
An update of our understanding of radiographic diagnostics for prevalent osteoporotic vertebral fracture in elderly women
Osteoporosis is characterized by low bone mass and micro-architectural deterioration, which leads to bone fragility and consequent increase in fracture risk. The bone composition of the spine, which is predominantly trabecular bone, is more prone to the thinning and microarchitectural changes associated with osteoporosis than regions of the hip that are richer in cortical bone. Osteoporotic vertebral fracture (OVF) is the most common osteoporotic fracture. Assessment of vertebral fracture (VF) status, in addition to bone mineral density (BMD), provides relevant clinical information to aid in predicting fracture risk in postmenopausal women. Siris et al. (1) reported that at any given BMD T-score, the risk of incident vertebral, non-vertebral, and any fracture depended heavily on prevalent radiographic OVF status (1). Johansson et al. (2) reported that, in older women and after adjustment for clinical risk factors and BMD, grade-1 OVFs identified on lateral spine imaging with dual-energy X-ray absorptiometry are associated with incident major osteoporotic fractures. Though BMD has been commonly used in decision-making for the diagnosis of osteoporosis, it is associated with many inherent limitations. BMD is a biomarker rather than a clinical endpoint (3,4). There is no ground truth justification for the classification of osteoporosis by the prevailing cutpoint T-score, particularly for non-Caucasian populations (5). Cutpoint T-score is also affected by the quality of the BMD reference database (6). Moreover, osteoporotic fracture commonly occurs in subjects not in the category of BMD-defined osteoporosis. On the other hand, OVF is a highly prevalent clinical endpoint. By analyzing a population-based sample of 303 Hong Kong Chinese women and 303 Italian women (mean age: 73.5 years for both groups), we reported that endplate and/or cortex fracture (ECF) sign positive OVF was detected among 27.1% of the Chinese subjects and 45.2% of the Italian subjects. Approximately a similar proportion of subjects had ‘apparent’ OVF with ≥20% measured vertebral height loss (7,8). Therefore, OVF appears to be a more sensitive clinical endpoint for compromised bone strength than BMD as a biomarker. OVF can be considered as a “gateway” to other more serious fractures, such as hip fracture (9,10). Some guidelines recommend spine imaging as a screening tool for osteoporosis (11). In this article, we update our understanding of radiographic diagnostics for OVF in women. The focus of discussion is on OVF detected during osteoporosis screening or epidemiological study for elderly women, rather than on traumatic vertebral fracture (TVF) occurred in osteoporotic patients.
Terminology: OVF or osteoporotic vertebral deformity (OVD)
It is known that OVF and their repair/healing occur frequently even in the absence of any appreciable radiographic change in vertebral shape. Microscopic fractures as shown by histology are common in radiographically normal vertebrae (12). Microscopic trabecula fracture and repair, vertebral wedging, ECF, and vertebral crushing are a spectrum of presentations of compromised vertebral bone strength. OVFs can be divided into two categories: (I) those truly without a distinct trauma incident, and (II) those associated with a distinct low energy trauma incident though some patients may not recall the incident (13,14). Thus, OVF can be classified into traumatic OVF and non-traumatic OVF. The former includes those who forgot the related low energy trauma history, and also can in appearance be similar to those with high energy trauma in an osteoporotic subject. For OVFs truly without a trauma incident, the fracture or vertebral deformity (VD) process may be a slowly progressive process without a distinct event. From this point of view, there is no distinct separation between elderly women without OVF and elderly women with mild OVF. Thus, diagnosing a woman to have mild OVF or not have an OVF would depend on the chosen criteria for OVF. This viewpoint is supported by our own experience that mild endplate depression is very commonly seen among elderly women’s CT scans. For a radiographically detected mild OVF in a woman truly without trauma incident, it can be considered that the slowly progressive microscopic fractures have progressed so much that the shape of this vertebra becomes distinctly different from the adjacent normal appearing vertebrae.
For screening detected OVFs without accompanying symptoms and without a fracture line, we argue that they are better called OVD. In addition, we feel the term ‘fracture’ gives the impression that it is still in the acute or subacute phase, which may not be the case for many OVFs. The detectability and the appearance of OVF heavily depend on the chosen imaging techniques, thus for radiographic studies, radiographic osteoporotic vertebral deformity (ROVD) may be the suitable term. However, the term ‘OVF’ has been commonly used for OVD, and indeed at least at the microscopic level, fractures are very common among vertebrae with OVD, thus we do not necessarily argue against the use of OVF for screening detected asymptomatic OVDs. The term OVF is therefore used in this article.
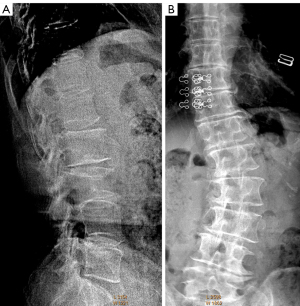
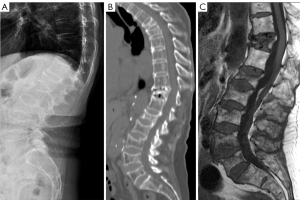
OVFs associated with a low energy trauma event are better called fracture when they are in the acute or subacute phase (Figure 1).
Morphology of OVF
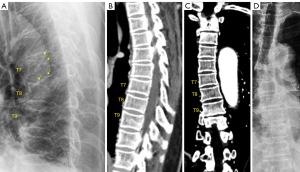
While typical OVFs are bi-concaved (Figure 2), atypical OVFs can have various shapes. One common appearance of OVF, which can be considered as a combination (or an intermediate or a ‘mixed shape’) of anterior wedging, bi-concaving, and vertebral ‘stair-step’, is shown in Figure 3. Note the anterior portion of a vertebra can demonstrate a ‘stair-step’ appearance (15,16). The anterior portion of a vertebra is more resistant to compressive force than the middle portion of an endplate.
