
Personalized dosimetry for a deeper understanding of metastatic response to high activity 131I-mIBG therapy in high risk relapsed refractory neuroblastoma
Introduction
Treatment of relapsed/refractory metastatic high-risk neuroblastoma (rrmHR-NBL) after multimodality therapy remains a clinical challenge; despite recent advances in our understanding of neuroblastoma biology and new treatment strategies, the outcome for children affected by rrmHR-NBL remains poor with a 5 years overall survival around 20% (1,2).
Approximately 90% of neuroblastoma cases accumulate the noradrenalin analogue metaiodobenzylguanidine (mIBG) by a specific uptake mechanism and 131I-mIBG can be used for targeted radiotherapy delivering a focal dose of radiation to the tumor sites. A 30–40% response rate has been observed in refractory and relapsed neuroblastoma (3,4) and the application of target radiotherapy in new sequential treatments with various drugs is under clinical assessment. Among sequential treatment strategies for rrmHR-NBL, high activity mIBG can be combined with chemotherapy followed by autologous stem cell transplantation (ASCT) (5,6). Thus, children with rrmHR-NBL continue to need combinations of different therapeutic modalities to prolong survival and minimize the toxicities of additional therapies.
In 2005, Gaze et al. (7) demonstrated the feasibility of high activity mIBG therapy based on dosimetric approach combined with chemotherapy. The rationale of this study protocol is based on two high activity 131I-mIBG administrations guided by a dosimetric assessment to achieve a whole-body (WB) absorbed dose of 4 Gy.
This paper will focus on reporting our experience in dosimetric assessment in children affected by rrmHR-NBL treated with high activity mIBG therapy, underlying the correlation between WB and red marrow (RM) absorbed dose and demonstrating the usefulness of lesions dosimetry.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/qims-21-548).
Methods
Patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of IRCCS Bambino Gesù Children’s Hospital and informed consent was taken from all individual participants. In the present case series study, 14 patients (7 boys and 7 girls with ages ranging from 3 to 17 years) with rrmHR-NBL (previously treated according standard procedure for HR-NBL) have been enrolled and submitted to high activity 131I-mIBG administrations at “Bambino Gesù” Children Hospital, recruited over a period of 3 years since 2017. The age at the time of the initial diagnosis ranged from 1 to 17 years (median 4 years). The treatment protocol was based on a scheme consisting of two 131I-mIBG administrations 2 weeks apart followed by a single dose of Melphalan (110 mg/m2) 96 hours after the second 131I-mIBG administration and autologous stem cell transplantation 24 hours after the end of chemotherapy.
First 131I-mIBG administration was weight-based (approximately 444 MBq/kg, excepted for 5 patients over 50 kg of weight who received no more than 17 GBq due to legal limit), whereas the second administration was dosimetry based in order to achieve an absorbed dose to WB (DWB) equals to 4 Gy as suggested by Gaze et al. (7). Patients over 50 kg of weight received a second administration of 17 GBq achieving a total absorbed dose to WB as high as possible.
Thyroid protection block was performed in all patients by administration of Lugol solution and triiodothyronine since 72 h before the first mIBG treatment to 15 days after the second one. mIBG avid disease was proven by 123I-mIBG scan to evaluate basal status disease and clinical response after treatment. 123I-mIBG scans were assessed according to the SIOPEN-mIBG scoring system (8) and the clinical response after 131I-mIBG therapy was classified as progressve and stable disease (PD and SD) or partial and complete response (PR and CR) according to the International Neuroblastoma Response Criteria (INRC) Definition of Response (9) within a range from 1 to 3 months.
All patients who had a therapeutic effect (such as complete response, partial response or stable disease) were included in the “Responder group” while patients in progression disease were assigned to “Non-Responder group”.
WB/RM dosimetry
Each patient was submitted to 6 or 7 dose rate measurements (R) to assess whole-body cumulated activity (ÃWB). Measurements were performed in anterior and posterior views, using a Geiger counter placed 2 m from the patient for a duration of 3 min. The first measurements [RA(0) and RP(0)] were performed immediately after 131I-mIBG administration, in order to avoid bladder emptying and measure the dose rate of the total administered activity; the next measurements were performed after bladder emptying with the following time sampling 1, 6, 24, 48, 54, 166 h after tracer administration; background activity was registered before each time point. The activity at time t was calculated using the following formula:
where AAdm is the activity administer and RA and RP are the dose rates, in anterior and posterior views, considering background correction.
