
A new logistic regression model for early prediction of severity of acute pancreatitis using magnetic resonance imaging and Acute Physiology and Chronic Health Evaluation II scoring systems
Introduction
Acute pancreatitis (AP) is a common inflammatory disorder of the pancreas with a growing incidence (1,2). The etiology and pathological changes of AP are complicated, and its course, clinical manifestations, and prognosis vary greatly. Approximately 20% of patients develop moderate or severe AP, and the mortality rates is very high, ranging from 20% to 40% (1,3). Therefore, the early diagnosis and evaluation of the severity of AP would support a personalized approach to its management.
Knowing when to perform imaging in AP remains unclear even though the careful evaluation of the application of diagnostic imaging in the course of AP is mandated. In terms of the economic costs associated with diagnostic imaging, early imaging examination may be not recommended for patients with typical clinical symptoms and laboratory presentation of AP (4). However, early imaging examination is usually used to diagnose suspected AP when the clinical presentation is unclear, discover the underlying cause of AP, diagnose complications, evaluate its severity, and guide management (5,6). It has been proven that early magnetic resonance imaging (MRI) can facilitate the early prediction of organ failure and the severity of AP (7-9). MRI can provide more information than computed tomography (CT) and, without ionizing radiation, is relatively safe. MRI is better able to detect the mildest alteration of AP and can characterize the contents of mild extrapancreatic inflammatory effusion that may be overlooked on CT (7,10,11). There are several radiologic prognostic scoring systems used to evaluate the severity of AP on MRI. The magnetic resonance severity index (MRSI), modified MRSI (MMRSI), and extrapancreatic inflammation on magnetic resonance (EPIM) are all derived from CT (12,13) and can all clearly reveal the local context of AP.
Several clinically relevant scoring systems, which can reflect systemic complications to some extent, have good predictive capabilities for disease severity and mortality; these include the Bedside Index of Severity in Acute Pancreatitis (BISAP), Ranson’s Criteria for Pancreatitis Mortality, and the Acute Physiology and Chronic Health Evaluation II (APACHE II) scoring system. Compared to the APACHE II scoring system, the Ranson score does not include the component of the Chronic Health Evaluation assessment; meanwhile, BISAP is convenient for quick evaluation but has lower sensitivity and specificity for predicting the disease severity of AP (14). Thus, the APACHE II is the most valuable scoring system for the early evaluation of AP severity (15,16).
However, despite the variety of scoring systems, no one tool works well for all forms of AP. Imaging scoring systems and clinical scoring system are not opposed to each other but are interrelated, and they have distinct advantages. Imaging scoring systems reflect the local conditions of AP patients, as clinical parameters apply to systemic conditions. It is not unusual to encounter some patients with a high imaging score that coincides with a low clinical score, potentially confusing clinicians and making it difficult for them to maintain overall control of the disease. At present, the most common approach is to use a single scoring system in research related to AP (16,17), and while some studies use a combined scoring system, they use mainly clinical and laboratory parameters (18). Only one paper exists regarding combining radiologic and clinical parameters to predict the severity of AP in its early stage. These authors of this paper drew upon CT-derived radiologic images and used a classification tree analysis (CTA) model that included both clinical and radiologic parameters. Their results showed that using a certain combination of these parameters could improve the efficiency of the early prediction of severe AP compared to using each parameter alone (19).
In our research, we used a MRI scoring system and a clinical scoring system, which could reflect systemic and local conditions. To our knowledge, no other study has focused on the early prediction of severe AP based on a regression model that incorporates both MRI and clinical parameters. Therefore, we aimed to develop a possible superior model for AP on the basis of clinical and MRI parameters and to evaluate its performance. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-158/rc).
Methods
Patients
The study was a retrospective study of AP patients who were admitted to the Affiliated Hospital of North Sichuan Medical College from March 2016 to December 2018. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the institutional review board of our hospital (No. 2019 ER[A] 223), and individual consent for this retrospective analysis was waived.
The diagnosis of AP for this study was based on the presence of two of the following three criteria: (I) acute upper abdominal pain; (II) an at least 3-fold elevation of serum levels of amylase or lipase; and/or (III) imaging findings characteristic of AP. The inclusion criteria for patients were the following: (I) hospitalization for AP; (II) experiencing a first episode of AP; and (III) undergoing an abdominal magnetic resonance (MR) examination within the first 3 days of hospitalization. Patients were excluded in following cases: (I) a documented history of chronic pancreatitis; (II) AP due to pancreatic carcinoma; (III) presence of retroperitoneal infection, neoplasia, or hemorrhagic diseases; (IV) presentation with comorbidities of chronic liver disease, hypoalbuminemia, or an underlying disease that may cause peritoneal effusion; and (V) a scan with poor image quality (Figure S1).