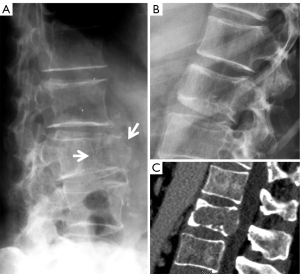
We consider that an OVF can also appear as simple wedging without radiographic endplate depression (Figures 4,5). In a recent study, we reported that more than 2/3 of the minimal radiographic osteoporotic wedgings with height loss of <20% had recognizable endplate depression on CT, thus strongly suggesting these deformities were of osteoporotic cause (17). We consider that, after excluding known OVF mimics, singular vertebral wedging in elderly women is most likely osteoporotic. Moreover, vertebrae with CT osteoporotic endplate depression may appear normal shaped on a radiograph. For the results described in our recent paper, it should be considered that CT negative for endplate depression also does not rule out the possibility of a vertebra having OVF (17). In a study of lateral chest radiographs, Yu et al. (18) noted that vertebral shape change is very rare among young subjects, and vertebral shape changes seen among older adults are less likely to be due to normal variation or congenital deformities.
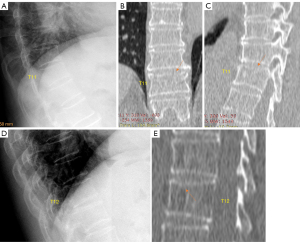
Of note, though slight posterior wedging can be rarely seen, posterior crushing OVF probably does not exist unless there is a special type of external force involved.
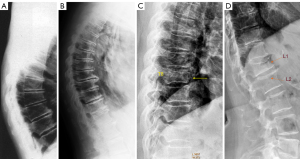
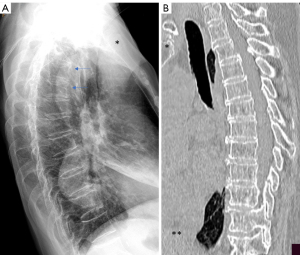
In terms of OVF location, in addition to the thoracolumbar junction and mid-thoracic region, another common location is the junction of upper segment of the thoracic spine (where the scapular bones provide protection in addition to the ribs) and mid-segment of the thoracic spine (Figure 6). The junction of upper segment and mid-segment of the thoracic spine may not be a smooth transition in some subjects, instead a slight angle may be formed (Figure 6A). This junction is likely under additional stress. Vertebra wedging at this junction can be observed in some subjects, and we consider these wedgings are osteoporotic and being OVF.
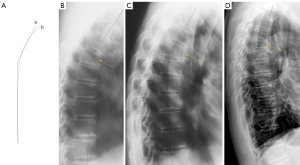
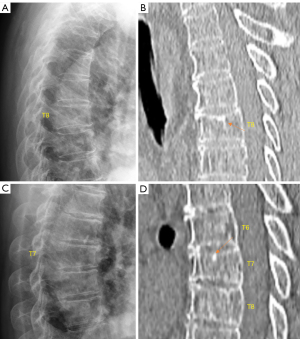
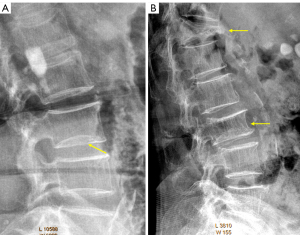
The term ‘short vertebra’ has been frequently discussed (19,20). We define short vertebrae as those with decreased vertebral anterior and middle heights, while without anterior wedging or bi-concave changes, i.e., middle height and anterior height are reduced to a similar extent (17). In addition, other apparent OVF mimics, such as apparent degenerative changes, are excluded. Our definition of short height vertebrae is likely different from other reports (19,20), since apparent mimics have been excluded (17). True congenital short vertebra does exit (15), but it is very rare. In our recent report, our limited experience suggests that, singular short height vertebra is often associated with CT endplate depression thus likely to be osteoporotic, while multiple adjacent short height vertebrae are without CT endplate depression and are likely due to other causes such as degenerative changes (Figure 7) (17). While we admit a larger sample size is required to confirm our result, our finding is reasonable, as ‘osteoarthritic’ degenerative changes more likely involve more than a single vertebra.
A portion of OVFs may be similar in appearance to TVF when a distinct low-energy trauma event had been involved, and anterior cortex fracture is commonly seen in these cases (Figure 1) (13,14). Note the spine regions with the highest prevalence of OVF are also the regions of highest prevalence of TVF (21,22). In an analysis of traumatic endplate fractures, we noted that, except traumatic endplate fractures involved both upper endplate and lower endplate, the vast majority of singular traumatic endplate fractures involved upper endplate, with only approximately 1.5% involved lower endplate alone; while 9.8% and 17.4% of osteoporotic endplate fractures involve lower endplate alone for males and females respectively. Unlike osteoporotic endplate fractures, traumatic endplate fracture’s features did not show gender differences. TVF can involve posterior vertebral wall with 68.5% of those involved breaking the bony wall surrounding the basivertebral vein (non-traumatic OVFs do not involve posterior wall), and 20% of the most depressed points of traumatic superior endplate fractures involved the most anterior portion of the endplate (this portion of endplate would not be the case in a non-traumatic osteoporotic vertebra) (21-23).
Critiques for morphometric methods
Now it is well recognized that purely morphometric methods can lead to falsely classifying physiological and degenerative wedging as OVF. On the other hand, true OVFs without meeting the morphometric thresholds may be missed. For example, Leidig-Bruckner et al. (24) applied an algorithm for radiological differential classification (RDC) and noted that, in female cases, 31–68% of cases with morphometric VD were classified by RDC as non-osteoporotic, and up to 48% in women were missed by morphometry. Purely morphometric methods are not used as a standalone method for OVF evaluation.
Though theoretically quantitative methods are based on objective and reproducible measurements, in practice, the results heavily depend on how the measurement is conducted, i.e., where the measurement cursors are placed. The selection of points for the measurement of vertebral heights is a quite subjective procedure. In addition, we argue that to compare the height loss of a vertebra with its adjacent normal appearing vertebrae will be more reasonable, as the ratio of anterior height to posterior height varies among different vertebral levels. Additionally, due to the existence of the uncinate process (or posterior lipping), the posterior height measure can cause high degree inconsistencies. Morphometric measures performed by different operators may not allow inter-study comparison.