With an analogous time sampling, six blood samples have been collected (with a volume of about 3 mL) using a central line catheter previously placed for clinical purpose. Blood sample activities were assessed after 2 weeks to reduce dead-time effects, thanks to a gamma counter. Blood activity concentration {[ABlood(t)]} was calculated as follows:
where CPS (Counts Per Second) is the output signal of the gamma counter after a measurement of 10 min, CGC is the gamma counter calibration factor which transforms CPS into activity taking into account the geometric deviations due to different masses for each blood sample (mBlood).
The calculation of ÃWB and [ÃBlood] has been performed using by NukFit software (10). With this software it was possible to choose the best fit model, competing a mono and bi exponential fit using the AICc method (11), and to calculate the area under the curve.
According to EANM guidelines DWB has been calculated using the following formula (12):
Using an S-factors given by the following formula (13,14):
where mp is the patient’s weight.
Whereas, the DRM is the sum of two contributions: (I) direct irradiation due to blood activity (self blood contribution) and (II) indirect irradiation due to rest of body activity. It has been calculated using the formula reported in (15,16):
where mWB and mRM are the body mass and RM, the mass of a standard phantom, respectively; RMBLR is the radioactive concentration ratio between marrow and blood, set equal to 1 and S-Factors are taken by Olinda (17).
Lesion dosimetry
The conjugate views method described in Pamphlet MIRD 16 (18) was used to calculate the absorbed dose to patient’s lesions using static planar scintigraphic images. Evaluation of 13 lesions in 9 patients detailed the following features: (I) lesions were qualitatively detectable by tumor delineation both on CT images and on scintigrafic images after the administration of 131I-mIBG; (II) lesions were to be non-retrohepatic.
Each of these patients underwent a specific acquisition protocol consisting of a CT scan, a trasmissive acquisition (before the first administration of 131I-mIBG) and a series of static images after the first and the second therapeutic administration.
A CT scan of the body’s segment including the lesion’s site was performed within a range of 1–3 months before 131I-mIBG therapy. By contouring the tumor’s edges on this scan, the volume and the mass of the lesion were calculated considering a density equal to 1 in case of soft tissue.
In order to estimate the patient’s body thickness along lesion’s view (T), 1 h before 131I-mIBG administration, two transmissive images (one without the patient - blank - and the other with the patient) were acquired using a source of 99mTc of about 37 MBq and LEHR collimators.
A series of static scintigraphic acquisitions were also collected after the first and the second administration. The acquisition parameters are reported in Table 1.
Table 1
| Parameters | Data |
|---|---|
| Time | 5 min |
| Time sampling | 2, 6, 24, 36, 144–166 h |
| Matrix | 128×128 |
| Collimator | HE |
| Primary Energy Window | 363.8 keV ±7.5% |
| Lower Energy Window | 309.2 keV ±7.5% |
| Upper Energy Window | 418.3 keV ±7.5% |
The scintigraphic images were subsequently corrected for scatter, using the Ogawa method (19), and for dead time placing a known source (of about 11.1 MBq) in the field of view, avoiding the overlap on patient’s body and normalising the total counts acquired within the image to those obtained from the source without the patient present (20). The lesion was delineated on the first image of the series and, in order to improve the placement of lesion’s region of interest (ROI), all (scintigraphic and trasmissive) planar images were co-registered referring to the first scintigraphic image acquired.
Following the Pamphlet MIRD 16 lesion’s activity at time t was calculated according to the formula showed in (18):
where IAnt and IPost represent counts inside lesion’s ROI in anterior and posterior views respectively, corrected for background; fj is the self attenuation correction; C and µ(I-131) are the sensitivity of the gamma camera and the attenuation coefficient for 131I obtained using an abosolute quantification described in (15,21) and are equal to 12.83 cps/MBq and 0.106 cm−1, respectively.
Finally the absorbed dose to lesion (DLesion) was calculated using the formula:
where ÃLesion is the cumulated activity, calculated by Nukfit in the same way as described for the WB and SLesion←Lesion using the values obtained by the interpolation of S-value of the spheres taken from the Olinda software.
