
Right-to-left shunts in unexplained syncope: an age- and sex-matched case-control study
Introduction
Syncope is a transient loss of consciousness (LOC) due to transient global cerebral hypoperfusion with rapid onset, short duration, and spontaneous complete recovery (1), and is a clinically common presentation. The Framingham Heart Study observed that the incidence of syncope is 6.2 per 1,000 person-years (2). A Dutch study found that in the general population, the lifetime cumulative incidence of syncope is 35% (3). According to the European syncope guidelines (version 2009) (1), syncope is pathophysiologically classified into 3 categories: reflex syncope, syncope due to orthostatic hypotension, and cardiac syncope. Disorders with LOC that are not caused by global cerebral hypoperfusion, such as epilepsy, vertebrobasilar transient ischemic attack, and hypoglycemia, were listed as differential diagnoses (1). Despite a thorough medical history inquiry and relevant examination, up to 30–50% of patients with syncope leave hospital without a clear explanation of cause, which is called unexplained syncope (2,4). Unexplained syncope does not indicate the absence of risk. Prospective cohort studies have observed an increased risk for both all-cause death [hazard ratio (HR) =1.22] and cardiovascular death (HR =1.72) in people with unexplained syncope in comparison to non-syncope cases (2,5). Therefore, exploration of the underlying causes is of great significance for people with unexplained syncope to enable them to take precautionary measures.
Right-to-left shunt (RLS) indicates abnormal communication between the right circulation and the left circulation (venous to arterial). It occurs as a sequela of structural abnormalities at both cardiac and extracardiac levels, such as patent foramen ovale (PFO), atrial/ventricular septal defects, and pulmonary arteriovenous malformations or fistulae (PAVMs), among which PFO is the most frequent cause (6). Cryptogenic stroke and migraine are the most widely studied clinical relevance of RLS (7,8). A previous study found that cerebral autoregulation (a control mechanism maintaining cerebral blood flow despite changes in arterial blood pressure) was impaired in RLS, potentially leading to cerebral hypoperfusion (9). Cerebral autoregulation may play a part in the pathophysiological mechanisms of syncope (10,11). A few case reports have shown that RLS is related to syncope (12,13). Platypnea-orthodeoxia and hypoxemia may account for this link (12-15). Therefore, RLS is suspected to be related to unexplained syncope, but limited supportive evidence is available (13).
Cerebral small vessel diseases (SVD) comprise a syndrome identified by neuroimaging, pathological, and clinical findings that is thought to arise from diseases affecting small vessels in the brain (16). During brain magnetic resonance imaging (MRI), SVD presents as white matter hyperintensity (WMH), enlarged perivascular spaces (EPVS), lacunes, and cerebral microbleeds (CMBs) (16). Both syncope (17) and RLS (18) have been observed to be related with SVD in the brain. Therefore, cerebral SVD is a potential confounding factor when investigating the association between RLS and syncope.
Here, we used the transcranial Doppler ultrasonography (TCD) bubble test to ascertain the presence and degree of RLS and investigated the association between RLS and unexplained syncope in an age-and gender-matched case study containing patients with unexplained syncope and a control group that was free of syncope. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1060/rc).
Methods
Sample size calculation
It is estimated that RLS can be found in 15–25% of the general population (19); however, the prevalence of RLS in patients with unexplained syncope is still unclear. The clinical relevance of RLS mainly involves migraine and cryptogenic stroke (ischemic stroke with unknown causes) (7,8,20). Therefore, we refer to the prevalence of RLS in cases of migraine [ranging from 48% to 67% (18,21)] or cryptogenic stroke [ranging from 42% to 66% (22,23)] as an estimation/reference of the prevalence of RLS in cases of unexplained syncope. We used a significance level of α=0.05 and an equal sample size between cases and controls. A 20% probability of the estimated prevalence of RLS in controls, and a 50% estimated prevalence of RLS in cases) were considered sufficiently different to warrant rejecting the null hypothesis. Accordingly, the required sample size to achieve an 80% power (β=0.2) was determined to be 39 participants in each group (39 cases and 39 controls; G*Power 3.1.9.2) (24).
Cases and clinical approach to making diagnostic decisions
Patients visited the outpatient clinics of the Department of Neurology at the first Affiliated Hospital of Nanjing Medical University. Our clinical approach to making diagnostic decisions regarding syncope patients was established according to the European Society of Cardiology (ESC) guidelines and a previously verified diagnostic pathway with a diagnostic accuracy of 88% (1,25,26). From January 2019 to February 2020, all syncope patients underwent a standard initial evaluation including a thorough medical history inquiry (according to the ESC guidelines), careful physical examination, active stand test, and a resting standard 12-lead electrocardiogram (ECG). Based on the initial evaluation, a certain, highly likely, or no diagnosis of a particular category (reflex syncope, syncope due to orthostatic hypotension, or cardiac syncope) was made. A highly likely diagnosis received a further work-up aiming to identify the suspected cause (e.g., additional cardiological tests and autonomic function tests, as appropriate). Patients with no diagnosis received additional testing (e.g., 24 h Holter ECG, echocardiogram, electroencephalogram, brain MRI, and TCD) in accordance with the ESC guidelines for further evaluation or differential diagnosis (e.g., epilepsy) (1). In addition, a cardiologist reviewed the medical data. The unexplained syncope diagnosis was made via consensus of a neurologist and cardiologist.