Critiques for Genant semi-quantitative (SQ) criteria
In the early ‘90s, Genant and colleagues at the University of California at San Francisco (UCSF), USA, proposed the SQ grading scheme to evaluate OVF (25). As it is difficult to estimate vertebral lateral area reduction, the percentage area reduction requirement, which was described in Genant’s original article, has been mostly dropped by users of SQ criteria over the years. The morphological description of Genant et al. appears to be reasonable and valid (26,27). However, it is also important to emphasize that it is difficult to apply Genant’s SQ criteria by reading the text description of Genant et al. For example, despite that a standardized protocol of radiograph acquisition techniques and of interpretation criteria was applied, Diacinti et al. (28) reported a study that, among 562 OVFs identified by radiologist readers in peripheral hospitals, 102 were classified as normal vertebrae by the experienced radiologist readers in a central hospital; while 205 OVFs were incorrectly evaluated by local readings as (false) negatives. Wu et al. (29) reported inter-reader kappa scores based on the dichotomous fracture/non-fracture decision was 0.69 agreement between experienced readers. Buckens et al. (30) assessed the agreement of four observers with different levels of experience, and sagittal reformatted CT images were used. For fracture presence the interobserver kappa scores ranged from 0.56 to 0.81 (29). These inter-reader agreements appear to be insufficient.
Genant SQ method relies on the estimation of vertebral dimensions for grading, which is a source of both poor inter- and intra-reader agreement. We argue that the grading of vertebral height loss for research purposes has to be measurement-based. According to the original description, SQ mild grade includes the range of vertebral height loss of 5% (i.e., 20–25%) which could hardly be reliably estimated. Even by measurement, a 24% vertebral height loss could be easily measured as 26% height loss thus in the moderate grade, or 21% vertebral height loss could be easily measured as 18% height loss thus below the threshold of mild grade OVF. In addition, consensus cannot be practically reached for visual estimation. We also noted a general trend that, compared to the measured results, the visually estimated results tend to overestimate the severity of vertebral height loss (31). While upper and lower endplates and their rings may show double-lines or multiple-lines, it appears that readers tend to use the most depressed line to visually estimate; this can be one of the sources for over-estimation of vertebral height loss (31).
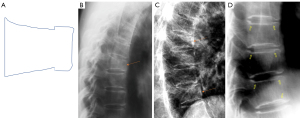
Genant et al. described mild OVFs have ≥20% height loss, and OVFs without achieving this threshold are classified as SQ grade-0.5. This causes some confusions such as whether an OVD with 15% height loss would qualify as an OVF. To our knowledge, OVDs without achieving 20% height loss threshold have been commonly classified as SQ grade-1 OVF by colleagues trained in UCSF (Figure 8, and personal communications). If a diagnostic reader strictly follows the description of ≥20% vertebral height loss as the threshold for OVF, then in practice some clinically relevant OVFs which has <20% vertebral height loss would be missed. If for research purposes, a reader like to follow SQ criteria, we suggest that mild grade should include OVFs with ≥10–25% vertebral height loss.
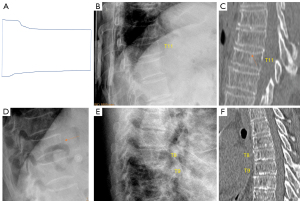
Though the initial description of Genant et al. did not include vertebra L5 assessment, actually L5 can be included in the analysis (though OVF of vertebra L5 is less common). It appears that posterior crushing OVF as illustrated in Fig. 1 of Genant et al. 1993 article may not exist.
Note, SQ criteria are not suitable for acute/subacute traumatic care setting. Application of SQ criteria in back pain clinics patients can be problematic.
Critiques for algorithm-based qualitative (ABQ) criteria
In vertebral osteoporosis, the endplate becomes weakened due to the loss of support from trabecular bone, and due to thinning of the endplate itself. The importance of identifying endplate deformities has been well recognized during earlier works (25,32). Jiang et al. (33) proposed that all OVFs should demonstrate endplate fracture. This contribution further emphasized the importance of reading the endplate during OVF assessment. However, radiograph is not a sensitive method to detect ECF, due to its resolution and due to the projectional overlay (17,34). CT can detect more endplate depressions than radiograph. Moreover, ECF negative OVF can turn into ECF positive over time, and a higher OVF severity (i.e., higher extent of vertebral height loss) is associated with an increased probability of turning into ECF positive during follow-up (35). This itself suggests that ECF negative OVD is already an osteoporotic phenomenon distinct from those vertebrae without OVD. We consider positive ECF as an additional sign of OVF, and a positive ECF sign would increase the confidence for OVF diagnosis, but ECF is not an essential sign of OVF (36).
Though ABQ criteria were proposed with the consideration that some OVFs do not display alterations of vertebral height. However, our experience is that OVF is usually associated with some extent of anterior vertebral height loss. We have reported that, radiographical OVFs with ≥20% measured vertebral height loss are often associated with ECF (7), and OVFs with >1/3 measured vertebral height loss are always associated with ECF (37). In fact, deformities without any anterior height loss are more likely due to X-ray projection artefacts.
It has been suggested that, compared with those OVFs without ECF, OVFs with ECF better predict further osteoporotic fracture risk (35,38). In addition to that some of VDs without ECF could be false positive readings, within the same grade as defined by vertebral height loss, those with ECF would have a higher extent of vertebral height loss. For example, for the range of 25–40% vertebral height loss (i.e., Genant moderate grade), some of the OVFs with ECF will be in the range of 33–40% vertebral height loss, while all of the OVFs without ECF will be in the range of 25–33% vertebral height loss (37). The extent of vertebral height loss is positively associated with the probability of having ECF (37), while the extent of vertebral height loss is well known positively associated with further osteoporotic fracture risk (35,39).
The ABQ approach heavily depends on the diagnosis of endplate depression. While moderate and severe endplate depression can be diagnosed with a high degree of confidence, diagnosing mild or minimal degree of endplate depression is highly subjective. We expect this subjectivity for mild endplate depression assessment will be an important obstacle for inter-reader agreement and hinder inter-study comparison.
Another issue of the initial ABQ criteria is that it did not consider vertebral cortex fracture. Anterior cortex fracture is very common among OVFs associated with a trauma history, and it is a risk factor for OVF progression (14,40-42). We hypothesize that this may be a reason that anterior cortex fracture is less commonly seen among screening detected mild OVFs, as those traumatic OVFs with anterior cortex disruption are more likely to progress. What we see for screening detected OVFs are the consequence of the initial traumatic OVFs. Vertebral posterior wall break is also a risk factor for traumatic OVF progression (42).
