Late postpartum eclampsia complicated with posterior reversible encephalopathy syndrome: a case report and a literature review
Background
Posterior reversible encephalopathy syndrome (PRES) is a rare but serious clinical-neuroradiological entity, with manifestation of a series of nonspecific clinical signs and symptoms, including headache, vomiting, visual disturbances, altered mental status, seizures, and unconsciousness. The characteristic imaging findings include sub-cortical vasogenic edema at the bilateral parietal and occipital lobes (1,2). Pre-eclampsia and eclampsia are the most common causes of PRES (1-3). Recent studies have shown that almost all patients with eclampsia occur together with PRES (4). Pre-eclampsia is a systemic syndrome in pregnancy characterized by hypertension and proteinuria. If seizures occurs together with pre-eclampsia which cannot be explained by other causes, the diagnosis of eclampsia is established (5).
Most of pre-eclampsia or eclampsia occurs between 20 weeks of pregnancy and 48 hours postpartum. A few cases occur between 48 hours postpartum to four weeks postpartum, and those are called late postpartum pre-eclampsia or eclampsia. Late postpartum eclampsia accounts for less than 16% of the postpartum eclampsia (6-8). Late postpartum eclampsia concurrent with PRES is rare, and most obstetricians and neurologists have limited awareness of this entity (9). In clinical practice, both health care professionals and pregnant women tend to pay sufficient care to antenatal and intrapartum changes, however, insufficient attention is paid to postnatal care (10). Most diagnosis of postpartum pre-eclampsia is made after discharge and necessitates re-hospitalization. The average time from birth to re-hospitalization is 6.9 days on average (11).
Although in more than 90% cases of delayed postpartum eclampsia seizure there will be at least one early symptom suggesting pre-eclampsia, most of these patients do not report the symptoms to health care staff. Only less than 22% of the patients are diagnosed with pre-eclampsia. This results in most patients lost the opportunity to prevent eclampsia (10,11), thereby increases the risk of onset of PRES. Hereby we report a rare case of PRES with late postpartum eclampsia. Initially this patient missed the diagnosis of pre-eclampsia and PRES. In order to raise awareness of this entity, we also carried out a literature review for PRES.
Case presentation
It was a 28-year-old pregnant woman with full-term pregnancy. The patient had no history of hypertension or neurological, mental illness. Blood pressure was normal during the pregnancy period. Cesarean section was performed due to the fetal cord wrapped around the neck of the baby. The surgery was smooth. Mildly elevated blood pressure (with the highest measurement being 134/94 mmHg) was found during the postoperative day. There was no other obvious discomfort and no treatment was administered. In the 6th day after surgery, the patient was discharged with abdominal incision healed well.
The next day after the patient was discharged, due to that her husband left home to work elsewhere, the patient had mood swings and did not sleep for one night. The followed morning the patient had blurred vision, headache, fever, with a blood pressure of 160/90 mmHg and body temperature of 38.0 °C. At noon the patient appeared wondering minded, and she was re-administered to the department of obstetrics at 18:30 pm.
At the admission, the patient’s vital signs included blood pressure 150/100 mmHg, body temperature 38.6 °C, pulse 108 beats/min, breathing 18 times/min, blurred consciousness, indifferent to questions. Bilateral pupils were round with normal size, and the light reflex was normal. The lungs had coarse breath sounds. Heart rate was 108 beats/min with regular rhythm. The patient was able to have free physical activity. Preliminary diagnosis was hypertensive disorder of pregnancy.
Patient had a sudden seizure at 19:20 pm, showing eyes hanging, convulsion of the limbs, cyanotic lips, foaming at the mouth, blood pressure 143/97 mmHg, heart beat 131 beats/min, SPO2 90%. The diagnosis of postpartum eclampsia was considered. Immediate rescue measures included: oxygen administration, magnesium sulfate 5 g intravenous bolus, followed by continue intravenous drip magnesium sulfate 1.5 g/hour to relieve spasms, diazepam 10 mg intramuscular injection, lytic cocktail slow intravenous push initially, then followed by an intravenous drip. Further medication included intravenous mannitol, furosemide 20 mg intravenous injection, dexamethasone 10 mg intravenous injection. Patient’s seizure lasted about 2 minutes and then remission occurred and the patient went to sleep.