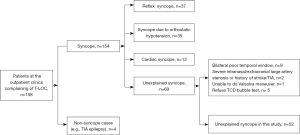
From January 2019 to February 2020, consecutive unexplained syncope patients were recruited. The case inclusion criteria were as follows: (I) age ≥15 years; and (II) unexplained syncope according to the diagnostic pathway above. The case exclusion criteria were as follows: (I) severe intracranial/extracranial large artery stenosis or brain tumor; (II) a history of acute stroke/transient ischemic attack; (III) inability to perform the Valsalva maneuver; and (IV) bilateral poor temporal window on TCD. In addition, age- (within 1 year) and gender-matched controls (free of syncope) were selected from consecutive patients with dizziness at the outpatient clinics of the Department of Neurology in the First Affiliated Hospital of Nanjing Medical University, from January 2019 to February 2020. The inclusion criteria in controls were as follows: (I) age ≥15 years old; and (II) dizziness upon presentation. The exclusion criteria in controls were as follows: (I) patients with syncope; (II) patients with headache; (III) patients with severe intracranial/extracranial large artery stenosis or brain tumor; (IV) patients with a history of acute stroke/transient ischemic attack; (V) patients unable to perform the Valsalva maneuver; and (VI) patients with a bilaterally poor temporal window on TCD. All methods were performed in accordance with the relevant guidelines and regulations according to the Declaration of Helsinki (as revised in 2013) and the Nanjing Medical University policies. Ethical approval was obtained from the Nanjing Medical University and written informed consent was provided by each participant.
Demography and vascular risk factors
We collected data on basic demographic (age and gender) and vascular risk factors. Hypertension, diabetes mellitus (DM), and hyperlipidemia were defined according to our previous criteria (27). Smoking and alcohol intake were dichotomized as ever or never smoked and ever or never drank alcohol.
TCD bubble test
We evaluated RLS using an MVU-6300 multifunctional vascular ultrasonic instrument equipped with XL2 intelligent TCD bubble test voice guidance software (Delica Medical Equipment, Shenzhen, China). The presence of RLS was detected both at rest and after the Valsalva maneuver. If RLS occurred during rest, it was considered persistent, and latent if it only occurred after a Valsalva maneuver. A mixture of 9 mL isotonic saline with 1 mL air and 0.5 mL of the patient’s blood was made. After 30 mixing cycles with two 10 mL syringes, this mixture was injected as a rapid bolus into the patient’s right antecubital vein, and the left middle cerebral artery (MCA) spectrum was recorded. If there was an RLS, typical high-intensity transient signals [microbubbles (MB)] were detected during TCD monitoring of the MCA (Figure 1). The degree of RLS was categorized into 5 grades (Grade 0–4) according to the number of MB: no shunt (Grade 0), <10 MB (Grade 1), 11–25 MB (Grade 2), >25 MB single spots pattern (Grade 3), and MB shower/curtain pattern (Grade 4) (28). Grades 3 and 4 were regarded as severe RLS. The TCD bubble test is extremely accurate, with sensitivity and specificity of almost 100% compared to the currently considered standard reference transesophageal echocardiography for detection of RLS (20). A recent study showed that the TCD bubble test can even have a higher sensitivity to detect RLS than transthoracic or transesophageal echocardiography (29).
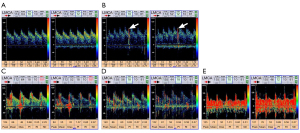
Neuroimaging examinations of SVD
Brain MRI (3.0 Tesla, TIM Trio; Siemens, Erlangen, Germany) was performed to detect SVD. We rated WMH, lacunes, and EPVS according to our previously published criteria (27). All ratings were performed blind to patients’ clinical data. Both intra- and inter-observer agreements between 2 raters were good or excellent when rating these neuroimaging markers on 30 randomly selected patients [the intraclass correlation coefficient (ICC) ranged from 0.81 to 0.99]. The presence of lacunes, WMH, or EPVS was defined according to the previous criteria (30). One point was awarded for each of the above 3 neuroimaging presences, which comprised a score ranging from 0 to 3 to reflect the total burden of cerebral SVD (27,30).
Statistics
Data were presented as means (standard deviations) or frequencies (percentages). Comparisons between cases and controls were carried out using a Students’ t-test, Mann–Whitney U test, and the chi-square or Fisher’s exact test, as appropriate. Variables in univariate analysis with a P<0.2 were entered into a multivariate conditional logistic regression, which was used to investigate the association between RLS and unexplained syncope. We used R software (The R Foundation for Statistical Computing, Vienna, Austria), and α was set at 0.05 for all statistical analyses.
Results
This study recruited 104 participants, including 52 cases (unexplained syncope; Figure 2) and 52 controls (free of syncope). The mean age was 42.5 years, with 20 (38.5%) males in each group. Among the 52 cases, 4 patients had migraines. Among these 4 migraineurs, 1 had frequent visual aura. Among the 52 cases, 20 (38.5%) participants had experienced syncope only once, 15 (28.8%) had experienced it twice, 5 (9.6%) had experienced 3 episodes, 10 (19.2%) had experienced 4 episodes, 1 (1.9%) had experienced 6 episodes, and 1 (1.9%) had experienced 7 episodes.
