
The significance of dual-mode elastography in the diagnosis of breast lesions by physicians with different levels of experience
Introduction
Breast cancer is a serious disease that threatens the health of women worldwide and is the primary cause of death in females (1,2). In recent years, the incidence of breast cancer has risen (3). At the same time, with the continuous improvement of medical technology, the survival rate of breast cancer has also continued to rise (4). However, early diagnosis and treatment of breast cancer are still necessary, and it is therefore urgent to find an effective detection technology for breast cancer.
Mammography is a valuable tool to detect early-stage breast cancer (5). However, high breast density significantly reduces the accuracy of mammographic diagnosis (6). Conventional ultrasound is a valuable auxiliary imaging technique, as it is low cost and does not expose patients to ionizing radiation. It also provides high sensitivity in differentiating benign from malignant breast lesions (7-9). For these reasons, conventional ultrasound has been widely used for early breast cancer examinations. However, it is also highly subjective, has poor specificity, and exhibits some limitations in differentiating certain benign and malignant breast lesions with no salient sonographic features (10). Elastography can provide information about tissues that cannot be obtained using conventional ultrasound. It is also an effective tool for obtaining important information for the differential diagnosis of breast cancer (11). The breast imaging reporting and data system (BI-RADS) provides standardised terminology descriptions, evaluations and recommendations for breast lesions according to their characteristics (12).
At present, there are two main elastography methods used to assess breast lesions: shear wave elastography (SWE) and strain elastography (SE) (13,14). SE can evaluate any shape changes of lesions resulting from changes in the degree of external compression (15). Pressure can be applied by the patient’s own physiologic movements, such as their breathing or heartbeat, or by the rhythmic movement of an ultrasound transducer. As the pressure applied is not quantifiable, SE is a qualitative index, and as such, may lead to subjective judgments. To improve the diagnosis, the strain ratio (SR) can be introduced as a quantitative index. To assess the SR, regions of interest (ROIs) are placed in the fatty tissue and the lesion. Software can then automatically calculate the relative elasticity of the two ROIs (16). Earlier studies reported that the SR was effective in the diagnosis of benign and malignant breast lesions (17-20).
In SWE, the vibration generated by an acoustic radiation force impulse causes the tissue to vibrate parallel to the direction of the sound beam. This generates a shear wave vibration in the surrounding tissue, which oscillates perpendicular to the direction of the sound beam and propagates in the form of transverse waves (21). By observing the speed at which the shear waves reach different fronts, the stiffness of the corresponding tissue can be calculated. Shear waves travel faster in harder tissue and more slowly in softer tissue (22-24). The maximum elasticity, mean elasticity, minimum elasticity, and elasticity standard deviation for the shear modulus (G), Young’s modulus (E), and shear wave velocity (Cs) of lesions can be quantitatively calculated using SWE (25,26). Different elasticity moduli in SWE can provide effective means for differentiating between benign and malignant breast lesions (27).
The current study set out to investigate the diagnostic value of different elastography modes in SE and SWE for benign and malignant breast lesions. Further, the diagnostic efficiency of physicians with different levels of experience (one senior physician, one intermediate-level physician, and one junior physician) was compared before and after using dual-mode elastography for breast lesions to determine whether there was any improvement.
The following article is presented in accordance with the Standards for Reporting Diagnostic Accuracy (STARD) guidelines (available at https://dx.doi.org/10.21037/qims-21-636).
Methods
Patient information
This study retrospectively analyzed 171 consecutive female patients with a total of 183 breast lesions. All the women were enrolled in the study from June 2019 to January 2021. Their ages ranged from 19 to 79 years old, with a mean age (± standard deviation) of 44 (±13) years. All the patients had breast lesions that had previously been identified using gray scale ultrasound and dual-mode elastography. The women underwent an ultrasound-guided needle biopsy or surgery within a week of their diagnosis. The inclusion criteria for this study were as follows: (I) breast lesions could be palpated or detected by conventional ultrasound; (II) breast lesions were solid or approximately solid (solid component >80%); and (III) breast lesions had either surgical or puncture pathological results. The exclusion criteria for the study were as follows: (I) pregnant or lactating women; (II) male patients with breast cancer; (III) lesions that did not have pathological results; and (IV) lesions that had undergone biopsy intervention, chemotherapy, or radiofrequency ablation. After screening, 183 breast lesions were included in the study.
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by Shenzhen People’ s Hospital Ethics Committee. Written informed consent was obtained from each patient participating in the study.