Emergency laboratory test showed: neutrophils (76.5%) slightly higher than normal range; potassium decreased slightly (3.38 mmol/L); lactate dehydrogenase (LDH) 272 U/L slightly above the normal range (50-240 U/L); proteinuria 3+. Red blood cell count, platelet count, hemoglobin levels, renal function, blood coagulation, blood glucose were all within normal range.
Brain CT showed low density in bilateral occipital lobes and basal ganglia, left temporal lobe and left parietal lobe, suggesting possible infarction. Lung CT demonstrated bilateral lung partial consolidation, bilateral pleural effusions, pericardial effusion, suggesting bilateral lung infection.
Urgent consultation was sought from neurology, neurosurgery, ophthalmology, respiratory medicine and cardiology. During examination the patient was uncooperative, bilateral pupil diameter was about 2.0 mm, light reflex existed. Neurologic examination showed normal limb tendon reflexes, bilateral Babinski’s sing (−), bilateral Chaddock’s sing (+). Pain stimulation was induced activity in the four limbs, but with significantly reduced activity of the right limbs than the left limbs. Taking into account that the patient might have cerebral infarction, she was transferred to the Department of Neurology at 21:30 pm for cerebral infarction treatment. Treatment of anticoagulation, anti-platelet aggregation, cerebral edema reduction, anti-inflammatory treatment and other supportive treatment were administered.
The second day after admission, the patient's condition improved markedly with a clear mind and no headaches, though spirit remained still poor. The blood pressure was 130/80 mmHg. Neurological examination showed the left Babinski’s sign (+), the left Chaddock’s sign (+), with no other significant abnormality. Liver function test showed total protein 49.8 g/L, albumin 30.8 g/L, globulin 19.0 g/L, all were lower than normal, while liver enzymes were within the normal range. Considering bilateral pleural effusion and pericardial effusion might be associated with hypoalbuminemia, complement human serum albumin was administered to correct hypoproteinemia.
Brain MRI was performed to further assess brain lesions. MRI reported left frontal, bilateral parietal lobe, basal ganglia, temporal lobe and occipital lobe showed low signal on T1-weighted images, high signals on T2-weighted images and fluid-attenuated inversion application recovery (FLAIR) images. Diffusion weighted imaging (DWI) showed slightly higher signal and apparent diffusion coefficient (ADC) map showed high signal (Figure 1A-F). MR angiography examination showed internal carotid arteries, middle cerebral arteries (MCA), vertebral basilar artery, and bilateral posterior cerebral artery had clear display but with stiff course and slender branches. Bilateral anterior cerebral artery lumen A1 segment was slender and the signal was reduced meeting cerebral vasospasm appearance (Figure 1G). MR Venography examination showed no abnormalities.
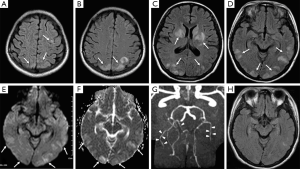
On the third day, the patient was generally in good condition with no obvious symptoms, neurological examination showed no positive signs. Vital signs were normal. Lung breath sounded rough, with slight moist rales. Cerebrospinal fluid showed colorless, transparent, with protein 0.64 mmol/L (normal: 0.10–0.40 mmol/L), LDH 18 U/L (normal: 50–240 U/L), and the remaining tests were in the normal range.
With the improvement of the symptoms and signs after treatment, together with the MRI characteristics, cerebral vasospasm and PRES was considered. Further treatment was to relieve cerebral vasospasm, while continuing to provide anti-inflammatory treatment, human serum albumin supplementation and other supportive treatments. Follow-up brain MRI 6 days later demonstrated edema lesions mostly resolved (Figure 1H).