Modified semi-quantitative (mSQ) criteria and extended semi-quantitative (eSQ) criteria
Based on Genant’s original SQ criteria, we proposed an mSQ grading scheme for OVF evaluation in elderly women (43). Mild OVFs include those of vertebral height loss of <20%, moderate OVFs include those of 20–34% vertebral height loss, and severe OVFs are those of >34% vertebral height loss. With the mSQ, as long as a qualitative OVF is diagnosed, the 20% vertebral height loss requirement is removed for the mild grade. Without the requirement for a minimum amount of a vertebral height loss may direct the attention of readers to the actual fractural deformities (25,26,33,44). Though the higher degree of vertebral height loss is overall associated with higher positive ECF prevalence, our data showed, compared with Genant SQ grade-2 OVF, SQ grade-1 OVF do not show distinctively lower ECF prevalence; indeed, Genant grade-1 OVFs show radiographic ECF prevalence similar to OVFs with 25–34% height loss [estimated from table 6 of reference 45 and Fig. 1 of reference (37)] (45,37). Thus, we proposed to group OVFs with 20–34% height loss together as a singular moderate grade. This mSQ scheme may be more practical to implement, such as, if height loss is more than 1/3 of the vertebra, then the OVF is severe grade (approximately >34%); if height loss is less than 1/5 of the vertebra, then the OVF is mild grade (<20%). Note mSQ is intended for clinical application of OVF screening and back pain clinics. However, primarily it is not intended for traumatic OVF classification. For traumatic OVF classification in their acute or subacute phases, other criteria may be more suitable (14,40,46,47).
For research purposes and to better record OVF and allow inter-study comparison, we proposed an eSQ criteria: (I) minimal grade refers to radiological OVF with <20% height loss, theoretically equivalent to Genant SQ grade-0.5; (II) mild grade is the same as Genant SQ mild grade (20–25% height loss); (III) SQ moderate grade is divided into two subgrades: 1/4–1/3 height loss and 1/3–2/5 height loss; (IV) SQ severe grade is divided into two subgrades: 2/5–2/3 height loss and ≥2/3 height loss (collapsed grade) (31). We have prepared pictorial materials for this grading scheme to facilitate self-learning (31,48). To avoid inconsistency of vertebral height loss estimation by different readers (49), eSQ evaluates vertebral height loss by measurement, with the heights of neighboring normal-appearing vertebrae as references. SQ moderate grade was subdivided into two grades because OVFs with ≥1/3 height loss always demonstrate ECF radiographically (37). A subdivision of SQ grade-3 facilitates the recording of progression of a severe OVF (such as a 45% height loss OVF progresses to 75% height loss). The eSQ criteria may look too complicated for application (40), however, we argue that if it is for research purposes and it is measurement-based for OVF grading, then this complexity would be acceptable. Moreover, a flexible approach for eSQ can be applied. For example, a reader may decide that minimal grade should have ≥10% vertebral height loss (this may be practical for reporting epidemiological study results), or a reader may decide that 1/5–1/3 vertebral height loss should be one grade (mild grade of 20–25% vertebral height loss was kept so the results can be theoretically comparable with existing literature using Genant SQ grading), or reader may still choose to only count those OVFs with ≥20% vertebral height loss (which we call ‘apparent’ OVF). Overall, we believe a measurement-based approach with detailed gradings will allow better inter-study comparison. The same as mSQ, primarily eSQ is not intended for traumatic OVF classification in their acute or subacute phases. Both mSQ and eSQ use the extent of vertebral height loss as the primary classification criterion. The extent of vertebral height loss may also be a late-stage consequence of traumatic OVF after progression risk factors contributed to the outcome.
We argue for the importance of recognizing OVFs with <20% vertebral height loss, particularly for traumatic OVF (14,32). The clinical management of such OVF cases would depend on the clinical data such as BMD or other fragility fracture history. Though minimal grade OVFs may not have immediate further fragility fracture consequences, they are a biomarker of compromised bone quality. From the further VF risk perspective, singular minimal OVF in a patient may not be associated with increased fracture risk in the short term groupwisely (i.e., statistically) (35), but may be associated with increased fracture risk in the long term. In our MsOS (Hong Kong) year-14 follow-up, out of 150 female participants, five women were identified as having baseline minimal OVF and among them three had osteopenia and two had osteoporosis. There was a trend that these minimal OVF subjects had incident OVF risk similar to that of the subjects with baseline apparent OVF (i.e., ≥20% height loss), higher than female subjects without baseline OVF (50). The real-world importance of minimal/mild OVFs may depend on patients individually, and a wait-and-see strategy with follow-up imaging may be sufficient for many non-traumatic cases.
In one recent study, we used a combination of ECF and eSQ criteria to evaluate OVF prevalence among elderly women (7).
Most important OVF mimics: scoliosis, oblique X-ray projection, and degenerative changes
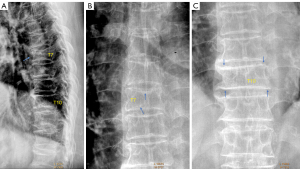
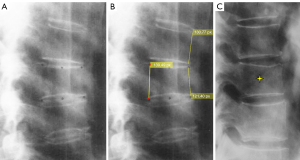
Scoliosis and oblique X-ray projection are among the most common causes of diagnostic confusion for OVF (Figure 9). If a frontal view radiograph is also simultaneously taken, then an additional checking on the frontal radiograph may solve the problem. One tip for differential diagnosis on lateral imaging appears to be that, OVFs are often associated with various degrees of vertebral anterior height loss, while artificial ‘deformities’ due to scoliosis and X-ray projection more likely show the vertebral anterior height maintained (Figure 10).
Sometimes, the visual height loss estimation and thus the grading of an OVF may depend on how ‘off-center’ of this vertebra is to the X-ray beam focus (Figure 11).

Another common differential diagnosis for OVF is ‘osteoarthritic’ degenerative changes [in the articles by Lentle et al. (51), it appears that the term of ‘morphometric deformity’ was used to describe these degenerative changes]. Degenerative deformities are not associated with increased further osteoporotic fracture risk (51,52). Degenerative deformities often involve multiple adjacent vertebrae appearing similarly deformed, while fractural deformities tend more often to be singular appearing as a distinct loss of expected shape (Figures 11,12). Degenerative deformities are also often associated with disc space narrowing and osteophytes. Of course, sometimes degenerative changes and OVF can occur in the same vertebra. Our experience is that these ‘osteoarthritic’ degenerative changes are much more common in Caucasian subjects than in Chinese subjects.