The comparison of clinical characteristics between cases and matched controls is shown in Table 1. Among the 52 cases, 11 (21.2%) participants had hypertension, 3 (5.8%) had diabetes, 15 (28.8%) had hyperlipidemia, 7 (13.5%) were smokers, and 1 (1.9%) was an alcohol consumer. Among the 52 controls, 8 (15.4%) had hypertension, 2 (3.8) had diabetes, 16 (30.8) had hyperlipidemia, 9 (17.3%) were smokers, and 3 (5.8%) were alcohol consumers. There were no differences between cases and controls in terms of these clinical factors. In terms of cerebral SVD, cases had a higher burden (P=0.018); 19 participants had at least 1 neuroimaging marker of SVD compared with 7 in the controls. Cases more frequently exhibited RLS than did controls (P=0.013), including 7 Grade 1, 2 Grade 2, 6 Grade 3, and 10 Grade 4 cases of RLS. In contrast, the controls had 6 Grade 1, 2 Grade 2, 1 Grade 3, and 2 Grade 4 cases of RLS. Among 25 cases with RLS, 14 instances were persistent, while the other 11 were latent. Among 11 of the controls with RLS, 7 instances were persistent, while the other 4 were latent (Table 1; Table S1). The prevalence of any RLS (Grade 1–4) was 48.1% (25/52) and 21.2% (11/52) in cases and controls (P=0.004), respectively. The prevalence of severe RLS (Grade 3 and 4) was significantly higher in cases than that in controls (16/52 vs. 3/52; P=0.001). There was no difference (P=0.323) in the prevalence of non-severe RLS (Grade 1 and 2) between cases (n=9) and controls (n=8).
Table 1
| Variables | Cases (n=52) | Control (n=52) | P value |
|---|---|---|---|
| Age (years) | 42.5 (17.4) | 42.4 (17.1) | 0.986 |
| Male, n (%) | 20 (38.5) | 20 (38.5) | 1.000 |
| Hypertension, n (%) | 11 (21.2) | 8 (15.4) | 0.446 |
| Diabetes, n (%) | 3 (5.8) | 2 (3.8) | 0.647 |
| Hyperlipidemia, n (%) | 15(28.8) | 16(30.8) | 0.830 |
| Smoking, n (%) | 7 (13.5) | 9 (17.3) | 0.587 |
| Alcohol consumption, n (%) | 1 (1.9) | 3 (5.8) | 0.618 |
| Cerebral SVD burden, n (%) | 0.018 | ||
| 0 | 33 (63.5) | 45 (86.5) | |
| 1 | 10 (19.2) | 6 (11.5) | |
| 2 | 5 (9.6) | 1 (1.9) | |
| 3 | 4 (7.7) | 0 (0) | |
| RLS, n (%) | 0.013 | ||
| Grade 0 | 27 (51.9) | 41 (78.8) | |
| Grade 1 | 7 (13.5) | 6 (11.5) | |
| Grade 2 | 2 (3.8) | 2 (3.8) | |
| Grade 3 | 6 (11.5) | 1 (1.9) | |
| Grade 4 | 10 (19.2) | 2 (3.8) | |
| Presence of any RLS (Grade 1–4), n (%) | 25 (48.1) | 11 (21.2%) | 0.004 |
| Presence of severe RLS (Grade 3–4), n (%) | 16 (30.8) | 3 (5.8) | 0.001 |
Continuous and categorical variables are presented as mean (SD) and percentages, respectively. SVD, small vessel diseases; RLS, right-to-left shunt; SD, standard deviation.
Conditional logistic regression (Table 2) showed a significant association between RLS and unexplained syncope [odds ratio (OR) =1.988; 95% confidence interval (CI): 1.233–3.205; P=0.005] after adjusting for SVD burden. Further analysis revealed a bigger OR between severe RLS (Grade 3–4) and unexplained syncope (OR =8.699; 95% CI: 1.858–40.726; P=0.006; Table 2). Furthermore, SVD burden was associated with unexplained syncope (OR =4.973; 95% CI: 1.471–16.813; P=0.010). To exclude the possible confounding effect of migraine, we excluded these 4 pairs of participants (4 migraineurs and 4 corresponding controls), then re-analyzed conditional logistic regression, which showed similar results as above (data not shown).
Table 2
| Variables | OR (95% CI) | P value |
|---|---|---|
| RLS (Grade 0–4, as a continuous variable), together with SVD burden as independent variables in the model (n=104) | ||
| RLS | 1.988 (1.233–3.205) | 0.005 |
| Cerebral SVD burden | 4.973 (1.471–16.813) | 0.010 |
| Presence of severe RLS (Grade 3–4, yes or no), together with SVD burden as independent variables in the model (n=104) | ||
| Presence of severe RLS | 8.699 (1.858–40.726) | 0.006 |
| Cerebral SVD burden | 5.071 (1.524–16.869) | 0.008 |
Further adjusting for age and gender did not change this association. RLS, right-to-left shunt; SVD, small vessel diseases; OR, odds ratio; CI, confidence interval.