The patient was hospitalized for 2 weeks, besides the blurred vision was completely remised only 1 day before discharge, no other symptoms reappeared. Prior to the discharge, the patient had transcranial Doppler examination showing normal blood flow spectrum, with bilateral middle cerebral artery having a slow blood flow and peak constriction value lower than normal. Blood flow velocity in other arteries was normal, suggesting cerebral vasospasm disappeared. The final diagnosis of this patient was: (I) late postpartum eclampsia; (II) PRES; (III) lung infection.
Discussion
The recognition of PRES
In 1996 Hinchey et al. (12) reported 15 cases of reversible posterior leukoencephalopathy syndrome (RPLS). From then on, RPLS is gradually being recognized by clinicians. With the application of MRI FLAIR technology, Casey et al. (13) found that in 94% of RPLS patients cerebral cortex is also involved, and the number of lesions in the cortex accounted for 46% of all lesions. Therefore, in 2000 a new name was proposed and PRES was used to replace RPLS, and this has been widely recognized.
The etiology of PRES is not clear. A variety of conditions and predisposing factors are associated with PRES which include hypertension, pre-eclampsia/eclampsia, the application of organ transplantation immunosuppression agents (especially cyclosporine, tacrolimus), cancer chemotherapy, autoimmune diseases (e.g., systemic lupus erythematosus, Wegener’s granulomatosis, etc.), infection or sepsis (1). Among these factors, pre-eclampsia and eclampsia are most commonly reported in the literature (1,3). Pre-eclampsia has the incidence of between about 3–8.7%, the incidence of eclampsia is less than 10/10,000 birth in most countries (5). In addition to the typical pre-eclampsia/eclampsia, individual atypical cases may occur before 20 weeks of pregnancy or 48 hours after giving birth. There are a few atypical cases without the appearance of hypertension or proteinuria (6).
Pathophysiology of PRES
The exact mechanism of PRES is not yet fully elucidated. There exist two opposing pathophysiological mechanisms explanation. The first hypothesis is the vasospasm/hypoperfusion doctrine. This doctrine suggests the various risk factors cause vasospasm or vasoconstriction, followed by in turn causing brain hypoperfusion, cerebral ischemia, resulting in further brain vasogenic edema. The second hypothesis, on the contrary, is the hypertension/hyperperfusion hypothesis. This theory suggests that, when severe hypertension surpasses the vascular autoregulation range, it will cause small arteries passive expansion, vascular endothelial damage and excessive perfusion, leading to vasogenic edema in brain tissue (14).
Based on an intuitive understanding of hypertension hazards and 75% PRES patients had moderate to severe hypertension, as well as the patients improved after antihypertensive treatment, hypertension/hyperperfusion theory is popular currently (14,15). The previous main evidence was mostly from animal experiments and clinical evidence only come from a small number of isolated cases (14,16). Recently a large single center study supported this theory (17). Despite these, there are still some significant doctrine defects as the upper limit of autoregulation in human for mean arterial pressure is 150–160 mmHg, while the degree of blood pressure in most PRES patients are within the limits of self-regulation, and also 20-30% patients have normal or only mildly elevated blood pressure (14,18).
Following more and more imaging techniques being applied for the assessment of PRES, increasing evidences support vasospasm/hypoperfusion hypothesis. Both catheter angiography and MR angiography demonstrated vasospasm, vasoconstriction, and/or string-of-bead appearance (18-20). In addition, noninvasive transcranial Doppler examination found that most patients diagnosed with pre-eclampsia/eclampsia showed the hemodynamics characteristics of cerebral vasospasm (21,22). However, the exploration of pathophysiology of PRES is still ongoing, two mechanisms may co-exist in some cases (23).