OVF mimics: oncological deformities
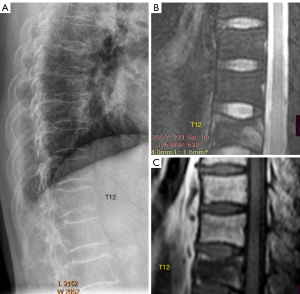
The most important differential diagnosis for OVF would be oncological deformities (metastatic bone diseases, multiple myeloma) and those due to some hematological diseases. Radiographic differential diagnosis between OVF and oncological deformities can be sometimes easy (Figure 13), or sometimes impossible and further imaging such as MRI is required (Figure 14). Generally speaking, OVFs most typically involve the thoracolumbar junction and mid-thoracic region, and OVFs usually have typical endplate changes or appear to be anteriorly wedged. We suspect a careful analysis of radiographic morphology will allow a correct differentiation for most cases.
A detailed discussion on the differentiation of OVF from oncological deformities is beyond the scope of this article.
Other OVF mimics
High-energy trauma induced old VD would not be rare but would be much less common than degenerative deformities or confusions due to scoliosis. Moreover, both low energy VF and high energy VF can occur in osteoporotic subjects. Trauma history is of course important for the diagnosis. At the individual subject’s levels, absolute differentiation of OVF and old high-energy trauma deformity is not always possible radiographically; however, we expect these ambiguities do not have a major impact on the statistical results of epidemiological studies for elderly women.
Many other OVF mimics, such as Schmorl’s nodes, lateral hyperosteogeny of vertebral body, Calvé’s disease (eosinophilic granuloma), Cupid’s bow, etc., have been described and illustrated previously (15,25). These changes can be relatively easily differentiated from OVF by an experienced reader. Note Schmorl’s nodes may co-exist with endplate fracture, actually Schmorl’s nodes likely predispose an osteoporotic endplate to fracture. Hereby we graphically illustrate two relatively common OVF mimics in Figures 15,16.
Role of frontal view radiograph for OVF assessment
Moderate to severe OVFs at mid-thoracic and lower thoracic spine as well as lumbar spine are mostly identifiable on frontal view spinal, chest, or abdominal radiographs, with a small proportion of ambiguous cases further clarified by additional lateral view imaging (53). We have prepared a pictorial review for reading OVF on frontal view radiograph (54). Frontal view radiograph can be sometimes of great help for confirming, or refuting, OVF suspicions on lateral radiograph.
The site of highest OVF prevalence, i.e., the thoracolumbar spine junction, is usually ‘off-center’ to the X-ray beam focus of T6 (as the custom) for taking chest frontal radiograph. We demonstrated that, if a chest frontal radiograph is taken with the X-ray beam focus approximately two vertebrae lower (i.e., the focus of X-ray beam is adjusted to towards T8), the visualization of thoracolumbar spine junction can be much improved, allowing better identification of OVF on chest frontal radiograph (55).
OVF prevalence in epidemiological research and inter-study comparison
Because of the issues described above, the previously reported OVF prevalences allow little opportunity for inter-study comparison. The inter-reader agreements are expected to be poor across different studies. This has led to some practical difficulties. For example, osteoporotic hip fracture prevalence among Chinese is generally no more than half that of Caucasians (6,56), and we demonstrated that both radiographic OVF and clinical OVF follow the same trend with their prevalences among Chinese being no more than half of those among Caucasians (6,7,36). However, earlier studies reported that OVF among East Asians is as prevalent as that of Caucasians (57,58). Another example is the two population studies on OVF prevalence of elderly Chinese women in Beijing (Table 1) (59,60), very different results were reported.
Table 1
| Age (years) | Ling et al. 2000# | Ling et al. 2000¶ | Cui et al. 2017§ |
|---|---|---|---|
| 50–59 | 3.9% | 4.9% | 13.4% |
| 60–69 | 10.5% | 16.2% | 22.6% |
| 70–79 | 15.0% | 19.0% | 31.4% |
| >80 | 31.2% | 36.6% | 58.1% |
For epidemiological research, unless otherwise justified, we argue that OVF prevalence should follow the following patterns: (I) as the age increases, the prevalence of OVF should increase exponentially (i.e., not linearly), both for men and women (61); (II) the location of highest OVF prevalence is the thoracolumbar junction, both for men and women (7). OVF prevalence at the mid-thoracic region should be lower than that of thoracolumbar spine junction. Note the mid-thoracic region is the site with a high prevalence of degenerative changes. If the OVF prevalence at mid-thoracic region is higher than that in the thoracolumbar spine junction, then an overcall of OVF at mid-thoracic region might have happened (22). This appears to be a common problem for many published works; (III) the prevalence of both radiographic OVF and clinical OVF in men should be approximately half of those in women (36,45,61,62). Our results suggest that, while minimal VDs in men likely have no long-term consequence, minimal VDs in women are more likely to be osteoporotic and associated with increased further fracture risk in the long term (50). The clinical relevance of apparent OVFs in elderly men is also less than that of elderly women (61,63,64); (IV) men before the age of 60 years should have few prevalent primary (senile) OVFs (61).
We argue that, for research purposes, it may not be sufficient for the radiographs being conveniently read by a local musculoskeletal radiologist unless this radiologist is experienced in OVF assessment (28), as the reading results may not allow inter-study comparison. Considering the efforts and costs associated with conducting the study and sampling the radiographs, it may well be worthwhile to seek an external experienced reader for additional calibration. While the discussions in this article are focused on radiograph, we consider our updates are also relevant for bone densitometric imaging [vertebral fracture assessment (VFA)].
Acknowledgments
I thank my colleagues in the Department of Imaging and Interventional Radiology and the Jockey Club Centre for Osteoporosis Care and Control, the Chinese University of Hong Kong, China, Professor Daniele Diacinti at the Sapienza University of Rome, Italy, Dr. Er-Zhu Du at the Dongguan TCM Hospital, China, and Professor Wei Yu at PUMC Hospital, China, for the collaborations. I thank Professor Brian C. Lentle at University of British Columbia, Canada, and Dr. Fernando Ruiz Santiago at University of Granada, Spain, for helpful discussions during the course of our studies.