Image acquisition
The instrument used to acquire the images was a Resona 7 diagnostic ultrasound system (Mindray, China), with a 3–11 MHz linear array transducer, which had both a shear wave mode and a strain mode. All ultrasound images were captured by a physician with over 5 years of experience in ultrasound diagnosis. Each patient underwent conventional ultrasound and dual-mode elastography on the same day. During the conventional ultrasound examination, the following characteristics of breast lesions were recorded: location, maximum diameter, shape, margin, internal echo, rear echotexture, and blood flow.
Dual-mode elastography with SWE and SE
The physician gently placed the transducer above the lesion and performed SWE. After centering the lesion in the image, the physician switched to the real-time SWE mode and stored the image in the instrument when the image quality surpassed 95%. In the image analysis stage, the physician traced the boundary of the lesion (A), and then turned the knob to mark the area around the lesion (Shell). The area comprising A and Shell is represented as A'. The following ultrasound parameters for Young’s modulus (E), shear wave velocity (Cs), and the shear modulus (G) of A, Shell, and A' were recorded with the shell set at −0.5, 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 mm: mean elasticity (Emean, Csmean, Gmean), maximum elasticity (Emax, Csmax, Gmax), minimum elasticity (Emin, Csmin, Gmin), standard deviation (ESD, CsSD, GSD), and elastic ratio (Shell mean/A mean, Shell max/A max, Shell min/A min, Shell SD/A SD) .
The physician also gently placed the transducer above the lesion and performed SE. After centering the lesion in the image, the physician activated the SE mode and stored the image in the instrument when the image quality surpassed 95%. In the image analysis stage, the physician traced the boundary of A and selected the bluest fat tissue as a reference (B). The physician then turned the knob to draw the Shell. With the Shell set at 0.5, 1.0, 1.5 and 2 mm, the physician recorded the following SE parameters: A, Shell, A'; and elastic ratios: A/Shell, B/A' and B/Shell.
Image analysis
Three physicians with 1, 3, and 5 years of experience, respectively, evaluated the breast lesions according to the breast imaging reporting and data system (BI-RADS) scores, without prior knowledge of the pathological results. They subsequently conducted a second assessment of the breast lesions based on the best selected elastic modulus value and modified their initial BI-RADS classification.
Pathology results
Pathology was the gold standard for breast lesion diagnosis. All lesion tissues were obtained through surgery or ultrasound-guided puncture. Diagnoses were made based on the final pathology results, by experienced pathologists without knowledge of the ultrasound results.
Statistical methods
Rstudio (Rstudio, Boston, MA, USA) and MedCalc 19 (MedCalc Software, Mariakerke, Belgium) were used to perform data analyses. Continuous variables including the patients’ age and the maximum lesion diameter were displayed as means and standard deviations. All elastography parameters were used to rank variables by importance in the random forest model with Rstudio. Any missing data were processed using a default value. The most diagnostic elasticity parameters were then used to draw the receiver operating characteristic (ROC) curve. With the optimal Youden index serving as the cutoff value, the following values were calculated: sensitivity, specificity, area under the curve (AUC) with [95% confidence interval (CI)], the positive likelihood ratio (PLR), the negative likelihood ratio (NLR), the positive predictive value (PPV), the negative predictive value (NPV), and the Youden index.
Breast lesions were diagnosed in series and in parallel using dual-mode elastography. When SE and SWE were malignant, the result in series was malignant. When either SE or SWE was benign, the result in series was benign. When SE and SWE were benign, the result in parallel was benign. When either SE or SWE was malignant, the result in parallel was malignant. ROC analysis of the three physicians’ diagnoses with conventional ultrasound and conventional ultrasound combined with dual-mode elastography was performed using MedCalc 19. The AUC, sensitivity, and specificity were calculated. The AUC was analyzed with a z-test. P<0.05 was considered to be statistically significant.
Results
General information
The patients ranged in age from 19 to 79 years, with the average age being 44±13 years. The lesions ranged in size from 5 to 73 mm, with the average lesion size being 18±10 mm. Of the 183 lesions, 45.9% were malignant (84/183) and 54.1% were benign (99/183), as shown in Table 1.