The highest blood pressure in our case was 150–160/90–100 mmHg, i.e., 113–117 mmHg mean arterial pressure (1/3 systolic + 2/3 diastolic pressure), below the upper limit of autoregulation of blood vessels, and also MRA showed arterial spasm characteristics in the cerebral edema regions. Therefore evidences in our case seem to support vasospasm/hypoperfusion hypothesis.
Although the specific pathogenesis of pre-eclampsia is not defined, but the placenta in its central role in the pathogenesis is clear (24). With the formation of the placenta, the placental ischemia caused more syncytial surface tissue apoptosis and necrosis and dropping off, and together with activated T-helper cells release large amounts of inflammatory cytokines, including tumor necrosis factor (TNF-α), interleukin (IL)-1, interferon (IFN)-γ and IL-6 into the maternal blood circulation causing severe maternal systemic immune response (toxemia of pregnancy), thereby leading to systemic endothelial cell activation and injury. Activated endothelial cells secrete large amounts of inflammatory mediators and vasoconstrictor substances, induce diffuse systemic vascular contraction, and then cause brain vasogenic edema (14,24-26). The occurrence of pre-eclampsia postpartum placental fragments residue is likely one of the causes (27). Endothelial cell injury can also cause platelet adhesion, hemolysis, and protein and body fluid extravasation. Severe cases can have thrombocytopenia, elevated LDH, abnormal red blood cell morphology and generalized edema. Glomerular endothelial dysfunction can cause loss of electrolytes, fluids and protein, leading to water and electrolyte imbalance and hypoproteinemia (1,28). Clinically red blood cell morphology and LDH levels are usually used as indicators of endothelial injury (29). Cerebrospinal fluid is usually normal, though mildly elevated protein has been occasionally reported (1).
In our case the laboratory results showed serum potassium 3.38 mmol/L (slightly decreased), LDH 272 U/L (normal range: 50–240 U/L), urine protein 3+. Liver function showed total protein 49.8 g/L, albumin 30.8 g/L, and globulin 19.0 g/L, all were lower than normal value. These results suggested serious vascular endothelial injury.
Clinical symptoms and imaging findings of PRES
PRES presents as a series of non-specific signs and symptoms, mainly headache, vomiting, visual disturbances, altered mental status, seizures, and unconsciousness, with the most common ones being seizure and headache (1,12,20,30). Seizure usually presents as generalized seizure (1,5,31). Visually impairment is also common including blurred vision, hemianopia, visual neglect and cortical blindness (1,12). These symptoms can be acute, and may occur gradually in a few days (20). Some patients showed decreased or asymmetric limb muscle strength, while tendon reflexes are usually active (31,32).
Typical imaging findings of PRES include reversible vasogenic subcortical edema at bilateral parietal lobes and occipital lobes. Frontal lobes, temporal lobes and cerebellar hemisphere are often involved. Some atypical sites may include the basal ganglia, brainstem and deep white matter. In addition, edema may also occur only in one side and showed asymmetry (2,33,34). Anatomically, compared with the internal carotid artery system, the distribution of sympathetic nerve fibers in the vertebrobasilar system is scarce (35), which may lead to self-regulation of arterial blood pressure depending on the sympathetic innervation is significantly lower in the posterior cerebrum than in the front cerebrum, which is probably the cause that edema most commonly occur in posterior cerebrum (29,36,37).