Medical records were reviewed. The clinical data of all patients were recorded, including age, sex, etiology, length of stay, occurrence of systemic inflammatory response syndrome (SIRS), occurrence of organ failure, and clinical severity of AP according to the modified Marshall scoring system as applied by two clinicians (who were blinded to the image data). All indicators at the worst value in the APAHCE II scoring system were recorded objectively within 3 days, and some of the missing data were scored as normal.
MRI techniques
Our hospital routinely performs MRI for AP patients. The MRI techniques examined in this study were similar to those reported in a previously published paper linked to our hospital (20). All patients underwent an MRI on a 3.0-T system (MR750; General Electric Medical Systems, Waukesha, WI, USA). The sequences included the following: coronal and axial single-shot fast spin-echo T2-weighted imaging (SSFSE T2WI), axial fast recovery fast spin-echo T2-weighted imaging (FRFSE T2WI) with fat saturation, T1-weighted in-phase and out-of-phase imaging obtained from three-dimensional liver acquisitions with volume acceleration flex (3D LAVA-flex), and dynamic contrast-enhanced 3D LAVA-flex with fat saturation imaging.
The parameters of the above sequences are listed in Table 1. 3D LAVA dynamic enhancement was performed with 20 mL of gadolinium (Magnevist; Bayer Schering, Guangzhou, China) administered intravenously at 2–3 mL/s, which was followed by a 20-mL saline solution flush. Dynamic enhancement was performed at 16 s (early hepatic arterial phase), 30 s (hepatic arterial phase), 60 s (venous phase), and 120 s (delayed phase) after the injection.
Table 1
| Sequences | TR (ms) | TE (ms) | Section thickness (mm) | Intersection gap (mm) | Matrix | FOV (cm2) |
|---|---|---|---|---|---|---|
| AX 3D LAVA-flex | 3.6–4.4 | 1.7–1.9 | 5.2 | 0 | 224×192 | 36×36 |
| AX FRFSE T2WI | 4,500–6,000 | 90–120 | 6 | 1 | 320×256 | 34×34 |
| AX FRFSE fs-T2WI | 2,500–3,000 | 90–110 | 6 | 1 | 384×384 | 34×34 |
| COR SSFSE T2WI | 4,500–6,000 | 90–120 | 5 | 1 | 384×256 | 36×36 |
| AX 3D LAVA C+* | 3.6–4.4 | 1.7–1.9 | 5.2 | 0 | 224×192 | 36×35 |
Dynamic enhanced imaging is indicated with *. MRI, magnetic resonance imaging; AX 3D LAVA-flex, axial three-dimensional liver acquisitions with volume acceleration flexible; AX FRFSE T2WI, axial fast recovery fast spin-echo T2-weighted imaging; fs, fat saturation; COR SSFSE T2WI, coronal single-shot fast spin-echo T2-weighted imaging; TR, repetition time; TE, echo time; FOV, field of view.
MRI interpretation
Two observers with at least 5 years of experience in abdominal MR images independently reviewed all MR images and were blinded to clinical data and outcomes. The severity of AP was graded according to the MRSI, EPIM, and MMRSI, all which were derived from the CT scoring system. AP was then graded as mild (0–3 points), moderate (4–6 points), or severe (7–10 points) (17,21) according to the MRSI or MMRSI. Although there is no research about MMRSI, in fact, in the contrast to the CT severity index (CTSI), the modified CTSI includes extrapancreatic complications in the assessment, which simplifies the evaluation of the extent of pancreatic parenchymal necrosis (none, ≤30%, or >30%) and peripancreatic inflammation (presence or absence of peripancreatic fluid). Moreover, extrapancreatic inflammation is a good indicator for evaluating the severity of AP, and the most common indicator is extrapancreatic inflammation on CT (EPIC) or EPIM (7,22). Hence, in 2004, MCTSI, which was alleged to be superior to the CTSI for assessing the severity of AP, was recommended for use in clinical practice (12,13,17).