Table 1
| Benign (n=99) | Malignant (n=84) | P value | |
|---|---|---|---|
| Age (years) | 37.78±11.04 | 51.31±11.32 | 0 |
| Size (mm) | 16.19±6.69 | 20.60±11.75 | 0.003 |
| BI-RADS | 0 | ||
| Senior physician | 76 | 107 | |
| Middle-aged physician | 69 | 114 | |
| Junior physician | 67 | 116 | |
| Elastography | 0 | ||
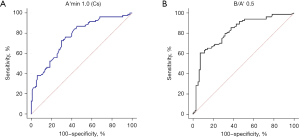
| A'Min 1.0 (Cs) | 2.14±1.00 | 0.88±0.90 | |
| B/A' 0.5 | 3.47±0.50 | 6.46±1.00 | |
| Pathology | |||
| Fibroadenoma | 65 (65.7%) | ||
| Mastopathy | 21 (21.2%) | ||
| Intraductal papilloma | 3 (3.0%) | ||
| Hyperplasia | 3 (3.0%) | ||
| Inflammation | 3 (3.0%) | ||
| Mammary duct ectasia | 2 (2.0%) | ||
| Tubular adenoma | 1 (1.0%) | ||
| Fat | 1 (1.0%) | ||
| Invasive non-specific cancer | 74 (88.1%) | ||
| Invasive lobular carcinoma | 5 (6.0%) | ||
| Ductal carcinoma in situ | 3 (3.6%) | ||
| Mucinous carcinoma | 2 (2.4%) |
Optimal elastography parameters
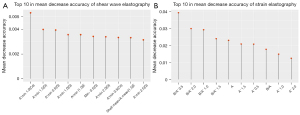
The elastography parameters were processed using a random forest model in Rstudio, and the OOB error rate of the random forest model was evaluated. When the number of trees was set to 1,000, the error rate remained stable. The variables of the random forest model were ranked according to their importance score, and the top 10 variables were selected for further research.
The final analysis results showed that the A'min 1.0 (Cs) mode for SWE and the B/A' 0.5 mode for SE performed best in the diagnosis of breast lesions (Figure 1). When the Youden index was optimal, the cutoff values were 1.5 m/s and 5.14, respectively. The values calculated for sensitivity, specificity, AUC (95% CI), PLR, NLR, PPV, NPV, and the Youden index for both modes were as follows: 73%, 71%, 0.78 (0.7101–0.8455), 2.6, 0.37, 70%, 75%, and 45%, respectively, for A'min 1.0 (Cs); and 61%, 93%, 0.82 (0.7571–0.8818), 8.5, 0.42, 88%, 73%, and 54%, respectively, for B/A' 0.5 (Figure 2).
Our results showed that when dual-mode elastography were used in series, they returned high specificity (98%), PLR (21.2), and PPV (95%). When the two methods were used in parallel, they returned high sensitivity (91%), NLR (0.15), and NPV (89%) (Table 2).
Table 2
| Elastography parameters | AUC | Sen (%) | Spe (%) | PLR | NLR | PPV (%) | NPV (%) | Youden (%) |
|---|---|---|---|---|---|---|---|---|
| A'min 1.0 (Cs) | 0.78 | 73 | 71 | 2.6 | 0.37 | 70 | 75 | 45 |
| B/A' 0.5 | 0.82 | 61 | 93 | 8.5 | 0.42 | 88 | 73 | 54 |
| Series | − | 43 | 98 | 21.2 | 0.58 | 95 | 67 | 40.8 |
| Parallel | − | 91 | 64 | 2.5 | 0.15 | 68 | 89 | 54.1 |
A'min 1.0 (Cs): minimum shear wave velocity of the area of interest and 1.0 mm around the area of interest; B/A' 0.5: ratio of fat to the elasticity of the area of interest and 0.5 mm around the area of interest. Series: when SE and SWE are malignant, the series result is malignant. When either SE or SWE is, the series result is benign. Parallel: When SE and SWE are benign, the result in parallel is benign. When either SE or SWE is malignant, the result in parallel is malignant. AUC (95% CI), area under the curve (95% confidence interval); Sen, sensitivity; Spe, specificity; PLR, positive likelihood ratio; NLR, negative likelihood ratio; PPV, positive predictive value; NPV, negative predictive value.
Results of physicians with different levels of experience
Three physicians classified the 183 lesions according to their BI-RADS scores. Diagnosis was most efficient when a BI-RADS score of 4a (low suspicion for malignancy (2–9%) was used as the critical value to distinguish between benign and malignant lesions.