Initial radiological diagnosis of PRES depends mainly brain CT scan and MRI. These examinations can determine the site of cerebral edema, but cannot distinguish the nature of cerebral edema. The diagnosis requires continuous observation of the clinical manifestations. Misdiagnose such as cerebral infarction might be made and lead to delayed treatment (12). Brain edema show low density in CT scans, and showed low signal or iso-signals on the T1-weighted image, high signal on the T2-weighted image (12,38). FLAIR sequence can effectively inhibit interference of high signal cerebrospinal fluid to surrounding tissue edema. It is the most sensitive method for detecting cerebral edema, and is particularly important for diagnosing mild cerebral edema. The same as on T2-weighted image, brain edema also shows high signal on FLAIR sequence, however, FLAIR sequence still can not identify the nature of cerebral edema (13). Based on the movement of water molecules, DWI simultaneously reflects the properties of water diffusion and T2 characteristic of the tissue, while ADC map reflect diffusion characteristics of water (39). With vasogenic edema, DWI manifests low signal or iso-signals, but sometimes due to the impact of the T2 signal can also be rendered slightly higher signal, this phenomenon is known as T2 “shine-through” effect. Vasogenic edema displays as high signal on ADC map, while cytotoxic edema show high signal on DWI and show as a low signal on ADC map (38-44).
Differential diagnosis of PRES
PRES needs to be differentiated from many diseases, mainly cerebral venous sinus thrombosis (CVST) and ischemic stroke (31,40), but also including acute or sub-acute cerebrovascular diseases, central nervous system infections and autoimmune diseases, and mitochondrial diseases. CVST is the most common puerperium cerebrovascular disease and sometimes has same clinical manifestations as PRES (45,46). The differential diagnosis of PRES and CVST is through MRI and MRA/MRV (31,46). The differentiation of PRES and ischemic stroke is of vital importance for the treatment and prognosis (41). In ischemic stroke, mild to moderate hypertension is not treated. While for PRES, aggressive treatment of hypertension should be taken to prevent the development of irreversible brain damage (31,47). In addition, early diagnosis may also provide for the opportunity of thrombolytic therapy for ischemic stroke (48,49).
Quantification of ADC, (ADC mapping) is necessary to differentiate cytotoxic edema from vasogenic edema. And this may be crucial for therapeutic and clinical outcome. Cytotoxic edema is caused by acute ischemia and infarction, with subsequently decreased ADC through a reduction in the diffusibility of protons. High ADC values are consistent with highly mobile water in areas of vasogenic edema (50). MRI shows changes to occur typically in the territory supplied by the posterior circulation, with anterior circulation abnormalities only seen in more severe cases. MRI can exclude differential diagnoses, such as infective encephalitis, sinus thrombosis, and cerebral ischemia. In patients following organ or bone marrow transplantation, the differential diagnosis is often infective encephalitis. The characteristic distribution of lesions on MRI is often helpful in this regard. However vasogenic edema and cytotoxic edema can co-exist. Koch et al. described one case with the presence of both cytotoxic and vasogenic edema, as detected by diffusion-weighted imaging, in a woman with eclampsia. Follow-up MR imaging showed that the regions of cytotoxic edema progressed to cerebral infarction (51).
Treatment of PRES
With correct diagnosis and timely and effective early treatment, the patient prognosis is usually good. Neurological symptoms, signs and radiographic changes usually disappear completely in 1–2 weeks, while delayed diagnosis and/or incorrect treatment likely cause irreversible neurological damage or even death (12,15,38). Treatment includes treatment of the primary disease, control of neurological symptoms and hypertension.
For pre-eclampsia/eclampsia induced PRES, the key treatment is to control hypertension and prevention or treatment of seizure and termination of pregnancy when necessary. When the contraction pressure rises up to and continues for more than 160 mmHg and/or diastolic blood pressure rises up to 105–110 mmHg, anti-hypertensive drugs, including labetalol, hydralazine, nicardipine or short-acting oral nifedipine, should be applied. The antispasmodic drug of choice is magnesium sulphate. For the treatment of epileptic seizures, an intravenous loading dose is administered initially, followed by a continuous maintenance dose infusion. Other drugs include magnesium sulphate with diazepam, phenytoin, or a lytic cocktail. Other treatments include diuretic agents and corticosteroids such as dexamethasone or betamethasone (3,4,25).