APACHE II score and clinical parameters
Medical records were reviewed, with the length of hospital stay and the incidence of SIRS or organ failure being extracted from the electronic file system. In order to ensure consistency of timing with the image data, all indicators at the worst value in the APACHE II scoring system were recorded objectively within the first 3 days of hospitalization. An APACHE II score of 8 was used as the cutoff point for differentiating predicted mild AP (0–7 points) from predicted severe AP (≥8 points) (23). Three organ systems were assessed: respiratory, cardiovascular, and renal. Organ failure was defined according to the 2012 Revised Atlanta Classification of AP and as a score of 2 or more for 1 of the 3 organ systems using the modified Marshall scoring system. In the 2012 Revised Atlanta Classification of AP (24), the presence of organ failure is a critical indicator of AP severity. Transient or persistent organ failure is important for differentiating the degrees of AP severity and their classification.
Construction of new groups
AP can be graded as the mild (subgroups A1, A1*) and the moderate and severe (subgroups A2, A2*) according to the MRSI or MMRSI, and was graded as the mild (subgroup B1) and the severe (subgroup B2) according to the APACHE II scoring system. In this study, we devised a novel grouping method based on combining the radiologic and APACHE II scoring systems. Hence, our new groups included group 1 (A1B1), group 2 (A1B2), group 3 (A2B1), group 4 (A2B2), group 1* (A1*B1), group 2* (A1*B2), group 3* (A2*B1), and group 4* (A2*B2), as shown in Figure S2.
Statistical analysis
MRI data are expressed as the average of the two observers’ findings. Kappa statistic was used to assess the interrater reliability between the two reviewers. Continuous variables are presented as the mean or median. Bivariate variables were compared using independent samples t-tests, Mann-Whitney U tests, or Wilcoxon tests. Rank and categorical variables are presented as frequencies and percentages and were compared using the χ2 test. The clinical and MRI variables were examined using multivariate logistic regression analyses and receiver operating characteristic (ROC) curve analysis. All statistical tests were calculated using SPSS v. 13.0 software (IBM Corp., Armonk, NY, USA). ROC analyses were performed using MedCalc v. 7.2.1.0 (MedCalc Software, Mariakerke, Belgium). A value of P<0.05 was considered statistically significant.
Results
Patient characteristics
The final study sample consisted of 363 patients with AP, 197 of them were male (54.27%) and 166 female (45.73%), with a mean age of 47.97±14.49 and 53.63±16.50 years, respectively. The etiology of AP included gallstones in 54.27% (197/363), hypertriglyceridemia in 22.87% (83/363), alcohol abuse in 8.0% (29/363), idiopathic causes in 6.33% (23/363), and other causes in 8.54% (31/363) of patients.
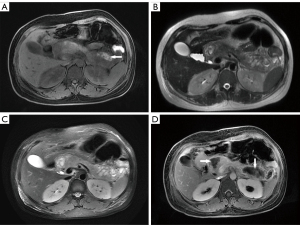
Of the 363 patients with AP, 306 patients (84.30%) had interstitial edematous pancreatitis and 57 patients (15.70%) had necrotizing pancreatitis. Of the 57 patients with necrotizing pancreatitis, 7.02% (4/57) had pancreatic necrosis alone, 38.60% (22/57) had extrapancreatic necrosis alone, and 54.39% (31/57) had combined necrosis according to the 2012 Revised Atlanta Classification proposed subtypes of necrotizing pancreatitis (Figure 1A-1D).
The interobserver agreement regarding the MRSI (k=0.89), MMRSI (k=0.91) and EPIM (k=0.83) were very good (all P<0.01). According to the MRSI, AP was graded as mild in 143 (39.40%), moderate in 213 (58.68%), and severe in 7 (1.93%) cases. For the MMRSI, AP was graded as mild in 54 (14.88%), moderate in 273 (75.21%), and severe in 36 (9.92%) cases. The EPIM score was 3.78±1.95, with a range of 1 to 7. AP was graded as mild in 301 (82.92%) and severe in 62 (17.08%) cases, according to the APACHE II scoring system.