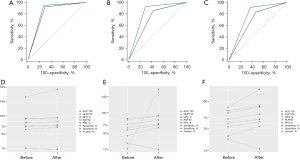
The sensitivity values of the senior physician’s diagnoses before and after the addition of dual-mode elastography were 92.9% and 95.2%, respectively, and the specificity values were 69.7% and 72.7%, respectively. The difference was not statistically significant (P=0.2602). The sensitivity values of the intermediate-level physician’s diagnoses before and after the addition of dual-mode elastography were 85.7% and 92.9%, respectively, and the specificity values were 57.6% and 70.7%, respectively. The difference was statistically significant (P=0.0001). The sensitivity values of the junior physician’s diagnoses before and after the addition of dual-mode elastography were 83.3% and 91.7%, respectively, and the specificity values were 53.5% and 62.6%, respectively. The difference was also statistically significant (P=0.0071). These results show that for all three physicians, the sensitivity and specificity in diagnosing benign and malignant lesions improved after the addition of dual-mode elastography. The senior physician modified the diagnosis from benign to malignant for two lesions, and from malignant to benign for another two lesions. The intermediate physician modified the diagnosis from benign to malignant for six lesions, and from malignant to benign for thirteen lesions. The junior physician modified the diagnosis from benign to malignant for seven lesions, and from malignant to benign for nine lesions. Finally, all lesions were confirmed by pathology (Tables 3,4, Figures 3,4).
Table 3
| Pathological type | BI-RADS classification | Senior physician | Intermediate physician | Junior physician | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | n | Before | After | n | Before | After | n | ||||
| Malignant | 3 | 6 | 4 | 10 | 12 | 6 | 18 | 14 | 7 | 21 | ||
| 4a | 4 | 5 | 9 | 24 | 9 | 33 | 7 | 10 | 17 | |||
| 4b | 24 | 25 | 49 | 32 | 27 | 59 | 31 | 12 | 43 | |||
| 4c | 39 | 39 | 78 | 13 | 30 | 43 | 31 | 30 | 61 | |||
| 5 | 11 | 11 | 22 | 3 | 12 | 15 | 1 | 25 | 26 | |||
| Benign | 3 | 70 | 72 | 142 | 57 | 70 | 127 | 53 | 62 | 115 | ||
| 4a | 22 | 21 | 43 | 38 | 18 | 56 | 32 | 15 | 47 | |||
| 4b | 5 | 4 | 9 | 4 | 10 | 14 | 13 | 15 | 28 | |||
| 4c | 2 | 2 | 4 | 0 | 1 | 1 | 1 | 7 | 8 | |||
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
Table 4
| Classification | Sen (%) | Spe (%) | AUC (95% CI) | PLR | NLR | PPV (%) | NPV (%) | Youden (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | ||||||||
| Senior Physician | 92.9 | 95.2 | 69.7 | 72.7 | 0.813 (0.749–0.867) | 0.84 (0.779–0.890) | 3.064 | 3.492 | 0.102 | 0.065 | 72.2 | 74.8 | 92 | 94.7 | 62.6 | 67.9 | |||||||
| Intermediate Physician | 85.7 | 92.9 | 57.6 | 70.7 | 0.716 (0.645–0.780) | 0.818 (0.754–0.871) | 2.201 | 3.17 | 0.248 | 0.101 | 63.2 | 72.9 | 82.6 | 92.1 | 43.3 | 63.6 | |||||||
| Junior Physician | 83.3 | 91.7 | 53.5 | 62.6 | 0.684 (0.612–0.751) | 0.771 (0.704–0.830) | 1.794 | 2.453 | 0.311 | 0.133 | 60.3 | 67.5 | 79.1 | 89.9 | 36.8 | 54.3 | |||||||
Sen, sensitivity; Spe, specificity; AUC (95% CI), area under the curve (95% confidence interval); PLR, positive likelihood ratio; NLR, negative likelihood ratio; PPV, positive predictive value; NPV, negative predictive value.
Discussion
In our study, the random forest method was used to select the optimal elasticity values for SWE and SE. Results showed that the A'min 1.0 (Cs) mode (1 out of 264) and the B/A' 0.5 mode (1 out of 23) achieved the best diagnostic performance among 287 elasticity values. The sensitivity of these two modes was 73% and 61%, respectively, and the specificity was 71% and 93%, respectively. By combining dual-mode elastography in series and in parallel, better diagnostic efficiency was achieved. Combining conventional ultrasound with elastography was found to improve the diagnostic efficiency of physicians with different levels of experience in the evaluation of breast lesions. The sensitivity of the intermediate-level and junior physicians increased by 7.2% and 8.4%, respectively, and the specificity increased by 13.1%, and 9.1%, respectively.