Our patient was a rare case of late postpartum eclampsia complicated with PRES. This patient had mild hypertension the same day after delivery. Because there was no other discomfort she was discharged from the hospital. In the morning of the seizure event, the patient had blurred vision, headaches and other neurological symptoms, and these symptoms gradually worsened. Based on that this patient have no history of hypertension, while there was postpartum high blood pressure as well as neurological and psychiatric symptoms, we considered this patient might have “pre-eclampsia”.
After eclampsia, in our case CT was first used to probe intracranial lesions, and the diagnosis of cerebral infarction was suggested and treatment started. Despite only on the third day after the hospital re-admission, with the completion of brain MRI, the diagnosis of PRES was established, the obstetricians’ treatment for eclampsia was timely and effective. Neurologists’ measures to reduce cerebral edema and cerebral vasospasm and other “infarction” treatment measures were beneficial for PRES, and therefore the patient recovered.
Conclusions
Puerpera are at the risk of postpartum pre-eclampsia and its complications. Health care workers and mothers should pay attention to physical changes in the postpartum period, with close monitoring of blood pressure and other neuropsychiatric symptoms. Obstetricians, neurologists, ophthalmologists and radiologists should be familiar with the clinical and imaging features of PRES. Radiological changes provide key characteristics for early diagnosis for PRES. When suspected, brain MRI should be performed, especially DWI and ADC map should be acquired. The key to full recovery of PRES is early diagnosis and treatment. Misdiagnosis and delayed treatment may cause neurological permanent damage.
Acknowledgements
Funding: Grant support from Science & Technology Support Program of Hebei Province (No. 122777142) is gratefully acknowledged.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Consents: Written consent was obtained from the patient for publication of this study.
References
- Staykov D, Schwab S. Posterior reversible encephalopathy syndrome. J Intensive Care Med 2012;27:11-24. [PubMed]
- Stevens CJ, Heran MK. The many faces of posterior reversible encephalopathy syndrome. Br J Radiol 2012;85:1566-75. [PubMed]
- Nasr R, Golara M, Berger J. Posterior reversible encephalopathy syndrome in a woman with pre-eclampsia. J Obstet Gynaecol 2010;30:730-2. [PubMed]
- Brewer J, Owens MY, Wallace K, Reeves AA, Morris R, Khan M, LaMarca B, Martin JN Jr. Posterior reversible encephalopathy syndrome in 46 of 47 patients with eclampsia. Am J Obstet Gynecol 2013;208:468.e1-6.
- Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2011;25:391-403. [PubMed]
- Sibai BM1, Stella CL. Diagnosis and management of atypical preeclampsia-eclampsia. Am J Obstet Gynecol 2009;200:481.e1-7.
- Martin J, Sidman R. Late postpartum eclampsia: a common presentation of an uncommon diagnosis. J Emerg Med 2003;25:387-90. [PubMed]
- Chhabra S, Tyagi S, Bhavani M, Gosawi M. Late postpartum eclampsia. J Obstet Gynaecol 2012;32:264-6. [PubMed]
- Chiou YH, Chen PH. Reversible posterior encephalopathy syndrome as the presentation of late postpartum eclampsia: a case report. Acta Neurol Taiwan 2007;16:158-62. [PubMed]
- Chames MC, Livingston JC, Ivester TS, Barton JR, Sibai BM. Late postpartum eclampsia: a preventable disease? Am J Obstet Gynecol 2002;186:1174-7. [PubMed]