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at available at https://qims.amegroups.com/article/view/10.21037/qims-22-360/coif). YXJW serves as the Editor-in-Chief of Quantitative Imaging in Medicine and Surgery. He is the founder of Yingran Medicals Co., Ltd., which develops medical image-based diagnostics software including those for vertebral compressive fracture evaluation. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siris ES, Genant HK, Laster AJ, Chen P, Misurski DA, Krege JH. Enhanced prediction of fracture risk combining vertebral fracture status and BMD. Osteoporos Int 2007;18:761-70. [Crossref] [PubMed]
- Johansson L, Sundh D, Magnusson P, Rukmangatharajan K, Mellström D, Nilsson AG, Lorentzon M. Grade 1 Vertebral Fractures Identified by Densitometric Lateral Spine Imaging Predict Incident Major Osteoporotic Fracture Independently of Clinical Risk Factors and Bone Mineral Density in Older Women. J Bone Miner Res 2020;35:1942-51. [Crossref] [PubMed]
- Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 1994;4:368-81. [Crossref] [PubMed]
- Wáng YXJ. Medical imaging in pharmaceutical clinical trials: what radiologists should know. Clin Radiol 2005;60:1051-7. [Crossref] [PubMed]
- Wáng YXJ, Xiao BH, Su Y, Leung JCS, Lam PMS, Kwok TCY. Fine-tuning the cutpoint T-score as an epidemiological index with high specificity for osteoporosis: methodological considerations for the Chinese population. Quant Imaging Med Surg 2022;12:882-5. [Crossref] [PubMed]
- Wáng YXJ, Xiao BH. Estimations of osteoporosis prevalence and cutpoint T-scores for defining osteoporosis among Chinese population: A framework based on relative fragility fracture risks. Quant Imaging Med Surg 2022; [Crossref]
- Wáng YXJ, Diacinti D, Leung JCS, Iannacone A, Kripa E, Kwok TCY, Diacinti D. Much lower prevalence and severity of radiographic osteoporotic vertebral fracture in elderly Hong Kong Chinese women than in age-matched Rome Caucasian women: a cross-sectional study. Arch Osteoporos 2021;16:174. [Crossref] [PubMed]
- Wáng YXJ, Diacinti D, Leung JCS, Iannacone A, Kripa E, Kwok TCY, Diacinti D. Further evidence supports a much lower prevalence of radiographic osteoporotic vertebral fracture in Hong Kong Chinese women than in Italian Caucasian women. Arch Osteoporos 2022;17:13. [Crossref] [PubMed]
- Kadowaki E, Tamaki J, Iki M, Sato Y, Chiba Y, Kajita E, Kagamimori S, Kagawa Y, Yoneshima H. Prevalent vertebral deformity independently increases incident vertebral fracture risk in middle-aged and elderly Japanese women: the Japanese Population-based Osteoporosis (JPOS) Cohort Study. Osteoporos Int 2010;21:1513-22. [Crossref] [PubMed]
- Black DM, Arden NK, Palermo L, Pearson J, Cummings SR. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. Study of Osteoporotic Fractures Research Group. J Bone Miner Res 1999;14:821-8. [Crossref] [PubMed]
- Cheng X, Yuan H, Cheng J, Weng X, Xu H, Gao J, Huang M, Wáng YXJ, Wu Y, Xu W, Liu L, Liu H, Huang C, Jin Z, Tian W. Bone and Joint Group of Chinese Society of Radiology, Chinese Medical Association (CMA), Musculoskeletal Radiology Society of Chinese Medical Doctors Association, Osteoporosis Group of Chinese Orthopedic Association, Bone Density Group of Chinese Society of Imaging Technology, CMA*. Chinese expert consensus on the diagnosis of osteoporosis by imaging and bone mineral density. Quant Imaging Med Surg 2020;10:2066-77. [Crossref] [PubMed]
- Antonacci MD, Mody DR, Rutz K, Weilbaecher D, Heggeness MH. A histologic study of fractured human vertebral bodies. J Spinal Disord Tech 2002;15:118-26. [Crossref] [PubMed]
- Wáng YXJ. Osteoporotic Vertebral Deformity: Radiological Appearances and Their Association With a History of Trauma and the Risk of Further Fragility Fracture. Can Assoc Radiol J 2021;72:585. [Crossref] [PubMed]
- Sugita M, Watanabe N, Mikami Y, Hase H, Kubo T. Classification of vertebral compression fractures in the osteoporotic spine. J Spinal Disord Tech 2005;18:376-81. [Crossref] [PubMed]
- Wáng YXJ, Santiago FR, Deng M, Nogueira-Barbosa MH. Identifying osteoporotic vertebral endplate and cortex fractures. Quant Imaging Med Surg 2017;7:555-91. [Crossref] [PubMed]
- Wáng YXJ, Deng M, He LC, Che-Nordin N, Santiago FR. Osteoporotic vertebral endplate and cortex fractures: A pictorial review. J Orthop Translat 2018;15:35-49. [Crossref] [PubMed]
- Du EZ, Wáng YXJ. CT detects more osteoporotic endplate depressions than radiograph: a descriptive comparison of 76 vertebrae. Osteoporos Int 2022; Epub ahead of print. [Crossref] [PubMed]
- Yu W, Lin Q, Zhou X, Shao H, Sun P. Reconsideration of the relevance of mild wedge or short vertebral height deformities across a broad age distribution. Osteoporos Int 2014;25:2609-15. [Crossref] [PubMed]
- Ferrar L, Jiang G, Cawthon PM, San Valentin R, Fullman R, Lambert L, Cummings SR, Black DM, Orwoll E, Barrett-Connor E, Ensrud K, Fink HA, Eastell R. Osteoporotic Fractures in Men (MrOS) Study. Identification of vertebral fracture and non-osteoporotic short vertebral height in men: the MrOS study. J Bone Miner Res 2007;22:1434-41. [Crossref] [PubMed]