A possible therapeutic dose-response correlation was also assessed comparing the cumulative doses to lesions in “Responder” and “Non-Responder” patients group by statistical analysis described in the next section.
Statistical analysis
Descriptive statistics tools (as mean, standard deviation and median) were used for describing the dosimetric results.
Correlation between DWB and DRM were determined by linear regression analysis.
Power-Law fit were used to estimate the correlation between DWB and mass of the patient.
Correlation between clinical response (categorical factor) and DLesion (continuous variable) was assessed by the Mann-Whitney test.
Results
Clinical results
Main patient’s characteristics and the relative clinical response to 131I-mIBG therapy are summarized in Table 2. No patients showed acute toxicity (hypertensive crisis or other heavy side effects); nausea and vomiting were well controlled by antiemetic drugs. Medullary aplasia was obtained in all patients as required by treatment protocol scheme. All patients had demonstrated progressive disease on the 123I-mIBG pre-therapy scan with a SIOPEN score greater than 3. After 131I-mIBG therapy 5/14 patients (35.7%) showed progressive disease while 4/14 (28.6%) patients showed stable disease. Two patients (14.3%) showed partial response and 3 (21.4%) patients showed a complete response.
Table 2
| Patient ID | Gender | Age at treatment, years | Weight, kg | Disease status after two 131I MIBG therapies |
|---|---|---|---|---|
| Pat - 1 | M | 8 | 27 | PD |
| Pat - 2 | M | 17 | 87 | CR |
| Pat - 3 | F | 9 | 21 | SD |
| Pat - 4 | F | 4 | 14 | CR |
| Pat - 5 | F | 4 | 18 | CR |
| Pat - 6 | M | 17 | 54 | PR |
| Pat - 7 | M | 12 | 68 | PR |
| Pat - 8 | F | 6 | 21 | PD |
| Pat - 9 | F | 16 | 57 | SD |
| Pat - 10 | M | 6 | 20 | SD |
| Pat - 11 | F | 3 | 12 | PD |
| Pat - 12 | M | 6 | 20 | SD |
| Pat - 13 | M | 13 | 71 | PD |
| Pat - 14 | F | 3 | 12 | PD |
The “Responder group” rate, considering all those patients who had a therapeutic effect (such as complete response, partial response or stable disease), was 64.3%. “Non Responder group” rate (progression disease) was 35.7%.
Absorbed dose to WB/RM results
The biokinetic chosen varied for each patient resulting 6/14 and 5/14 mono exponential fit for WB and RM respectively, remaing the same fit-model from the first to the second administration.
On the base of the DWB assessment at the first administration, 13 patients received a full treatment while only one patient received a single dose administration due to the delivery of a DWB value of 4.2 Gy. The injected activities at the second administration to obtain a complete treatment were in the range of 1.8 to 17.9 GBq (median 11.0 GBq) while the total activity administered was between 2.2 and 35.4 GBq (median 20.8 GBq).
The media (range) of DWB values for single administration was 1.7 (0.6–4.2) Gy. The absorbed dose obtained summing the results of the two administrations was 3.6 (1.5–4.5) Gy.
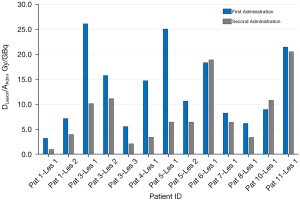
In Figure 1A, the cumulated absorbed doses to WB for the 14 treated patients are reported.
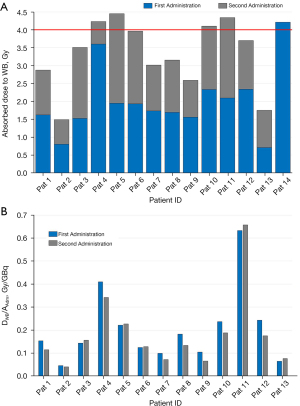
Considering the 13 patients who completed two administrations, in Figure 1B, DWB/AAdm values of 13 patients are reported obtaining values ranging to 0.15 (0.04–1.91) Gy/GBq. The percentage difference of DWB/AAdm (normalized dose to administered activity) between the first and second administration was between −37.0% and 18.5% with a median of −16.5%. Eight out of 13 patients (61.5%) showed a reduction in the absorbed dose per unit of administered activity between the first and second treatment, while 4 out of 13 patients (30.8%) showed an increase in DWB/AAdm of less than 10%.