Discussion
This age- and gender-matched case-control study observed an association between RLS (exposure) and unexplained syncope (outcome). This association was mainly driven by severe RLS (Grade 3–4), and the severity of RLS was related to the risk of unexplained syncope. In addition, we also found an association between unexplained syncope and a higher burden of cerebral SVD.
The TCD bubble test is a reliable and sufficient way to diagnose RLS and has high sensitivity (97–100%) and specificity (93%) (20,29,31). In contrast, transesophageal echocardiography missed 15% of RLS cases that were detected using TCD, and 40% of those missed were large shunts (32). The association between TCD-detected RLS and unexplained syncope in our study shed light on a new evaluation strategy (e.g., the TCD bubble test) to find potential causes of syncope. However, transesophageal ultrasound examination has its advantages in the diagnostic evaluation of PFO and plays a pivotal role in the indications for PFO closure, assessment of varying anatomies, and post-procedure follow-up (33).
As the most frequent cause of RLS, PFO was found to be a common cause of cryptogenic stroke and migraine (7,8,20). It has also been reported to be related to sleep apnea (34). The prevalence of RLS in cryptogenic stroke ranged from 42% to 66% (22,23). The prevalence of RLS in migraine cases ranged from 48% to 67% (18,21). Case-control studies have also observed a higher frequency of PFO in patients with cryptogenic stroke or migraine with aura compared with controls (35,36). Paradoxical embolism (37,38), intracardiac formed thrombus (39), direct transport through RLS of some vasoactive substances (40), and cortical spreading depression (CSD) evoked by microembli (40,41) may explain the association between RLS and stroke/migraine. Furthermore, RLS was also reported to be related to SVD lesions (e.g., WMH and juxtacortical spots on MRI), and cardiac embolism may account for this phenomenon (42,43). However, other studies have not observed an association between RLS and SVD lesions (18,21,44,45). A study found that RLS was associated with SVD lesions only in the frontal lobe, instead of other locations like basal ganglia or deep white matter (46). Further studies are needed to investigate the clinical relevance of RLS. Closure of PFO in carefully selected cryptogenic stroke patients significantly reduced the risk of subsequent ischemic stroke compared to antiplatelet treatment alone (7). Furthermore, 3 randomized controlled trials explored the role of PFO closure on cases of migraine, but failed to meet their primary endpoints (e.g., 50% reduction in migraine attacks) (8,47,48); however, they did demonstrate some benefit of PFO closure (e.g., reduction in headache days) (8). A recent pooled analysis also demonstrated the safety and clinical benefits of PFO closure in patients with migraine (49). The link between RLS and unexplained syncope in our case-control study further expanded the clinical relevance of RLS.
Cerebral autoregulation is a control mechanism that maintains cerebral blood flow despite changes in arterial blood pressure. Cerebral autoregulation was found to be impaired in patients with RLS, and its degree of impairment was associated with the severity of the RLS (the more severe the RLS, the worse the cerebral autoregulation) (9). Although controversial, impaired cerebral autoregulation may play a part in syncope, and the association between RLS and syncope may be accounted for by impaired cerebral autoregulation (9-11,50). Schondorf et al. (51) found that cerebrovascular resistance decreased during neutrally-mediated syncope, indicating that the integrity of cerebrovascular autoregulation was maintained during syncope. However, among patients with orthostatic hypotension, an association between the presence of syncope and impaired cerebral autoregulation was observed (11). Another study also demonstrated decreased cerebral autoregulation during fainting (10). In patients with RLS, carbon dioxide, serotonin, and other vasoactive substances, which bypass the lung and directly enter the arteries from the venous system, may cause abnormal cerebral artery dilation/contraction (52,53). This can lead to impaired cerebral autoregulation or cerebral hypoperfusion, especially in specific brain regions responsible for maintaining consciousness, and finally result in syncope. Standardized and complementary methods to assess cerebrovascular autoregulation are needed to further evaluate the incidence and significance of impaired autoregulation in syncope and RLS (50). In addition, the association between RLS and syncope may be mediated by CSD, which is a transient wave of near-complete neuronal/glial depolarization associated with massive transmembrane ionic and water shifts, followed by prolonged suppression of neuronal activity. In the context of neurovascular coupling, CSD leads to dramatic changes in cerebral blood flow (CBF) with compromised autoregulation of CBF (53-55). Previous studies have found that CSD is readily triggered by microemboli in rodent brains (41). Therefore, RLS-generated microemboli may trigger CSD and further cause a decrease of CBF related with syncope. In addition, PFO is associated with platypnea orthodeoxia, especially in the context of some acquired anatomical alterations, such as right pneumonectomy, lobectomy, localized bullous emphysema, kyphoscoliosis, and phrenic palsy (12,14,15). Platypnea orthodeoxia and hypoxemia may account for syncope episodes in RLS. However, mechanisms by which RLS might cause syncope are still unclear and largely speculative. Syncope was also reported to occur during migraine attacks, and unexplained syncope may be caused by migraine attack (56). Interestingly, syncopal migraineurs had longer periods of unconsciousness and recovery than those the syncope without headache group, suggesting a different etiology for LOC between the 2 groups (57). This suggests another plausible, but unknown mechanism for syncope. These studies show that there may be an interaction between migraine, syncope, and RLS. To exclude the possible confounding effect of migraine, we excluded these 4 pairs of participants (4 migraineurs and 4 corresponding controls), then re-analyzed our data, which yielded similar results to those attained before the exclusion.