Comparison of clinical characteristics and incidence of severe pancreatitis and SIRS in the new grouping system
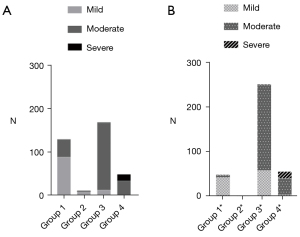
Of the 363 patients with AP, group 1, group 2, group 3, and group 4 had 131 (36.09%), 12 (3.31%), 170 (46.83%), and 50 (13.77%) patients, respectively. Group 1*, group 2*, group 3*, and group 4* had 50 (13.77%), 4 (1.10%), 252 (69.42%), and 57 (15.70%) patients, respectively. Group 3 and group 3* were the largest groups in each of their respective categories. The clinical characteristics in the categories are shown in Table 2. The BISAP score and the length of hospital stay in the group 4 and group 4* (patients with a high MRI score and a high APACHE II score) were significantly higher than those of the other three groups (all P<0.05). The level of high-sensitivity C-reactive protein (hs-CRP) was highest in group 4 and group 4*, but only some of the groups had statistically significant differences (group 1 vs. group 4, group 1* vs. group 4*, group 3* vs. group 4*). From group 1 (or group 1*) to group 4 (group 4*), the calcium level gradually decreased, but only some of the groups had statistically significant differences (group 1 vs. group 4, group 1* vs. group 4*). According to the 2012 Revised Atlanta Classification, the severity of AP was graded as mild in 115 (31.68%), moderately severe in 231 (63.64%), and severe in 17 (4.68%) cases. The prevalence of severe AP in group 4 and group 4* was significantly higher than that in the other three groups (Table 2 and Figure 2A,2B), as the same as the incidence of SIRS (all P<0.05).
Table 2
| Characteristics | All patients (n=363) | Group (MRSI-APACHE II) | Group (MMRSI-APACHE II) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 (n=131) | Group 2 (n=12) | Group 3 (n=170) | Group 4 (n=50) | Group 1* (n=50) | Group 2* (n=4) | Group 3* (n=252) | Group 4* (n=57) | |||
| Sex (male/female) | 197/166 | 71/60 | 5/7 | 98/72 | 23/27 | 26/24 | 1/3 | 144/108 | 26/31 | |
| Age (years), mean ± SD | 50.56±15.68 | 49.25±16.27 | 69.00±10.98 | 48.98±14.38 | 54.96±16.15 | 48.00±16.33 | 74.50±5.00 | 49.38±15.00 | 56.35±16.07 | |
| Etiology, n | ||||||||||
| Gallstones | 197 | 70 | 9 | 87 | 31 | 26 | 4 | 131 | 36 | |
| Hypertriglyceridemia | 83 | 25 | 0 | 48 | 10 | 8 | 0 | 65 | 10 | |
| Alcohol abuse | 29 | 10 | 1 | 13 | 5 | 6 | 0 | 18 | 5 | |
| Idiopathic cause | 23 | 13 | 2 | 7 | 1 | 3 | 0 | 17 | 3 | |
| Others | 31 | 13 | 0 | 15 | 3 | 7 | 0 | 21 | 3 | |
| BISAP, median [range] | 1 [0–4] | 1 [0–4]# | 1 [0–2]# | 1 [0–4]# | 2 [0–4] | 0 [0–3]# | 1 [0–1]# | 1 [0–4]# | 2 [0–4] | |
| Calcium (mmol/L), mean ± SD | 2.25±0.17 | 2.30±0.14# | 2.29±0.15 | 2.24±0.18 | 2.20±0.20 | 2.31±0.14# | 2.29±0.09 | 2.26±0.17 | 2.20±0.20 | |
| Hospital stay (days), median [range] | 12 [4–43] | 9 [4–29]# | 12 [4–20]# | 12 [4–36]# | 17 [9–43] | 9 [4–22]# | 12 [4–13]# | 11 [4–36]# | 16 [6–43] | |
| hs-CRP (mg/L), median [range] | 31.62 [0–278.69] | 15.57 [0.08–275]# | 34.39 [0.24–102.14] | 45.79 [0.22–277.66] | 54.06 [1.12–278.69] | 13.10 [0.15–275.00]# | 15.36 [0.47–88] | 36.95 [0.08–277.66]# | 52.65 [1.12–278.69] | |
| Severity of AP, n | ||||||||||
| Mild | 115 | 90 | 10 | 14 | 1 | 45 | 4 | 60 | 6 | |
| Moderate | 231 | 40 | 2 | 155 | 34 | 4 | 0 | 191 | 36 | |
| Severe | 17 | 1 | 0 | 1 | 15 | 1 | 0 | 1 | 15 | |
| SIRS, n | ||||||||||
| (+) | 144 | 20 | 6 | 74 | 44 | 3 | 2 | 92 | 47 | |
| (−) | 219 | 111 | 6 | 96 | 6 | 47 | 2 | 160 | 10 | |
Severity of AP based on the Revised Atlanta Classification. #, a statistically significant difference compared to group 4 or group 4*. SD, standard deviation; BISAP, Bedside Index of Severity in Acute Pancreatitis; hs-CRP, high-sensitivity C-reactive protein; AP, acute pancreatitis; SIRS, systemic inflammatory response syndrome; MRSI, magnetic resonance severity index; APACHE II, Acute Physiology and Chronic Health Evaluation II; MMRSI, modified magnetic resonance severity index.