SWE shows the different elasticity moduli of a lesion and is effective in differentiating benign from malignant breast lesions (27). Wang et al. reported that the Emax and Emean achieved the best diagnostic performance, with sensitivity of 60.9% and 45.7%, respectively, and specificity of 85.3% and 86.8%, respectively. There was no statistical difference for Emin (28). After studying lesions and a 2-mm area surrounding them, Moon et al. also found a statistical difference between the Emax and Emean, but the repeatability of the Emin was the lowest (29). Previous studies have shown that the E(max-3Shell) and the E(min-3Shell) are significant predictors of malignancy (30). In our study, we concluded that A'min 1.0 (Cs) achieved the best diagnostic performance in SWE. When A'min 1.0 (Cs) >1.5 m/s, breast lesions were classified as benign, and when A'min 1.0 (Cs) ≤1.5 m/s, breast lesions were classified as malignant. The AUC, sensitivity, and specificity were 0.78, 73%, and 71% respectively. Our results are not completely consistent with those of previous research, which may be related to the desmoplastic reaction formed by cancer cells eroding the surrounding tissues, which causes the tissue surrounding the nodule to harden and the internal structure to soften (31,32). In addition to the outward infiltration of the tumor tissue, liquefaction, necrosis, and cystic changes resulting from the reduced blood supply within the tumor can lead to a decrease in tumor tissue hardness. Studies have shown that when the inner area of the lesion is softer than the outer area, SWE can show a “stiff rim” at the edge of the lesion (33), which has the effects of increasing the attenuation of sound energy and reducing shear wave propagation and amplitude into the lesion. The system then misinterprets the reduction as a low-speed shear wave, and the minimum elasticity value becomes lower, which is more likely to lead to a diagnosis of malignancy.
In SE, the strain score and SR are the main parameters used to differentiate between benign and malignant breast lesions. The strain score is a subjective qualitative diagnostic tool and mainly depends on the operator. The SR is a semi-quantitative measure which describes the difference in stiffness between the lesion and normal tissue (fatty or glandular) (34). Previous studies had shown that in SE, the strain score (sensitivity 77.59–85%, specificity 75–86%) and SR (sensitivity 77.59–88.2%, specificity 63.7–78.68%) are significant in the diagnosis of benign and malignant breast lesions (17-20). However, the difference in the SR with the use of different shells has not been studied before. In the present study, the SR was used to obtain the elasticity values (a total of 23 values) of the lesion and surrounding fatty tissue under different shells, including A, Shell, and A', as well as the SRs A/shell, B/A' and B/shell. Random forest screening showed B/A' 0.5 to achieve the best diagnostic performance. When the cutoff value was 5.14, the AUC, sensitivity, and specificity were 0.82, 61%, and 93%, respectively. We believe that this may be because a malignant tumor has an outward pattern of infiltration, and the surrounding tissue becomes harder than the internal tissue. Furthermore, A' contains the lesion itself and the surrounding area, so it can better represent the tumor stiffness and achieves higher diagnostic efficiency.
In this study, 54 lesions were classified as positive with SWE and negative with SE, and 20 lesions were classified as negative with SWE and positive with SE. The discrepancy between the SWE and SE results may be related to their imaging principles. In SWE, the vibration generated by the acoustic radiation force impulse deforms the tissue. In SE, the elastic deformation of the tissue comes from the external force applied by the physician. Using the same method cannot guarantee that the result will be the same every time.
Our study found that the addition of dual-mode elastography can improve the diagnostic performance in differentiating benign and malignant breast lesions to some extent. When used in parallel, dual-mode elastography had a higher sensitivity (91%), NLR (0.15), and NPV (89%) than when used alone. The results show that the parallel method can effectively help to avoid unnecessary operations. When both elastography scans are negative, the parallel result is also negative, providing tangible proof for diagnosing benign lesions. Patients can then be given a recommendation for a regular follow-up. Further, the series method, with higher specificity (98%), PLR (21.2), and PPV (95%), is superior to separate elastography scans. In other words, if the results of the scans in series are positive (i.e., both SE and SWE are positive), the lesions should be considered to present a higher risk of breast cancer and patients should be recommended to undergo further testing in a timely manner. We did not calculate the AUC of the parallel and series methods, as our purpose was not to compare which of the two methods is better but to use their advantages to differentiate benign and malignant breast lesions. To detect a greater number of malignant breast lesions, the parallel method was used. However, the specificity of parallel scans is low, which may lead to some benign lesions being unnecessarily punctured. Although the possibility of over-treating benign lesions exists, malignant breast lesions can be identified in time and treated promptly, providing patients with a great advantage. Breast lesions can cause great anxiety in patients, and even when a benign lesion is diagnosed, the desire is to eliminate it as soon as possible to relieve that anxiety. When the SWE and SE results are malignant, the dual-mode elastography diagnosis is undoubtedly malignant. When the SWE and SE results are benign, the dual-mode elastography diagnosis is benign. However, when the results of SWE and SE are inconsistent, a final diagnosis should be based on the patient’s clinical characteristics, such as their age or family history, and the slide and texture of the lesion. In cases where the patient is over 40 years old, there is a family history, or the lesion cannot be slided or is hard, the parallel method can be used to diagnose the lesion as malignant. Otherwise, the lesion can be diagnosed as benign using the series method.