The term SVD refers to a group of pathological processes that affect the small arteries, arterioles, venules, and capillaries of the brain, which usually results in cerebral parenchyma damages demonstrated by MRI, including WMH, EPVS, lacunes, and CMBs (16). The clinical consequences of SVD include, but are not limited to, cognitive impairment, lacunar syndrome (stroke), gait disorder, incontinence, and depression (16). A previous study showed that syncope was an independent risk factor (OR =2.7) for a high load of deep WMH (17). Similarly, RLS was also found to be related to SVD in the brain, but the finding remains controversial (18,21). Our study also observed a significant association between unexplained syncope and SVD, which was in line with the previous study (17). Given the compromised CBF during syncope attacks, the association between syncope and a high burden of cerebral SVD is not surprising. Nevertheless, this case-control design could not ascertain a causal relationship between syncope and SVD, and this finding may be incidental.
This study has several strengths. First, this was an age- and gender-matched case-control study, and we used conditional logistic regression to compare the difference between groups and pairs. Conditional logistic regression is a specialized type of logistic regression usually employed in a matched case-control study and the matched factors (i.e., age and gender in our study) are evenly balanced between each pair (cases and controls) (58,59). To the best of our knowledge, this is among the first reports to explore the association between RLS and syncope. Second, we constructed a score to reflect the burden of cerebral SVD.
Our study also has limitations. First, this case-control study could not confirm a causal effect between RLS and unexplained syncope. This is our preliminary study, and a prospective study with a large sample size is being organized by our team. Second, RLS includes intracardiac (e.g., PFO) and extracardiac (i.e., PAVMs) shunts. Our study did not report on the prevalence of these types of shunts. However, PFO accounts for the vast majority (>95%) of RLS, while PAVMs is rare and has a prevalence of 0.038% in the general population (60,61). It is conceivable that almost all our RLS patients had PFO. Third, the pathophysiological mechanism between RLS and unexplained syncope is largely unknown. Both impaired cerebral autoregulation and CSD as causes mentioned above were speculative. Vasoactive substances (e.g., carbon dioxide, serotonin, norepinephrine, neuropeptide Y, acetylcholine, and substance P), cerebral autoregulation parameters (e.g., continuous recording of CBF velocity, and arterial blood pressure), oxygen saturation, and brain perfusion (e.g., MR perfusion imaging) should be measured in future studies. Fourth, this TCD bubble test for RLS should be interpreted only as a second line to a classical workup flowchart for syncope at present. The prevalence of RLS (15–25%) is also high in the asymptomatic population (19). Therefore, our findings should not prevent diagnosis of other severe causes that need to be promptly addressed. Fifth, given that this was a single hospital-based case-control study, findings in this study should be generalized to other population with caution.
In conclusion, this study demonstrated an association between RLS (especially severe RLS) and unexplained syncope. New strategies, including the TCD bubble test, should be carried out to evaluate patients with unexplained syncope and gain greater understanding of the pathophysiology and treatments. The benefits of PFO closure on these RLS syncope cases needs further study.
Acknowledgments
We thank Dr. Jing Shi for the help with the PFO closure information.
Funding: This work was supported by the Shuangchuang Plan of Jiangsu Province (2016, Zhaolu Wang) and the support plan for excellent young teachers in Nanjing Medical University (2017, Zhaolu Wang).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-21-1060/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1060/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Task Force for the Diagnosis and Management of Syncope. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J 2009;30:2631-71. [Crossref] [PubMed]
- Soteriades ES, Evans JC, Larson MG, Chen MH, Chen L, Benjamin EJ, Levy D. Incidence and prognosis of syncope. N Engl J Med 2002;347:878-85. [Crossref] [PubMed]
