [PubMed]
- Greffier J, Hoballah A, Sadate A, de Oliveira F, Claret PG, de Forges H, Loubet P, Mauboussin JM, Hamard A, Beregi JP, Frandon J. Ultra-low-dose chest CT performance for the detection of viral pneumonia patterns during the COVID-19 outbreak period: a monocentric experience. Quant Imaging Med Surg 2021;11:3190-9. [Crossref] [PubMed]
- Nicolan B, Greffier J, Dabli D, de Forges H, Arcis E, Al Zouabi N, Larbi A, Beregi JP, Frandon J. Diagnostic performance of ultra-low dose versus standard dose CT for non-traumatic abdominal emergencies. Diagn Interv Imaging 2021;102:379-87. [Crossref] [PubMed]
- Greffier J, Macri F, Larbi A, Fernandez A, Khasanova E, Pereira F, Mekkaoui C, Beregi JP. Dose reduction with iterative reconstruction: Optimization of CT protocols in clinical practice. Diagn Interv Imaging 2015;96:477-86. [Crossref] [PubMed]
- Hamard A, Greffier J, Bastide S, Larbi A, Addala T, Sadate A, Beregi JP, Frandon J. Ultra-low-dose CT versus radiographs for minor spine and pelvis trauma: a Bayesian analysis of accuracy. Eur Radiol 2021;31:2621-33. [Crossref] [PubMed]
- Alagic Z, Bujila R, Enocson A, Srivastava S, Koskinen SK. Ultra-low-dose CT for extremities in an acute setting: initial experience with 203 subjects. Skeletal Radiol 2020;49:531-9. [Crossref] [PubMed]
- Collins J, Huynh M. Estimation of diagnostic test accuracy without full verification: a review of latent class methods. Stat Med 2014;33:4141-69. [Crossref] [PubMed]
- Hui SL, Walter SD. Estimating the error rates of diagnostic tests. Biometrics 1980;36:167-71. [Crossref] [PubMed]
- Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR. Bayesian methods in health technology assessment: a review. Health Technol Assess 2000;4:1-130. [Crossref] [PubMed]
- Haapamaki VV, Kiuru MJ, Koskinen SK. Ankle and foot injuries: analysis of MDCT findings. AJR Am J Roentgenol 2004;183:615-22. [Crossref] [PubMed]
- Carpenter B, Gelman A, Hoffman MD, Lee D, Goodrich B, Betancourt M, Brubaker M, Guo J, Li P, Riddell A. Stan: A Probabilistic Programming Language. Journal of Statistical Software 2017;76:1-32. [Crossref]
- Yi JW, Park HJ, Lee SY, Rho MH, Hong HP, Choi YJ, Kim MS. Radiation dose reduction in multidetector CT in fracture evaluation. Br J Radiol 2017;90:20170240. [Crossref] [PubMed]
- Macri F, Greffier J, Khasanova E, Claret PG, Bastide S, Larbi A, Bobbia X, Pereira FR, de la Coussaye JE, Beregi JP. Minor Blunt Thoracic Trauma in the Emergency Department: Sensitivity and Specificity of Chest Ultralow-Dose Computed Tomography Compared With Conventional Radiography. Ann Emerg Med 2019;73:665-70. [Crossref] [PubMed]
- Macri F, Greffier J, Pereira FR, Mandoul C, Khasanova E, Gualdi G, Beregi JP. Ultra-low-dose chest CT with iterative reconstruction does not alter anatomical image quality. Diagn Interv Imaging 2016;97:1131-40. [Crossref] [PubMed]
- Koivisto J, van Eijnatten M, Kiljunen T, Shi XQ, Wolff J. Effective Radiation Dose in the Wrist Resulting from a Radiographic Device, Two CBCT Devices and One MSCT Device: A Comparative Study. Radiat Prot Dosimetry 2018;179:58-68. [Crossref] [PubMed]
- Konda SR, Goch AM, Leucht P, Christiano A, Gyftopoulos S, Yoeli G, Egol KA. The use of ultra-low-dose CT scans for the evaluation of limb fractures: is the reduced effective dose using ct in orthopaedic injury (REDUCTION) protocol effective? Bone Joint J 2016;98-B:1668-73. [Crossref] [PubMed]