In previous studies, conventional ultrasound combined with elastography was found to improve the diagnostic performance and specificity in the diagnosis of breast lesions. After the addition of elastography, the specificity of diagnosis in Lee et al.’s study increased from 17.4% to 73.8%, and that in Choi et al.’s study increased from 17.1% to 69.6% (35,36). In our study, the sensitivity and specificity of three physicians with different levels of experience using conventional ultrasound were 92.9% and 69.7% (senior), 85.7% and 57.6% (intermediate-level), and 83.3% and 53.5% (junior), which were consistent with the guidelines of the American Society of Radiology (37). The sensitivity, specificity, and AUC of dual-mode elastography were higher than those of conventional ultrasound alone. There was a statistical difference in the results of the intermediate-level and junior physicians (P<0.05), but no statistical significance was observed in relation to the results of the senior physician (P=0.2602). When the intermediate-level physician used conventional ultrasound combined with dual-mode elastography, the number of malignant lesions diagnosed as BI-RADS 3 decreased from 12 to 6, while the number of benign lesions diagnosed as BI-RADS 4a, 4b, 4c, and 5 decreased from 42 to 29. When the junior physician combined conventional ultrasound with dual-mode elastography, the number of malignant lesions diagnosed as BI-RADS 3 decreased from 14 to 7, and the number of benign lesions diagnosed as BI-RADS 4a, 4b, 4c, and 5 decreased from 46 to 37. These results show that the addition of dual-mode elastography can effectively improve the detection ability and diagnostic accuracy of intermediate-level and junior physicians for malignant breast lesions. This finding suggests that dual-mode elastography is more helpful to inexperienced physicians, but not necessarily to senior physicians. In the process of SE and SWE image acquisition, the equipment should be operated in strict accordance with the manufacturer’s specifications. If the operator does not have sufficient experience, the image quality will be poor, which will affect the diagnosis. During the acquisition process, the lesion should be centered in the image with moderate intensity, and the best quality image should be drawn along the edge of the lesion to avoid data bias, and then retained for analysis.
This study has some limitations. First, it was a retrospective study with a limited number of participants, who were mostly enrolled from the same center. Therefore, certain selection biases may exist. Multi-hospital and large-sample studies are necessary for further verification. In this study, the physician who acquired the images had extensive experience and was able to acquire suitable images, whereas the junior and intermediate-level physicians did not participate in the image acquisition. Therefore, the difference between the senior, intermediate-level, and junior physicians was not reflected in this process. Further, each experience level was only represented by one physician, which reflects the diagnostic abilities of the individual rather than that of a particular level of physician. More physicians should be included in future studies to increase the accuracy of results.
Conclusions
This study has shown that dual-mode elastography performs well in the diagnosis of breast lesions, and that more effective diagnoses can be achieved by using the series or parallel methods. Dual-mode elastography shows higher sensitivity when used in parallel, which can avoid unnecessary punctures and reduce the number of invasive examinations for patients. When used in series, dual-mode elastography shows high specificity and can detect high-risk nodules for further treatment in a timely manner. Most importantly, combining conventional ultrasound with dual-mode elastography can improve the sensitivity and specificity of physicians with different levels of experience in the diagnosis of breast lesions. Despite being more helpful to the intermediate-level and junior physicians in this study than for the senior physician, this combination is worth popularizing in the clinical setting.
Acknowledgments
We would like to thank our Dream Team at the Ultrasound Department of Shenzhen People’s Hospital for their help and support.
