Antisynthetase syndrome: a case report
Introduction
Antisynthetase syndrome (ASS) is quite a rare condition and early diagnosis is the key in management (1-3).
Case presentation
A 48-year-old United Kingdom born Caucasian male with no significant past medical history was admitted to the respiratory ward with a 1-week history of worsening shortness of breath, fever and polyarthralgia. He also described left sided chest pain, worse on the deep inspiration. His exercise tolerance had significantly reduced from a normal active life style to being unable to brush his teeth or comb his hair. He was an ex-smoker (20 pack years history), no alcohol use, no known allergies and did not take any regular medications.
Physical examination revealed bilateral fine basal crepitation, oxygen saturations of 95% on room air and blood pressure 118/75 mmHg. Cardiovascular, abdominal and neurological system examinations were unremarkable. The metacarpo-phalangeal and proximal interphalangeal joints of both hands were swollen and tender to joint margin palpation.
Laboratory and investigative findings
Full blood count was normal. Routine biochemistry and liver function tests were normal. His erythrocyte sedimentation rate was 25 (0-15 mm/hr) and C-reactive protein was 63 (0-10 mg/L). Creatine kinase (CK) levels were elevated at 646 (0-20 mg/L). Urinalysis, hepatitis serology, HIV screen, and serum ferritin levels were unremarkable.
Rheumatology workup showed a negative rheumatoid factor (<20.0) but positive anti-aminoacyl-transfer RNA synthetase result with positivity for anti-Ro and anti-Jo-1 antibodies (negative for anti-La, anti-RNP and anti-Sm antibodies). Antiglomerular basement membrane and scl-70 were negative. Serum angiotensin converting enzyme, complement, and cryoglobulins levels were normal. There were also negative results for blood cultures, influenza A and B, Mycoplasma pneumoniae, Chlamydia, Q fever, respiratory syncytial virus, adenovirus, legionella pneumophilia and pneumococcal antigen serology.
Electrocardiogram showed sinus tachycardia with no other changes. Dyspnoea precluded lung function testing and bronchoscopy. Chest radiograph showed subtle areas of opacification scattered throughout both lower lobes. A high resolution computed tomography (HRCT) scan showed lower lobe predominant multi-focal ground glass and reticular infiltrates with scattered regions of peri-bronchial consolidation and a few small scattered sub-5 mm nodules (Figure 1). There was no honeycomb change, cavitating lesions and no effusion. A few mildly enlarged mediastinal lymph nodes were noted and considered reactive. The spleen was not enlarged and the bones unremarkable. Overall appearances were most in keeping with non-specific interstitial pneumonitis (NSIP).
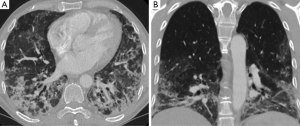
The clinical features in conjunction with elevated CK and anti-Ro and anti-Jo-1 positivity and the HRCT findings lead to a presumptive diagnosis of ASS. The patient was commenced on oral corticosteroids and a cyclophosphamide infusion fortnightly for 3 months, ambulatory and nocturnal oxygen. His CK levels gradually reduced and there was a significant improvement in exercise tolerance, oxygen requirements and breathing during this period.
Discussion
ASS is a rare systemic autoimmune syndrome characterized by a combination of interstitial lung disease (ILD), myopathy, fever and polyarthralgia. It has a reported prevalence of 1.5 per 100,000 populations. The hallmark of ASS are antibodies against aminoacyl-transfer RNA synthetase of which the most frequent is the anti-Jo-1 antibody which is present in approximately 80% of cases (1). Less commonly encountered antibodies in ASS are including anti-PL-7 and anti-EJ (2).
ILD is the most frequently occurring manifestation of ASS being reported in 80-90% of cases and as such lung involvement is considered to be the most important prognostic indicator. Indeed it has been suggested that ASS antibodies should be routinely tested in all patients with ILD, as the clinical presentation of ASS is frequently non-specific in the early stages (3). ILD in ASS is usually apparent on plain chest radiographs but high resolution CT is the imaging technique of choice to characterize the pattern and distribution of disease. Reported HRCT findings in ASS are regions of diffuse patchy ground-glass opacification with a lower lobe and sub-pleural predominance, peri-bronchial consolidations, reticular change and fine nodularity. HRCT is also helpful to exclude the presence of cavitating lesions which are more suggestive of a vasculitic process as well as signs of infective change. NSIP is the most frequently reported histological pattern in ASS and the presence of fibrosis (as opposed to cellular infiltrates) is an adverse predictor for response to immunosuppressive therapies (1-3).
Joint symptoms are reported in around 60% of patients with ASS and are more common in anti-Jo-1 positive ASS patients however they are non-specific and often mimic more common inflammatory arthropathies such as rheumatoid arthritis (1). Myositis is reported in 30-60% of ASS patients and anti-Jo-1 positivity can pre-date clinical myositis (1). Most treatment regimens for ASS describe a combination of glucocorticosteroids and other immunosuppressant such as cyclophosphamide with pulmonary disease response dictating treatment duration (1).
In summary ASS is a rare systemic autoimmune condition characterized by a combination of ILD, myopathy, fevers and polyarthralgia. HRCT typically shows an NSIP pattern and may help to suggest the diagnosis in a patient presenting with appropriate clinical features.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Katzap E, Barilla-LaBarca ML, Marder G. Antisynthetase syndrome. Curr Rheumatol Rep 2011;13:175-81. [Crossref] [PubMed]
- Solomon J, Swigris JJ, Brown KK. Myositis-related interstitial lung disease and antisynthetase syndrome. J Bras Pneumol 2011;37:100-9. [Crossref] [PubMed]
- Mielnik P, Wiesik-Szewczyk E, Olesinska M, et al. Clinical features and prognosis of patients with idiopathic inflammatory myopathies and anti-Jo-1 antibodies. Autoimmunity 2006;39:243-7. [Crossref] [PubMed]