- Ganzeboom KS, Mairuhu G, Reitsma JB, Linzer M, Wieling W, van Dijk N. Lifetime cumulative incidence of syncope in the general population: a study of 549 Dutch subjects aged 35-60 years. J Cardiovasc Electrophysiol 2006;17:1172-6. [Crossref] [PubMed]
- Grossman SA, Shapiro NI, Van Epp S, Kohen R, Arnold R, Moore R, Lee L, Wolfe RE, Lipsitz LA. Sex differences in the emergency department evaluation of elderly patients with syncope. J Gerontol A Biol Sci Med Sci 2005;60:1202-5. [Crossref] [PubMed]
- Yasa E, Ricci F, Magnusson M, Sutton R, Gallina S, Caterina R, Melander O, Fedorowski A. Cardiovascular risk after hospitalisation for unexplained syncope and orthostatic hypotension. Heart 2018;104:487-93. [Crossref] [PubMed]
- Koppen H, Palm-Meinders IH, Ferrari MD. Right-to-left shunts and micro-embolization in migraine. Curr Opin Neurol 2012;25:263-8. [Crossref] [PubMed]
- Søndergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, Settergren M, Sjöstrand C, Roine RO, Hildick-Smith D, Spence JD, Thomassen LGore REDUCE Clinical Study Investigators. Patent Foramen Ovale Closure or Antiplatelet Therapy for Cryptogenic Stroke. N Engl J Med 2017;377:1033-42. [Crossref] [PubMed]
- Tobis JM, Charles A, Silberstein SD, Sorensen S, Maini B, Horwitz PA, Gurley JC. Percutaneous Closure of Patent Foramen Ovale in Patients With Migraine: The PREMIUM Trial. J Am Coll Cardiol 2017;70:2766-74. [Crossref] [PubMed]
- Guo ZN, Xing Y, Liu J, Wang S, Yan S, Jin H, Yang Y. Compromised dynamic cerebral autoregulation in patients with a right-to-left shunt: a potential mechanism of migraine and cryptogenic stroke. PLoS One 2014;9:e104849. [Crossref] [PubMed]
- Ocon AJ, Kulesa J, Clarke D, Taneja I, Medow MS, Stewart JM. Increased phase synchronization and decreased cerebral autoregulation during fainting in the young. Am J Physiol Heart Circ Physiol 2009;297:H2084-95. [Crossref] [PubMed]
- Gur AY, Auriel E, Korczyn AD, Gadoth A, Shopin L, Giladi N, Bornstein NM, Gurevich T. Vasomotor reactivity as a predictor for syncope in patients with orthostatism. Acta Neurol Scand 2012;126:32-6. [Crossref] [PubMed]
- Cheaito R, Benamer H, Tritar A, Hage F, Haziza F, Piechaud JF, El-Amine S, Medkour F, Morice MC, Jessen P. Platypnea-orthodeoxia revealed by recurrent syncope episodes. Ann Cardiol Angeiol (Paris) 2014;63:451-4. [Crossref] [PubMed]
- Alvarez-Fernández JA, Blasco OA, Pérez-Quintero R. Clinical relevance of patent foramen ovale and right-to-left shunt. Rev Clin Esp 2006;206:202-4. [PubMed]
- Piéchaud JF. Hypoxemia related to right-to-left shunting through a patent foramen ovale: successful percutaneous treatment with the CardioSeal device. J Interv Cardiol 2001;14:57-60. [Crossref] [PubMed]
- Eicher JC, Bonniaud P, Baudouin N, Petit A, Bertaux G, Donal E, Piéchaud JF, David M, Louis P, Wolf JE. Hypoxaemia associated with an enlarged aortic root: a new syndrome? Heart 2005;91:1030-5. [Crossref] [PubMed]
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9:689-701. [Crossref] [PubMed]
- Kruit MC, Thijs RD, Ferrari MD, Launer LJ, van Buchem MA, van Dijk JG. Syncope and orthostatic intolerance increase risk of brain lesions in migraineurs and controls. Neurology 2013;80:1958-65. [Crossref] [PubMed]
- Jiang XH, Wang SB, Tian Q, Zhong C, Zhang GL, Li YJ, Lin P, You Y, Guo R, Cui YH, Xing YQ. Right-to-left shunt and subclinical ischemic brain lesions in Chinese migraineurs: a multicentre MRI study. BMC Neurol 2018;18:18. [Crossref] [PubMed]
- Ntaios G, Tzikas A, Vavouranakis E, Nikas D, Katsimagklis G, Koroboki E, Manolis AS, Milionis H, Papadopoulos K, Sideris S, Spengos K, Toutouzas K, Tziakas D, Vassilopoulou S, Kanakakis I, Vemmos K, Tsioufis K. Expert consensus statement for the management of patients with embolic stroke of undetermined source and patent foramen ovale: A clinical guide by the working group for stroke of the Hellenic Society of Cardiology and the Hellenic Stroke Organization. Hellenic J Cardiol 2020;61:435-41. [Crossref] [PubMed]
- Mojadidi MK, Roberts SC, Winoker JS, Romero J, Goodman-Meza D, Gevorgyan R, Tobis JM. Accuracy of transcranial Doppler for the diagnosis of intracardiac right-to-left shunt: a bivariate meta-analysis of prospective studies. JACC Cardiovasc Imaging 2014;7:236-50. [Crossref] [PubMed]
- Du W, Wu X, Xing Y, Geng Y, Bai J, Song X. Right-to-Left Shunt Does Not Increase the Incidence of Silent Lacunar Infarcts in Patients with Migraine. Biomed Res Int 2015;2015:749745. [Crossref] [PubMed]