Funding: This project was supported by the Commission of Science and Technology of Shenzhen (GJHZ20200731095401004).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://dx.doi.org/10.21037/qims-21-636
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/qims-21-636). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Shenzhen People’s Hospital Ethics Committee. Patients’ written informed consent was obtained for the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sakorafas GH, Farley DR, Peros G. Recent advances and current controversies in the management of DCIS of the breast. Cancer Treat Rev 2008;34:483-97. [Crossref] [PubMed]
- Chen DR, Chang RF, Kuo WJ, Chen MC, Huang YL. Diagnosis of breast tumors with sonographic texture analysis using wavelet transform and neural networks. Ultrasound Med Biol 2002;28:1301-10. [Crossref] [PubMed]
- Li T, Mello-Thoms C, Brennan PC. Descriptive epidemiology of breast cancer in China: incidence, mortality, survival and prevalence. Breast Cancer Res Treat 2016;159:395-406. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Tabár L, Vitak B, Chen TH, Yen AM, Cohen A, Tot T, Chiu SY, Chen SL, Fann JC, Rosell J, Fohlin H, Smith RA, Duffy SW. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology 2011;260:658-63. [Crossref] [PubMed]
- Checka CM, Chun JE, Schnabel FR, Lee J, Toth H. The relationship of mammographic density and age: implications for breast cancer screening. AJR Am J Roentgenol 2012;198:W292-5. [Crossref] [PubMed]
- Zonderland HM, Coerkamp EG, Hermans J, van de Vijver MJ, van Voorthuisen AE. Diagnosis of breast cancer: contribution of US as an adjunct to mammography. Radiology 1999;213:413-22. [Crossref] [PubMed]
- Lister D, Evans AJ, Burrell HC, Blamey RW, Wilson AR, Pinder SE, Ellis IO, Elston CW, Kollias J. The accuracy of breast ultrasound in the evaluation of clinically benign discrete, symptomatic breast lumps. Clin Radiol 1998;53:490-2. [Crossref] [PubMed]
- Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology 1995;196:123-34. [Crossref] [PubMed]
- Mendelson EB, Berg WA, Merritt CR. Toward a standardized breast ultrasound lexicon, BI-RADS: ultrasound. Semin Roentgenol 2001;36:217-25. [Crossref] [PubMed]
- Stachs A, Hartmann S, Stubert J, Dieterich M, Martin A, Kundt G, Reimer T, Gerber B. Differentiating between malignant and benign breast masses: factors limiting sonoelastographic strain ratio. Ultraschall Med 2013;34:131-6. [PubMed]
- Berg WA. Supplemental screening sonography in dense breasts. Radiol Clin North Am 2004;42:845-51. vi. [Crossref] [PubMed]
- Barr RG. Sonographic breast elastography: a primer. J Ultrasound Med 2012;31:773-83. [Crossref] [PubMed]
- Barr RG, Nakashima K, Amy D, Cosgrove D, Farrokh A, Schafer F, Bamber JC, Castera L, Choi BI, Chou YH, Dietrich CF, Ding H, Ferraioli G, Filice C, Friedrich-Rust M, Hall TJ, Nightingale KR, Palmeri ML, Shiina T, Suzuki S, Sporea I, Wilson S, Kudo M. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 2: breast. Ultrasound Med Biol 2015;41:1148-60. [Crossref] [PubMed]
- Wells PN, Liang HD. Medical ultrasound: imaging of soft tissue strain and elasticity. J R Soc Interface 2011;8:1521-49. [Crossref] [PubMed]
- Barr RG, De Silvestri A, Scotti V, Manzoni F, Rebuffi C, Capittini C, Tinelli C. Diagnostic Performance and Accuracy of the 3 Interpreting Methods of Breast Strain Elastography: A Systematic Review and Meta-analysis. J Ultrasound Med 2019;38:1397-404. [Crossref] [PubMed]
- Jia W, Luo T, Dong Y, Zhang X, Zhan W, Zhou J. Breast Elasticity Imaging Techniques: Comparison of Strain Elastography and Shear-Wave Elastography in the Same Population. Ultrasound Med Biol 2021;47:104-13. [Crossref] [PubMed]
- Liu J, Wu JP, Wang N, Li GH, Wang XH, Wang Y, Zheng M, Zhang B. Value of Elastography Strain Ratio Combined with Breast Ultrasound Imaging Reporting and Data System in the Diagnosis of Breast Nodules. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2021;43:63-8. [PubMed]