- Ferrar L, Jiang G, Armbrecht G, Reid DM, Roux C, Glüer CC, Felsenberg D, Eastell R. Is short vertebral height always an osteoporotic fracture? The Osteoporosis and Ultrasound Study (OPUS). Bone 2007;41:5-12. [Crossref] [PubMed]
- Wang XR, Xu FR, Huang QL, Wáng YXJ. Radiological features of traumatic vertebral endplate fracture: an analysis of 194 cases with 263 vertebral fractures. Chin Med J (Engl) 2020;133:2696-702. [Crossref] [PubMed]
- Wáng YXJ, Wang XR, Che-Nordin N, Xu FR, Huang QL. On the possibility of over-diagnosis of osteoporotic vertebral fracture at mid-thoracic level. J Thorac Dis 2019;11:5708-11. [Crossref] [PubMed]
- Che-Nordin N, Deng M, Griffith JF, Leung JCS, Kwok AWL, Zhu YQ, So RHY, Kwok TCY, Leung PC, Wáng YXJ. Prevalent osteoporotic vertebral fractures more likely involve the upper endplate than the lower endplate and even more so in males. Ann Transl Med 2018;6:442. [Crossref] [PubMed]
- Leidig-Bruckner G, Limberg B, Felsenberg D, Bruckner T, Holder S, Kather A, Miksch J, Wüster C, Ziegler R, Scheidt-Nave C. Sex difference in the validity of vertebral deformities as an index of prevalent vertebral osteoporotic fractures: a population survey of older men and women. Osteoporos Int 2000;11:102-19. [Crossref] [PubMed]
- Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 1993;8:1137-48. [Crossref] [PubMed]
- Genant HK, Jergas M. Assessment of prevalent and incident vertebral fractures in osteoporosis research. Osteoporos Int 2003;14:S43-55. [Crossref] [PubMed]
- Wáng YXJ, Che-Nordin N. Informed communication with study subjects of radiographically detected osteoporotic vertebral deformity. Quant Imaging Med Surg 2018;8:876-80. [Crossref] [PubMed]
- Diacinti D, Vitali C, Gussoni G, Pisani D, Sinigaglia L, Bianchi G, Nuti R, Gennari L, Pederzoli S, Grazzini M, Valerio A, Mazzone A, Nozzoli C, Campanini M, Albanese CVResearch Department of FADOI. Misdiagnosis of vertebral fractures on local radiographic readings of the multicentre POINT (Prevalence of Osteoporosis in INTernal medicine) study. Bone 2017;101:230-5. [Crossref] [PubMed]
- Wu CY, Li J, Jergas M, Genant HK. Comparison of semiquantitative and quantitative techniques for the assessment of prevalent and incident vertebral fractures. Osteoporos Int 1995;5:354-70. [Crossref] [PubMed]
- Buckens CF, de Jong PA, Mol C, Bakker E, Stallman HP, Mali WP, van der Graaf Y, Verkooijen HM. Intra and interobserver reliability and agreement of semiquantitative vertebral fracture assessment on chest computed tomography. PLoS One 2013;8:e71204. [Crossref] [PubMed]
- Wáng YXJ, Diacinti D, Yu W, Cheng XG, Nogueira-Barbosa MH, Che-Nordin N, Guglielmi G, Ruiz Santiago F. Semi-quantitative grading and extended semi-quantitative grading for osteoporotic vertebral deformity: a radiographic image database for education and calibration. Ann Transl Med 2020;8:398. [Crossref] [PubMed]
- Yoshida T, Nanba H, Mimatsu K, Kasai T. Treatment of osteoporotic spinal compression fractures. Conservative therapy and its limitation. Clin Calcium 2000;10:53-8.
- Jiang G, Eastell R, Barrington NA, Ferrar L. Comparison of methods for the visual identification of prevalent vertebral fracture in osteoporosis. Osteoporos Int 2004;15:887-96. [Crossref] [PubMed]
- Wáng YXJ. Osteoporotic vertebral endplate and/or cortex fracture is not always radiographically detectable. Osteoporos Int 2022; Epub ahead of print. [Crossref] [PubMed]
- Wáng YXJ, Che-Nordin N, Deng M, Leung JCS, Kwok AWL, He LC, Griffith JF, Kwok TCY, Leung PC. Osteoporotic vertebral deformity with endplate/cortex fracture is associated with higher further vertebral fracture risk: the Ms. OS (Hong Kong) study results. Osteoporos Int 2019;30:897-905. [Crossref] [PubMed]
- Wáng YXJ, Deng M, Griffith JF, Kwok AWL, Leung JCS, Lam PMS, Yu BWM, Leung PC, Kwok TCY. 'Healthier Chinese spine': an update of osteoporotic fractures in men (MrOS) and in women (MsOS) Hong Kong spine radiograph studies. Quant Imaging Med Surg 2022;12:2090-105. [Crossref] [PubMed]
- Deng M, Kwok TCY, Leung JCS, Leung PC, Wáng YXJ. All osteoporotically deformed vertebrae with >34% height loss have radiographically identifiable endplate/cortex fracture. J Orthop Translat 2018;14:63-6. [Crossref] [PubMed]
- Lentle BC, Berger C, Probyn L, Brown JP, Langsetmo L, Fine B, Lian K, Shergill AK, Trollip J, Jackson S, Leslie WD, Prior JC, Kaiser SM, Hanley DA, Adachi JD, Towheed T, Davison KS, Cheung AM, Goltzman D. CaMos Research Group. Comparative Analysis of the Radiology of Osteoporotic Vertebral Fractures in Women and Men: Cross-Sectional and Longitudinal Observations from the Canadian Multicentre Osteoporosis Study (CaMos). J Bone Miner Res 2018;33:569-79. [Crossref] [PubMed]
- Delmas PD, Genant HK, Crans GG, Stock JL, Wong M, Siris E, Adachi JD. Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone 2003;33:522-32. [Crossref] [PubMed]
- López Zúñiga D, Láinez-Ramos-Bossini AJ, Ruiz Santiago F. Radiographic diagnosis of osteoporotic vertebral fractures. An updated review. Med Clin (Barc) 2022;158:125-32. [Crossref] [PubMed]
- Ruiz Santiago F, Láinez Ramos-Bossini AJ, Wáng YXJ, López Zúñiga D. The role of radiography in the study of spinal disorders. Quant Imaging Med Surg 2020;10:2322-55. [Crossref] [PubMed]