- Kent DM, Ruthazer R, Weimar C, Mas JL, Serena J, Homma S, Di Angelantonio E, Di Tullio MR, Lutz JS, Elkind MS, Griffith J, Jaigobin C, Mattle HP, Michel P, Mono ML, Nedeltchev K, Papetti F, Thaler DE. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology 2013;81:619-25. [Crossref] [PubMed]
- Job FP, Ringelstein EB, Grafen Y, Flachskampf FA, Doherty C, Stockmanns A, Hanrath P. Comparison of transcranial contrast Doppler sonography and transesophageal contrast echocardiography for the detection of patent foramen ovale in young stroke patients. Am J Cardiol 1994;74:381-4. [Crossref] [PubMed]
- Faul F, Erdfelder E, Lang AG, Buchner A G. *Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175-91. [Crossref] [PubMed]
- Brignole M, Alboni P, Benditt DG, Bergfeldt L, Blanc JJ, Thomsen PE, et al. Guidelines on management (diagnosis and treatment) of syncope-update 2004. Executive Summary. Eur Heart J 2004;25:2054-72. [Crossref] [PubMed]
- van Dijk N, Boer KR, Colman N, Bakker A, Stam J, van Grieken JJ, Wilde AA, Linzer M, Reitsma JB, Wieling W. High diagnostic yield and accuracy of history, physical examination, and ECG in patients with transient loss of consciousness in FAST: the Fainting Assessment study. J Cardiovasc Electrophysiol 2008;19:48-55. [PubMed]
- Wang Z, Qin H, Chen G, Mok VCT, Dai Y, Cai Y, Cheng X, Qian Y, Chu M, Lu X. Association between advanced interatrial block and small vessel diseases in the brain. Quant Imaging Med Surg 2020;10:585-91. [Crossref] [PubMed]
- Wessler BS, Kent DM, Thaler DE, Ruthazer R, Lutz JS, Serena J. The RoPE Score and Right-to-Left Shunt Severity by Transcranial Doppler in the CODICIA Study. Cerebrovasc Dis 2015;40:52-8. [Crossref] [PubMed]
- Mahmoud AN, Elgendy IY, Agarwal N, Tobis JM, Mojadidi MK. Identification and Quantification of Patent Foramen Ovale-Mediated Shunts: Echocardiography and Transcranial Doppler. Interv Cardiol Clin 2017;6:495-504. [Crossref] [PubMed]
- Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 2014;83:1228-34. [Crossref] [PubMed]
- Zetola VF, Lange MC, Scavasine VC, Bazan R, Braga GP, Leite ACCB, et al. Latin American Consensus Statement for the Use of Contrast-Enhanced Transcranial Ultrasound as a Diagnostic Test for Detection of Right-to-Left Shunt. Cerebrovasc Dis 2019;48:99-108. [Crossref] [PubMed]
- Tobe J, Bogiatzi C, Munoz C, Tamayo A, Spence JD. Transcranial Doppler is Complementary to Echocardiography for Detection and Risk Stratification of Patent Foramen Ovale. Can J Cardiol 2016;32:986.e9-986.e16. [Crossref] [PubMed]
- Vitarelli A. Patent Foramen Ovale: Pivotal Role of Transesophageal Echocardiography in the Indications for Closure, Assessment of Varying Anatomies and Post-procedure Follow-up. Ultrasound Med Biol 2019;45:1882-95. [Crossref] [PubMed]
- Mojadidi MK, Bokhoor PI, Gevorgyan R, Noureddin N, MacLellan WC, Wen E, Aysola R, Tobis JM. Sleep Apnea in Patients with and without a Right-to-Left Shunt. J Clin Sleep Med 2015;11:1299-304. [Crossref] [PubMed]
- Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology 2000;55:1172-9. [Crossref] [PubMed]
- Del Sette M, Angeli S, Leandri M, Ferriero G, Bruzzone GL, Finocchi C, Gandolfo C. Migraine with aura and right-to-left shunt on transcranial Doppler: a case-control study. Cerebrovasc Dis 1998;8:327-30. [Crossref] [PubMed]
- Ranoux D, Cohen A, Cabanes L, Amarenco P, Bousser MG, Mas JL. Patent foramen ovale: is stroke due to paradoxical embolism? Stroke 1993;24:31-4. [Crossref] [PubMed]
- Elmariah S, Furlan AJ, Reisman M, Burke D, Vardi M, Wimmer NJ, Ling S, Chen X, Kent DM, Massaro J, Mauri L. CLOSURE I Investigators. Predictors of recurrent events in patients with cryptogenic stroke and patent foramen ovale within the CLOSURE I (Evaluation of the STARFlex Septal Closure System in Patients With a Stroke and/or Transient Ischemic Attack Due to Presumed Paradoxical Embolism Through a Patent Foramen Ovale) trial. JACC Cardiovasc Interv 2014;7:913-20. [Crossref] [PubMed]
- Ning M, Lo EH, Ning PC, Xu SY, McMullin D, Demirjian Z, Inglessis I, Dec GW, Palacios I, Buonanno FS. The brain's heart - therapeutic opportunities for patent foramen ovale (PFO) and neurovascular disease. Pharmacol Ther 2013;139:111-23. [Crossref] [PubMed]
- Kumar P, Kijima Y, West BH, Tobis JM. The Connection Between Patent Foramen Ovale and Migraine. Neuroimaging Clin N Am 2019;29:261-70. [Crossref] [PubMed]
- Nozari A, Dilekoz E, Sukhotinsky I, Stein T, Eikermann-Haerter K, Liu C, Wang Y, Frosch MP, Waeber C, Ayata C, Moskowitz MA. Microemboli may link spreading depression, migraine aura, and patent foramen ovale. Ann Neurol 2010;67:221-9. [Crossref] [PubMed]