- Matthys LA, Coppage KH, Lambers DS, Barton JR, Sibai BM. Delayed postpartum preeclampsia: an experience of 151 cases. Am J Obstet Gynecol 2004;190:1464-6. [PubMed]
- Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas JL, Caplan LR. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996;334:494-500. [PubMed]
- Casey SO, Sampaio RC, Michel E, Truwit CL. Posterior reversible encephalopathy syndrome: utility of fluid-attenuated inversion recovery MR imaging in the detection of cortical and subcortical lesions. AJNR Am J Neuroradiol 2000;21:1199-206. [PubMed]
- Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol 2008;29:1043-9. [PubMed]
- Striano P, Striano S, Tortora F, De Robertis E, Palumbo D, Elefante A, Servillo G. Clinical spectrum and critical care management of Posterior Reversible Encephalopathy Syndrome (PRES). Med Sci Monit 2005;11:CR549-53. [PubMed]
- Apollon KM, Robinson JN, Schwartz RB, Norwitz ER. Cortical blindness in severe preeclampsia: computed tomography, magnetic resonance imaging, and single-photon-emission computed tomography findings. Obstet Gynecol 2000;95:1017-9. [PubMed]
- Li Y, Gor D, Walicki D, Jenny D, Jones D, Barbour P, Castaldo J. Spectrum and potential pathogenesis of reversible posterior leukoencephalopathy syndrome. J Stroke Cerebrovasc Dis 2012;21:873-82. [PubMed]
- Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol 2008;29:447-55. [PubMed]
- Brubaker LM, Smith JK, Lee YZ, Lin W, Castillo M. Hemodynamic and permeability changes in posterior reversible encephalopathy syndrome measured by dynamic susceptibility perfusion-weighted MR imaging. AJNR Am J Neuroradiol 2005;26:825-30. [PubMed]
- Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol 2008;29:1036-42. [PubMed]
- Hansen WF, Burnham SJ, Svendsen TO, Katz VL, Thorp JM Jr, Hansen AR. Transcranial Doppler findings of cerebral vasospasm in preeclampsia. J Matern Fetal Med 1996;5:194-200. [PubMed]
- Williams K, Galerneau F. Maternal transcranial Doppler in pre-eclampsia and eclampsia. Ultrasound Obstet Gynecol 2003;21:507-13. [PubMed]
- Pula JH, Eggenberger E. Posterior reversible encephalopathy syndrome. Curr Opin Ophthalmol 2008;19:479-84. [PubMed]
- Redman CW, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response--a review. Placenta 2003;24 Suppl A:S21-7.
- Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet 2005;365:785-99. [PubMed]
- Noris M, Perico N, Remuzzi G. Mechanisms of disease: Pre-eclampsia. Nat Clin Pract Nephrol 2005;1:98-114. [PubMed]
- Delefosse D, Samain E, Helias A, Regimbeau JM, Deval B, Farah E, Marty J. Late onset of cortical blindness in a patient with severe preeclampsia related to retained placental fragments. Anesthesiology 2003;98:261-3. [PubMed]
- Hypertensive Disorders in Pregnancy. In: Cunningham FG, Hauth JC, Leveno KJ, Gilstrap L, Bloom SL, Wenstrom KD, editors. Williams Obstetrics. 22nd ed. New York: McGraw-Hill, 2005;761-808.
- Schwartz RB, Feske SK, Polak JF, DeGirolami U, Iaia A, Beckner KM, Bravo SM, Klufas RA, Chai RY, Repke JT. Preeclampsia-eclampsia: clinical and neuroradiographic correlates and insights into the pathogenesis of hypertensive encephalopathy. Radiology 2000;217:371-6. [PubMed]
- Liman TG, Bohner G, Heuschmann PU, Scheel M, Endres M, Siebert E. Clinical and radiological differences in posterior reversible encephalopathy syndrome between patients with preeclampsia-eclampsia and other predisposing diseases. Eur J Neurol 2012;19:935-43. [PubMed]
- Servillo G, Bifulco F, De Robertis E, Piazza O, Striano P, Tortora F, Striano S, Tufano R. Posterior reversible encephalopathy syndrome in intensive care medicine. Intensive Care Med 2007;33:230-6. [PubMed]