Logistic regression models predicting the severity of AP according to the Revised Atlanta Classification
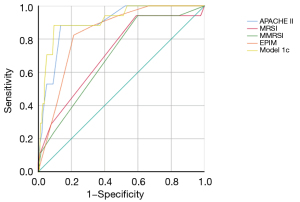
We built logistic regression models to predict severe AP by combining the MRI and clinical evaluation scoring systems. APACHE II and MRSI were combined and labeled as model 1a, APACHE II and MMRSI were combined and labeled as model 1b, and APACHE II and EPIM were combined and labeled as model 1c. Only model 1c was built successfully, and the regression equation was as follows: logit(y) = −8.601 + 0.417 × (APACHE II score) + 0.528 × (EPIM score). The odds ratio (OR) values of the APACHE II and EPIM were 1.518 [95% confidence interval (CI): 1.234–1.852] and 1.695 (95% CI: 1.112–2.582), respectively. The area under the ROC curve (AUC) for model 1c was 0.912 (95% CI: 0.844–0.980), higher than that of the above single parameters (Figure 3). The AUC for MRSI, MMRSI, EPIM, and APACHE II were, respectively, 0.715, 0.694, 0.836, and 0.896. Compared to the single parameter, there were significant differences between model 1c and MRSI/MMRSI, but not for EPIM and APACHE II.
Comparison of the occurrence of SIRS complications and logistic regression modelling predictions of SIRS complications
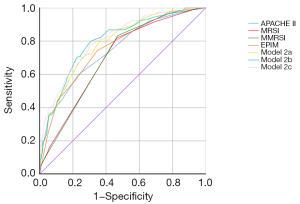
SIRS occurred in 144 (39.67%) patients. The occurrence of SIRS was as high as 88.00% (44/50) in group 4, which was similar to the result in group 4* (Table 2 and Figure 4A,4B). The logistic regression models were built as described previously. APACHE II and MRSI were combined and labeled as model 2a, APACHE II and MMRSI were combined and labeled as model 2b, and APACHE II and EPIM were combined and labeled as model 2c. All models were built successfully, and all the regression coefficient-related image parameters were higher than those of the APACHE II parameters (2a: 0.468 vs. 0.320; 2b: 0.388 vs. 0.318; 2c: 0.414 vs. 0.294) in these regression equations. The AUC of model 2c was the highest than these single parameters (AUC =0.806; P<0.05), but there were no significant differences between these models (Tables 3,4 and Figure 5).

Table 3
| Model | Regression equation | OR (95% CI) | OR’ (95% CI) |
|---|---|---|---|
| 2a | Logit(y) = −3.745 + 0.320 × (APACHE II score) + 0.468 × (MRSI score) | 1.377 (1.245–1.532) | 1.597 (1.271–2.006) |
| 2b | Logit(y) = −4.097 + 0.318 × (APACHE II score) + 0.388 × (MMRSI score) | 1.375 (1.242–1.522) | 1.473 (1.256–1.728) |
| 2c | Logit(y) = −3.583 + 0.294 × (APACHE II score) + 0.414 × (EPIM score) | 1.342 (1.210–1.488) | 1.513 (1.317–1.738) |
OR and OR’ represented respectively APACHE II score-related and image-related (MRSI, MMRSI, EPIM) parameters. SIRS, systemic inflammatory response syndrome; APACHE II, Acute Physiology and Chronic Health Evaluation II; MRSI, magnetic resonance severity index; MMRSI, modified magnetic resonance severity index; EPIM, extrapancreatic inflammation on magnetic resonance; OR, odds ratio; CI, confidence interval.
Table 4
| Parameter/model | AUC | 95% CI | Cutoff | Sensitivity, % | Specificity, % |
|---|---|---|---|---|---|
| MRSI | 0.698 | 0.648 to 0.745 | >3 | 81.94 | 53.42 |
| MMRSI | 0.701 | 0.651 to 0.748 | >4 | 83.33 | 52.97 |
| EPIM | 0.756 | 0.708 to 0.799 | >3 | 74.31 | 65.30 |
| APACHE II | 0.748 | 0.700 to 0.791 | >5 | 59.72 | 76.71 |
| 2a | 0.788 | 0.742 to 0.829 | >0.4262 | 70.14 | 74.43 |
| 2b | 0.798 | 0.753 to 0.838 | >0.3458 | 79.86 | 69.41 |
| 2c | 0.806 | 0.761 to 0.845 | >0.3428 | 77.78 | 69.86 |
SIRS, systemic inflammatory response syndrome; MRSI, magnetic resonance severity index; MMRSI, modified magnetic resonance severity index; EPIM, extrapancreatic inflammation on magnetic resonance; APACHE II, Acute Physiology and Chronic Health Evaluation II; AUC, area under the ROC curve; ROC, receiver operating characteristic; OR, odds ratio; CI, confidence interval.