- Ha KY, Kim YH. Risk factors affecting progressive collapse of acute osteoporotic spinal fractures. Osteoporos Int 2013;24:1207-13. [Crossref] [PubMed]
- Wáng YXJ. A modified semi-quantitative (mSQ) grading scheme for osteoporotic vertebral fracture in elderly women. Quant Imaging Med Surg 2019;9:146-50. [Crossref] [PubMed]
- Wáng YXJ, Che-Nordin N. Some radiographically 'occult' osteoporotic vertebral fractures can be evidential if we look carefully. Quant Imaging Med Surg 2019;9:1992-5. [Crossref] [PubMed]
- Deng M, Zeng XJ, He LC, Leung JCS, Kwok AWL, Griffith JF, Kwok T, Leung PC, Wáng YXJ. Osteoporotic Vertebral Fracture Prevalence in Elderly Chinese Men and Women: A Comparison of Endplate/Cortex Fracture-Based and Morphometrical Deformity-Based Methods. J Clin Densitom 2019;22:409-19. [Crossref] [PubMed]
- Ruiz Santiago F, Tomás Muñoz P, Moya Sánchez E, Revelles Paniza M, Martínez Martínez A, Pérez Abela AL. Classifying thoracolumbar fractures: role of quantitative imaging. Quant Imaging Med Surg 2016;6:772-84. [Crossref] [PubMed]
- Schnake KJ, Blattert TR, Hahn P, Franck A, Hartmann F, Ullrich B, et al. Classification of Osteoporotic Thoracolumbar Spine Fractures: Recommendations of the Spine Section of the German Society for Orthopaedics and Trauma (DGOU). Global Spine J 2018;8:46S-9S. [Crossref] [PubMed]
- Wáng YXJ, Liu WH, Diacinti D, Yang DW, Iannacone A, Wang XR, Kripa E, Che-Nordin N, Diacinti D. Diagnosis and grading of radiographic osteoporotic vertebral deformity by general radiologists after a brief self-learning period. J Thorac Dis 2020;12:4702-10. [Crossref] [PubMed]
- Lentle B, Koromani F, Brown JP, Oei L, Ward L, Goltzman D, Rivadeneira F, Leslie WD, Probyn L, Prior J, Hammond I, Cheung AM, Oei EH. Vertebral Fracture Research Groups of the CaMos, STOPP, and Rotterdam Studies. The Radiology of Osteoporotic Vertebral Fractures Revisited. J Bone Miner Res 2019;34:409-18. [Crossref] [PubMed]
- Wáng YXJ, Che-Nordin N, Leung JCS, Man Yu BW, Griffith JF, Kwok TCY. Elderly men have much lower vertebral fracture risk than elderly women even at advanced age: the MrOS and MsOS (Hong Kong) year 14 follow-up radiology results. Arch Osteoporos 2020;15:176. [Crossref] [PubMed]
- Lentle BC, Berger C, Brown JP, Probyn L, Langsetmo L, Hammond I, Hu J, Leslie WD, Prior JC, Hanley DA, Adachi JD, Josse RG, Cheung AM, Kaiser SM, Towheed T, Kovacs CS, Wong AKO, Goltzman D. Vertebral Fractures: Which Radiological Criteria Are Better Associated With the Clinical Course of Osteoporosis? Can Assoc Radiol J 2021;72:150-8. [Crossref] [PubMed]
- Abdel-Hamid Osman A, Bassiouni H, Koutri R, Nijs J, Geusens P, Dequeker J. Aging of the thoracic spine: distinction between wedging in osteoarthritis and fracture in osteoporosis--a cross-sectional and longitudinal study. Bone 1994;15:437-42. [Crossref] [PubMed]
- Wáng YXJ, Du MM, Che-Nordin N, Ye PP, Qiu SW, Griffith JF, Yan ZH. Recognizing osteoporotic vertebral deformity on frontal view radiograph: a cohort analysis and a pictorial review. Arch Osteoporos 2020;15:41. [Crossref] [PubMed]
- Wáng YXJ, Du EZ, Gong J, Cheng X. Interpretation of osteoporotic vertebral deformity on frontal view radiographs of the chest and abdomen: a pictorial review. Quant Imaging Med Surg 2021;11:423-42. [Crossref] [PubMed]
- Du EZ, Liu WH, Wáng YXJ. Improving osteoporotic vertebral deformity detection on chest frontal view radiograph by adjusted X-ray beam positioning. J Orthop Translat 2021;28:169-78. [Crossref] [PubMed]
- Wáng YXJ. Fragility fracture prevalence among elderly Chinese is no more than half of that of elderly Caucasians. Quant Imaging Med Surg 2022;12:874-81. [Crossref] [PubMed]
- Kung AW. Epidemiology and diagnostic approaches to vertebral fractures in Asia. J Bone Miner Metab 2004;22:170-5. [Crossref] [PubMed]
- Ballane G, Cauley JA, Luckey MM, El-Hajj Fuleihan G. Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporos Int 2017;28:1531-42. [Crossref] [PubMed]
- Ling X, Cummings SR, Mingwei Q, Xihe Z, Xioashu C, Nevitt M, Stone K. Vertebral fractures in Beijing, China: the Beijing Osteoporosis Project. J Bone Miner Res 2000;15:2019-25. [Crossref] [PubMed]
- Cui L, Chen L, Xia W, Jiang Y, Cui L, Huang W, Wang W, Wang X, Pei Y, Zheng X, Wang Q, Ning Z, Li M, Wang O, Xing X, Lin Q, Yu W, Weng X, Xu L, Cummings SR. Vertebral fracture in postmenopausal Chinese women: a population-based study. Osteoporos Int 2017;28:2583-90. [Crossref] [PubMed]
- Wáng YXJ, Lentle BC. Radiographic osteoporotic vertebral fractures in elderly men: a brief review focusing on differences between the sexes. Quant Imaging Med Surg 2020;10:1863-76. [Crossref] [PubMed]
- Lentle BC. Gender and the recognition of vertebral fractures. Quant Imaging Med Surg 2020;10:1401-7. [Crossref] [PubMed]
- Waterloo S, Søgaard AJ, Ahmed LA, Damsgård E, Morseth B, Emaus N. Vertebral fractures and self-perceived health in elderly women and men in a population-based cross-sectional study: the Tromsø Study 2007-08. BMC Geriatr 2013;13:102. [Crossref] [PubMed]
- Wáng YXJ, Che-Nordin N, Leung JCS, Kwok TCY. Existing severe osteoporotic vertebral fractures in elderly Chinese males were only weakly associated with higher further vertebral fracture risk at year-4 follow-up. Osteoporos Int 2020;31:1593-4. [Crossref] [PubMed]