- Kwon S, Koo J, Lee S. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Pediatr Neurol 2001;24:361-4. [PubMed]
- Bartynski WS, Boardman JF. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol 2007;28:1320-7. [PubMed]
- McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS, Teksam M. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol 2007;189:904-12. [PubMed]
- Edvinsson L, Owman C, Sjöberg NO. Autonomic nerves, mast cells, and amine receptors in human brain vessels. A histochemical and pharmacological study. Brain Res 1976;115:377-93. [PubMed]
- Sheth RD, Riggs JE, Bodenstenier JB, Gutierrez AR, Ketonen LM, Ortiz OA. Parietal occipital edema in hypertensive encephalopathy: a pathogenic mechanism. Eur Neurol 1996;36:25-8. [PubMed]
- Vaughan CJ, Delanty N. Hypertensive emergencies. Lancet 2000;356:411-7. [PubMed]
- O'Hara McCoy H. Posterior reversible encephalopathy syndrome: an emerging clinical entity in adult, pediatric, and obstetric critical care. J Am Acad Nurse Pract 2008;20:100-6. [PubMed]
- Provenzale JM, Engelter ST, Petrella JR, Smith JS, MacFall JR. Use of MR exponential diffusion-weighted images to eradicate T2 "shine-through" effect. AJR Am J Roentgenol 1999;172:537-9. [PubMed]
- Lamy C, Oppenheim C, Méder JF, Mas JL. Neuroimaging in posterior reversible encephalopathy syndrome. J Neuroimaging 2004;14:89-96. [PubMed]
- Schaefer PW, Buonanno FS, Gonzalez RG, Schwamm LH. Diffusion-weighted imaging discriminates between cytotoxic and vasogenic edema in a patient with eclampsia. Stroke 1997;28:1082-5. [PubMed]
- Wang YX, Ng CK. The impact of quantitative imaging in medicine and surgery: Charting our course for the future. Quant Imaging Med Surg 2011;1:1-3. [PubMed]
- Chou MC, Lai PH, Yeh LR, Li JY, Yuan MK, Liang HL, Chen C, Pan HB, Lo YK, Yang CF. Posterior reversible encephalopathy syndrome: magnetic resonance imaging and diffusion-weighted imaging in 12 cases. Kaohsiung J Med Sci 2004;20:381-8. [PubMed]
- Winston GP. The physical and biological basis of quantitative parameters derived from diffusion MRI. Quant Imaging Med Surg 2012;2:254-65. [PubMed]
- Jeng JS, Tang SC, Yip PK. Incidence and etiologies of stroke during pregnancy and puerperium as evidenced in Taiwanese women. Cerebrovasc Dis 2004;18:290-5. [PubMed]
- Zis P, Tavernarakis A. Headache and status epilepticus in the postpartum period; posterior reversible encephalopathy syndrome or cerebral venous thrombosis? Case Rep Emerg Med 2013;2013:680327.
- Stott VL, Hurrell MA, Anderson TJ. Reversible posterior leukoencephalopathy syndrome: a misnomer reviewed. Intern Med J 2005;35:83-90. [PubMed]
- Soltes L, Schmalfuss IM, Bhatti MT. Cortical blindness due to reversible posterior leukoencephalopathy syndrome in a patient with thrombotic thrombocytopenic purpura and preeclampsia. Arch Ophthalmol 2004;122:1885-7. [PubMed]
- Servillo G, Striano P, Striano S, Tortora F, Boccella P, De Robertis E, Rossano F, Briganti F, Tufano R. Posterior reversible encephalopathy syndrome (PRES) in critically ill obstetric patients. Intensive Care Med 2003;29:2323-6. [PubMed]
- Doelken M, Lanz S, Rennert J, Alibek S, Richter G, Doerfler A. Differentiation of cytotoxic and vasogenic edema in a patient with reversible posterior leukoencephalopathy syndrome using diffusion-weighted MRI. Diagn Interv Radiol 2007;13:125-8. [PubMed]
- Koch S, Rabinstein A, Falcone S, Forteza A. Diffusion-weighted imaging shows cytotoxic and vasogenic edema in eclampsia. AJNR Am J Neuroradiol 2001;22:1068-70. [PubMed]