Discussion
In this study, we found that the combination of the MRI and APACHE II scoring systems to assess the severity of AP was feasible and more precise than the other scoring systems. The group 4 and group 4* participants sustained more clinically severe pancreatitis, which manifest as high MRSI (or MMRSI) and high APACHE II scores, and they were more likely to develop SIRS and have a longer hospital stay. To our knowledge, we are the first to develop a new model of combined MR scoring systems and clinical scoring systems. We found that all of the models achieved significantly high accuracy in the early prediction of AP severity compared to those models relying on only selected single scoring system. Moreover, the imaging scoring system had a more important role than the clinical scoring system. Hence, our models have the potential to support the early prediction of AP severity and to identify patients for whom close management or aggressive interventions can be considered.
The MRI and the APACHE-II scoring systems were recruited as major indicators in our study for the following reasons. Firstly, as is widely known, CT is a commonly used tool, but, compared to CT, MRI has been shown to be superior due to its superior tissue contrast resolution, especially for verifying the spread of extrapancreatic inflammation (10,11,25). So, MRI can detect mild alterations or mild AP that may be overlooked on CT. Secondly, MRI is safe and without radiation. Thirdly, the MRI scoring system can provide MRSI, EPIM, and MMRSI scores, which can better evaluate extrapancreatic necrosis and extrapancreatic inflammation. Finally, the application of the clinical values of the APACHE-II scores in the early prediction of AP severity has been well documented. Although the BISAP is convenient for a quick assessment, we did not include it in our models due to its relatively low sensitivity rate. The APACHE II scoring system was used to gauge the physiologic response to the inflammatory cascade in AP, which was related to systemic complications, whereas image parameters could assess the morphologic alteration that reflected local complications. Although the process of gauging the APACHE II score was relatively cumbersome, the APACHE II score has been shown to be a proven predictor of severe AP in the early stage and has been widely used (19).
The CRP and calcium levels are related to the progression of SAP (26), with hs-CRP levels being shown to increase nonspecifically in the event of inflammation in the body (27). In our new groups, patients in group 4 and group 4* with clinically relevant indicators all showed more severe AP, such as higher BISAP and hs-CRP and lower serum calcium level, and these patients were more likely to develop SIRS and have a longer hospital stay. Indeed, it was clinically obvious that these patients had poor local and systemic conditions. We have proposed a method by which clinicians can more accurately ascertain a patient’s condition in the early stages of AP. These models we designed were not only successful, but also more accurate than the other models. The performance of model 1c, which was derived from combining APACHE II and EPIM scores, for evaluating the severity of AP, was good, with an AUC of 0.912 (95% CI: 0.844–0.980), higher than any single parameter. In all the models used for predicting SIRS complications, the performance of model 2c, which was derived from the combination of APACHE II and EPIM scores, was the highest, with an AUC of 0.806 (95% CI: 0.761–0.845). The reasons for the success of our scoring system may be that the appearance of extrapancreatic inflammation is more pronounced than the morphological changes of the pancreas itself in the early stage of AP. Our previous research has also confirmed this point demonstrating the EPIM score to be more helpful in evaluating the severity of AP than either the MRSI and MMRSI in the early stage of AP (7,20). Another possible reason may be that MRI is more sensitive than CT in detecting slight changes of mild inflammation and effusion (28,29).
Furthermore, as we know, a value of OR >1 represents a risk factor. It was worth noting that all OR values in this study were greater than 1, and the related image parameters were higher than those of the corresponding APACHE II scores in all of the regression equations. This result reveals that the image parameters played an important role in the early prediction of AP severity and outcome, and were even superior to the clinical parameters, similar to the findings of a previous study (30). Thus, these results emphasize the importance of the associated image parameters, especially relating to peripancreatic changes, in the determination of AP prognosis.
There is much in the published literature concerning the use of these scoring systems in evaluating the severity of AP. We confirmed the value of the MRSI, MMRSI, EPIM, and APACHE II scoring systems for predicting outcomes in AP patients, and our findings are in line with previous research (16,20,31). The main strength or innovation of this study is that we constructed a new model of the early prediction of AP of using MR and APACHE II scoring systems and used the model to evaluate the severity of AP in its early stage. This is not only an innovation of a method, but also an important instantiation of multidisciplinary cooperation in disease diagnosis and treatment. The main limitation of our study is that this study was retrospective and some clinical parameters were incomplete, which might have caused some patients to have become lost from the study, thus influencing the results. Another limitation was the lack of validation of these models; hence, we plan to conduct a further prospective study with a larger sample size.