- Kokubu Y, Yamada K, Tanabe M, Izumori A, Kato C, Horii R, Ohno S, Matsueda K. Evaluating the usefulness of breast strain elastography for intraductal lesions. J Med Ultrason (2001) 2021;48:63-70. [Crossref] [PubMed]
- Wang H, Li CY, Zha HL, Xu D, Hu ZB. Diagnostic and Predictive Values of Strain Ratios in the Regions of Interests in Reference Tissue for Breast Tumor. Cancer Manag Res 2021;13:1017-28. [Crossref] [PubMed]
- Balleyguier C, Canale S, Ben Hassen W, Vielh P, Bayou EH, Mathieu MC, Uzan C, Bourgier C, Dromain C. Breast elasticity: principles, technique, results: an update and overview of commercially available software. Eur J Radiol 2013;82:427-34. [Crossref] [PubMed]
- Jeong WK, Lim HK, Lee HK, Jo JM, Kim Y. Principles and clinical application of ultrasound elastography for diffuse liver disease. Ultrasonography 2014;33:149-60. [Crossref] [PubMed]
- Athanasiou A, Tardivon A, Tanter M, Sigal-Zafrani B, Bercoff J, Deffieux T, Gennisson JL, Fink M, Neuenschwander S. Breast lesions: quantitative elastography with supersonic shear imaging--preliminary results. Radiology 2010;256:297-303. [Crossref] [PubMed]
- Youk JH, Gweon HM, Son EJ, Chung J, Kim JA, Kim EK. Three-dimensional shear-wave elastography for differentiating benign and malignant breast lesions: comparison with two-dimensional shear-wave elastography. Eur Radiol 2013;23:1519-27. [Crossref] [PubMed]
- Ko KH, Jung HK, Kim SJ, Kim H, Yoon JH. Potential role of shear-wave ultrasound elastography for the differential diagnosis of breast non-mass lesions: preliminary report. Eur Radiol 2014;24:305-11. [Crossref] [PubMed]
- Berg WA, Cosgrove DO, Dore CJ, Schafer FK, Svensson WE, Hooley RJ, Ohlinger R, Mendelson EB, Balu-Maestro C, Locatelli M, Tourasse C, Cavanaugh BC, Juhan V, Stavros AT, Tardivon A, Gay J, Henry JP, Cohen-Bacrie C, Investigators BE. Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology 2012;262:435-49. [Crossref] [PubMed]
- Hari S, Paul SB, Vidyasagar R, Dhamija E, Adarsh AD, Thulkar S, Mathur S, Sreenivas V, Sharma S, Srivastava A, Seenu V, Prashad R. Breast mass characterization using shear wave elastography and ultrasound. Diagn Interv Imaging 2018;99:699-707. [Crossref] [PubMed]
- Wang ZL, Li JL, Li M, Huang Y, Wan WB, Tang J. Study of quantitative elastography with supersonic shear imaging in the diagnosis of breast tumours. Radiol Med 2013;118:583-90. [Crossref] [PubMed]
- Moon JH, Hwang JY, Park JS, Koh SH, Park SY. Impact of region of interest (ROI) size on the diagnostic performance of shear wave elastography in differentiating solid breast lesions. Acta Radiol 2018;59:657-63. [Crossref] [PubMed]
- Yang H, Xu Y, Zhao Y, Yin J, Chen Z, Huang P. The role of tissue elasticity in the differential diagnosis of benign and malignant breast lesions using shear wave elastography. BMC Cancer 2020;20:930. [Crossref] [PubMed]
- Tozaki M, Fukuma E. Pattern classification of ShearWave Elastography images for differential diagnosis between benign and malignant solid breast masses. Acta Radiol 2011;52:1069-75. [Crossref] [PubMed]
- Evans A, Whelehan P, Thomson K, McLean D, Brauer K, Purdie C, Jordan L, Baker L, Thompson A. Quantitative shear wave ultrasound elastography: initial experience in solid breast masses. Breast Cancer Res 2010;12:R104. [Crossref] [PubMed]
- Zhou J, Zhan W, Dong Y, Yang Z, Zhou C. Stiffness of the surrounding tissue of breast lesions evaluated by ultrasound elastography. Eur Radiol 2014;24:1659-67. [Crossref] [PubMed]
- Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, Yamakawa M, Matsumura T. Breast disease: clinical application of US elastography for diagnosis. Radiology 2006;239:341-50. [Crossref] [PubMed]
- Lee SH, Chang JM, Kim WH, Bae MS, Seo M, Koo HR, Chu AJ, Gweon HM, Cho N, Moon WK. Added value of shear-wave elastography for evaluation of breast masses detected with screening US imaging. Radiology 2014;273:61-9. [Crossref] [PubMed]
- Goddi A, Bonardi M, Alessi S. Breast elastography: A literature review. J Ultrasound 2012;15:192-8. [Crossref] [PubMed]
- Magny SJ, Shikhman R, Keppke AL. Breast Imaging Reporting and Data System. Treasure Island (FL): StatPearls, 2021.