- Iwasaki A, Suzuki K, Takekawa H, Takashima R, Suzuki A, Suzuki S, Hirata K. The relationship between right-to-left shunt and brain white matter lesions in Japanese patients with migraine: a single center study. J Headache Pain 2017;18:3. [Crossref] [PubMed]
- Kim DE, Choi MJ, Kim JT, Chang J, Choi SM, Lee SH, Park MS, Cho KH. Juxtacortical spots on fluid-attenuated inversion recovery images in cryptogenic transient ischemic attack. J Clin Neurol 2013;9:103-10. [Crossref] [PubMed]
- Adami A, Anzola G. White matter lesions and right-to-left shunt in migraine. Eur J Neurol 2012;19:e79-author reply e80. [Crossref] [PubMed]
- Adami A, Rossato G, Cerini R, Thijs VN, Pozzi-Mucelli R, Anzola GP, Del Sette M, Finocchi C, Meneghetti G, Zanferrari CSAM Study Group. Right-to-left shunt does not increase white matter lesion load in migraine with aura patients. Neurology 2008;71:101-7. [Crossref] [PubMed]
- Liu JR, Plötz BM, Rohr A, Stingele R, Jansen O, Alfke K. Association of right-to-left shunt with frontal white matter lesions in T2-weighted MR imaging of stroke patients. Neuroradiology 2009;51:299-304. [Crossref] [PubMed]
- Dowson A, Mullen MJ, Peatfield R, Muir K, Khan AA, Wells C, Lipscombe SL, Rees T, De Giovanni JV, Morrison WL, Hildick-Smith D, Elrington G, Hillis WS, Malik IS, Rickards A. Migraine Intervention With STARFlex Technology (MIST) trial: a prospective, multicenter, double-blind, sham-controlled trial to evaluate the effectiveness of patent foramen ovale closure with STARFlex septal repair implant to resolve refractory migraine headache. Circulation 2008;117:1397-404. [Crossref] [PubMed]
- Mattle HP, Evers S, Hildick-Smith D, Becker WJ, Baumgartner H, Chataway J, Gawel M, Göbel H, Heinze A, Horlick E, Malik I, Ray S, Zermansky A, Findling O, Windecker S, Meier B. Percutaneous closure of patent foramen ovale in migraine with aura, a randomized controlled trial. Eur Heart J 2016;37:2029-36. [Crossref] [PubMed]
- Mojadidi MK, Kumar P, Mahmoud AN, Elgendy IY, Shapiro H, West B, Charles AC, Mattle HP, Sorensen S, Meier B, Silberstein SD, Tobis JM. Pooled Analysis of PFO Occluder Device Trials in Patients With PFO and Migraine. J Am Coll Cardiol 2021;77:667-76. [Crossref] [PubMed]
- Edwards MR, Schondorf R. Is cerebrovascular autoregulation impaired during neurally-mediated syncope? Clin Auton Res 2003;13:306-9. [Crossref] [PubMed]
- Schondorf R, Benoit J, Wein T. Cerebrovascular and cardiovascular measurements during neurally mediated syncope induced by head-up tilt. Stroke 1997;28:1564-8. [Crossref] [PubMed]
- Schwerzmann M, Nedeltchev K, Lagger F, Mattle HP, Windecker S, Meier B, Seiler C. Prevalence and size of directly detected patent foramen ovale in migraine with aura. Neurology 2005;65:1415-8. [Crossref] [PubMed]
- Lauritzen M. Long-lasting reduction of cortical blood flow of the brain after spreading depression with preserved autoregulation and impaired CO2 response. J Cereb Blood Flow Metab 1984;4:546-54. [Crossref] [PubMed]
- Østergaard L, Dreier JP, Hadjikhani N, Jespersen SN, Dirnagl U, Dalkara T. Neurovascular coupling during cortical spreading depolarization and -depression. Stroke 2015;46:1392-401. [Crossref] [PubMed]
- Ayata C, Lauritzen M. Spreading Depression, Spreading Depolarizations, and the Cerebral Vasculature. Physiol Rev 2015;95:953-93. [Crossref] [PubMed]
- Thijs RD, Kruit MC, van Buchem MA, Ferrari MD, Launer LJ, van Dijk JG. Syncope in migraine: the population-based CAMERA study. Neurology 2006;66:1034-7. [Crossref] [PubMed]
- Curfman D, Chilungu M, Daroff RB, Alshekhlee A, Chelimsky G, Chelimsky TC. Syncopal migraine. Clin Auton Res 2012;22:17-23. [Crossref] [PubMed]
- Rahman M, Sakamoto J, Fukui T. Conditional versus unconditional logistic regression in the medical literature. J Clin Epidemiol 2003;56:101-2. [Crossref] [PubMed]
- Koletsi D, Pandis N. Conditional logistic regression. Am J Orthod Dentofacial Orthop 2017;151:1191-2. [Crossref] [PubMed]
- Bodilsen J. Hereditary haemorrhagic telangiectasia and pulmonary arteriovenous malformations in brain abscess patients: a nationwide, population-based matched cohort study. Clin Microbiol Infect 2020;26:1093.e1-3. [Crossref] [PubMed]
- Nakayama M, Nawa T, Chonan T, Endo K, Morikawa S, Bando M, Wada Y, Shioya T, Sugiyama Y, Fukai S. Prevalence of pulmonary arteriovenous malformations as estimated by low-dose thoracic CT screening. Intern Med 2012;51:1677-81. [Crossref] [PubMed]