In conclusion, the newly constructed models for the early prediction of the outcome of AP using the MRI and APACHE II scoring systems proved viable, and the model using the EPIM and APACHE II scoring systems worked best. These new models would be helpful for clinicians in evaluating the conditions of AP patients more comprehensively and formulating informed diagnoses and treatment plans.
Acknowledgments
The authors would like to thank the funding agency and all the participants for participating in this study.
Funding: This study was supported by the Bureau of Science and Technology Nanchong City (No. 20SXQT0250) and the North Sichuan Medical College (No. CBY20-QA-Z07).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-158/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-158/coif). All authors report that this study was supported by the Bureau of Science and Technology Nanchong City (No. 20SXQT0250) and the North Sichuan Medical College (No. CBY20-QA-Z07). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the institutional review board of our hospital (No. 2019 ER[A] 223) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet 2020;396:726-34. [Crossref] [PubMed]
- Roberts SE, Morrison-Rees S, John A, Williams JG, Brown TH, Samuel DG. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology 2017;17:155-65. [Crossref] [PubMed]
- Schepers NJ, Bakker OJ, Besselink MG, Ahmed Ali U, Bollen TL, Gooszen HG, van Santvoort HC, Bruno MJDutch Pancreatitis Study Group. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut 2019;68:1044-51. [Crossref] [PubMed]
- Rocha APC, Schawkat K, Mortele KJ. Imaging guidelines for acute pancreatitis: when and when not to image. Abdom Radiol (NY) 2020;45:1338-49. [Crossref] [PubMed]
- Porter KK, Zaheer A, Kamel IR, Horowitz JM, Arif-Tiwari H, Bartel TB, Bashir MR, Camacho MA, Cash BD, Chernyak V, Goldstein A, Grajo JR, Gupta S, Hindman NM, Kamaya A, McNamara MM, Carucci LR. ACR Appropriateness Criteria® Acute Pancreatitis. J Am Coll Radiol 2019;16:S316-30. [Crossref] [PubMed]
- Porter KK, Cason DE, Morgan DE. Acute Pancreatitis: How Can MR Imaging Help. Magn Reson Imaging Clin N Am 2018;26:439-50. [Crossref] [PubMed]
- Zhou T, Chen Y, Wu JL, Deng Y, Zhang J, Sun H, Lan C, Zhang XM. Extrapancreatic Inflammation on Magnetic Resonance Imaging for the Early Prediction of Acute Pancreatitis Severity. Pancreas 2020;49:46-52. [Crossref] [PubMed]
- Tang MY, Chen TW, Bollen TL, Wang YX, Xue HD, Jin ZY, Huang XH, Xiao B, Li XH, Ji YF, Zhang XM. MR imaging of hemorrhage associated with acute pancreatitis. Pancreatology 2018;18:363-9. [Crossref] [PubMed]
- Jiang ZQ, Xiao B, Zhang XM, Xu HB. Early-phase vascular involvement is associated with acute pancreatitis severity: a magnetic resonance imaging study. Quant Imaging Med Surg 2021;11:1909-20. [Crossref] [PubMed]
- Xiao B, Xu HB, Jiang ZQ, Zhang J, Zhang XM. Current concepts for the diagnosis of acute pancreatitis by multiparametric magnetic resonance imaging. Quant Imaging Med Surg 2019;9:1973-85. [Crossref] [PubMed]
- Dhaka N, Samanta J, Kochhar S, Kalra N, Appasani S, Manrai M, Kochhar R. Pancreatic fluid collections: What is the ideal imaging technique? World J Gastroenterol 2015;21:13403-10. [Crossref] [PubMed]
- Mortele KJ, Wiesner W, Intriere L, Shankar S, Zou KH, Kalantari BN, Perez A, vanSonnenberg E, Ros PR, Banks PA, Silverman SG. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol 2004;183:1261-5. [Crossref] [PubMed]
- De Waele JJ, Delrue L, Hoste EA, De Vos M, Duyck P, Colardyn FA. Extrapancreatic inflammation on abdominal computed tomography as an early predictor of disease severity in acute pancreatitis: evaluation of a new scoring system. Pancreas 2007;34:185-90. [Crossref] [PubMed]
- Mederos MA, Reber HA, Girgis MD. Acute Pancreatitis: A Review. JAMA 2021;325:382-90. [Crossref] [PubMed]
- Simoes M, Alves P, Esperto H, Canha C, Meira E, Ferreira E, Gomes M, Fonseca I, Barbosa B, Costa JN. Predicting Acute Pancreatitis Severity: Comparison of Prognostic Scores. Gastroenterology Res 2011;4:216-22. [Crossref] [PubMed]
- Harshit Kumar A, Singh Griwan M. A comparison of APACHE II, BISAP, Ranson's score and modified CTSI in predicting the severity of acute pancreatitis based on the 2012 revised Atlanta Classification. Gastroenterol Rep (Oxf) 2018;6:127-31. [Crossref] [PubMed]
- Bollen TL, Singh VK, Maurer R, Repas K, van Es HW, Banks PA, Mortele KJ. Comparative evaluation of the modified CT severity index and CT severity index in assessing severity of acute pancreatitis. AJR Am J Roentgenol 2011;197:386-92. [Crossref] [PubMed]
- Hong W, Dong L, Huang Q, Wu W, Wu J, Wang Y. Prediction of severe acute pancreatitis using classification and regression tree analysis. Dig Dis Sci 2011;56:3664-71. [Crossref] [PubMed]
- Choi HW, Park HJ, Choi SY, Do JH, Yoon NY, Ko A, Lee ES. Early Prediction of the Severity of Acute Pancreatitis Using Radiologic and Clinical Scoring Systems With Classification Tree Analysis. AJR Am J Roentgenol 2018;211:1035-43. [Crossref] [PubMed]
- Zhou T, Tang MY, Deng Y, Wu JL, Sun H, Chen Y, Chen TW, Zhang XM. MR Imaging for Early Extrapancreatic Necrosis in Acute Pancreatitis. Acad Radiol 2021;28:S225-33. [Crossref] [PubMed]
- Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990;174:331-6. [Crossref] [PubMed]
- Peng R, Zhang L, Zhang ZM, Wang ZQ, Liu GY, Zhang XM. Chest computed tomography semi-quantitative pleural effusion and pulmonary consolidation are early predictors of acute pancreatitis severity. Quant Imaging Med Surg 2020;10:451-63. [Crossref] [PubMed]
- Bollen TL. Imaging of acute pancreatitis: update of the revised Atlanta classification. Radiol Clin North Am 2012;50:429-45. [Crossref] [PubMed]
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SSAcute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11. [Crossref] [PubMed]
- Sandrasegaran K, Heller MT, Panda A, Shetty A, Menias CO. MRI in acute pancreatitis. Abdom Radiol (NY) 2020;45:1232-42. [Crossref] [PubMed]
- Tachyla SA, Marochkov AV, Lipnitski AL, Nikiforova YG. The prognostic value of procalcitonin, C-reactive protein and cholesterol in patients with an infection and multiple organ dysfunction. Korean J Anesthesiol 2017;70:305-10. [Crossref] [PubMed]
- Park SW, Park SS, Kim EJ, Sung WS, Ha IH, Jung B. Sex differences in the association between self-rated health and high-sensitivity C-reactive protein levels in Koreans: a cross-sectional study using data from the Korea National Health and Nutrition Examination Survey. Health Qual Life Outcomes 2020;18:341. [Crossref] [PubMed]
- Shyu JY, Sainani NI, Sahni VA, Chick JF, Chauhan NR, Conwell DL, Clancy TE, Banks PA, Silverman SG. Necrotizing pancreatitis: diagnosis, imaging, and intervention. Radiographics 2014;34:1218-39. [Crossref] [PubMed]
- Sun H, Jian S, Peng B, Hou J. Comparison of magnetic resonance imaging and computed tomography in the diagnosis of acute pancreatitis: a systematic review and meta-analysis of diagnostic test accuracy studies. Ann Transl Med 2022;10:410. [Crossref] [PubMed]
- Leung TK, Lee CM, Lin SY, Chen HC, Wang HJ, Shen LK, Chen YY. Balthazar computed tomography severity index is superior to Ranson criteria and APACHE II scoring system in predicting acute pancreatitis outcome. World J Gastroenterol 2005;11:6049-52. [Crossref] [PubMed]
- Sharma V, Rana SS, Sharma RK, Kang M, Gupta R, Bhasin DK. A study of radiological scoring system evaluating extrapancreatic inflammation with conventional radiological and clinical scores in predicting outcomes in acute pancreatitis. Ann Gastroenterol 2015;28:399-404. [PubMed]