A tight range of the blood samples mass was measured 2.5–3.1 g minimizing geometric effects. DRM values for each administration was 1.0 (0.4–2.5) Gy. The absorbed dose obtained summing the results of the two administrations was 2.1 (1.0–2.6) Gy. In Figure 2, DRM/AAdm values of 13 patients who received two administrations are reported showing a blood self contribution ranging from 5.7% to 19.2% with a median value of 10.0% respect to the DRM value. The percentage difference of DRM/AAdm between the first and the second administration were between −57.0% and 13.1% with a median value of −23.8%. In particular, 9/13 patients (69.2%) showed a reduction of DRM/AAdm, while 3/13 patients (23.1%) showed an increase of DRM/AAdm inferior to 10%.
In Figure 3, the trend of the absorbed dose to RM respect to the dose to WB are reported. The graph shows a strong linear correlation between the two parameters with an angular coefficient a R2 equal to 1.7, 0.9922 respectively (P<0.01).
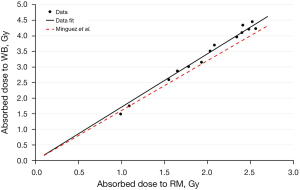
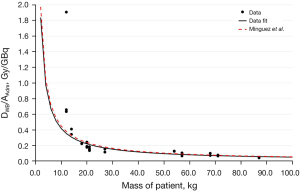
Figure 4 showed the correlation between DWB/AAdm versus the mass of the patient obtaining the following result:
Absorbed dose to lesion results
The number of detected lesion following the mentioned inclusion criteria was 13 in 9 patients and their anatomical features and dosimetric results were reported in Table 3.
Table 3
| Lesion ID | Contouring lesion site | Volume (cm3) | First administration | Second administration | |||
|---|---|---|---|---|---|---|---|
| D/A (Gy/GBq) | D (Gy) | D/A (Gy/GBq) | D (Gy) | ||||
| Pat 1 - Les 1 | Latero-cervical lymph-nodes | 83.9 | 3.2 | 33.9 | 0.9 | 10.3 | |
| Pat 1 - Les 2 | Pelvic tissue | 21.9 | 7.2 | 75.8 | 4.0 | 43.1 | |
| Pat 3 - Les 1 | Left retroclavear lymph-nodes | 4.3 | 26.1 | 276.7 | 10.1 | 128.3 | |
| Pat 3 - Les 2 | Right retroclavear lymph-nodes | 0.4 | 15.8 | 167.1 | 11.1 | 141.1 | |
| Pat 3 - Les 3 | Thoracic paravertebral tissue | 61.4 | 5.6 | 58.8 | 2.1 | 26.7 | |
| Pat 4 - Les 1 | Cervico-mediastinic lymph-nodes | 4.8 | 14.7 | 129.2 | 3.4 | 6.3 | |
| Pat 5 - Les 1 | Mediastinic lymph-nodes | 0.4 | 25.1 | 220.9 | 6.4 | 70.6 | |
| Pat 5 - Les 2 | Left retroclavear lymph-nodes | 0.9 | 10.6 | 93.7 | 6.7 | 73.6 | |
| Pat 6 - Les 1 | Abdominal paravertebral tissue | 14.4 | 18.3 | 284.8 | 18.9 | 300.9 | |
| Pat 7 - Les 1 | Cervico-mediastinic lymph-nodes | 246.4 | 8.2 | 143.9 | 6.4 | 114.3 | |
| Pat 8 - Les 1 | Mediastinic lymph-nodes | 2.8 | 6.2 | 57.3 | 3.3 | 36.3 | |
| Pat 10 - Les 1 | Abdominal mass | 31.9 | 8.9 | 87.8 | 10.8 | 101.6 | |
| Pat 11 - Les 1 | Latero-cervical lymph-nodes | 98.6 | 21.4 | 70.8 | 20.5 | 70.2 | |
The distribution of lesions in different anatomical districts was heterogeneous with a volume range of 0.4–246.4 cm3 from the cervical district to the low abdomen.
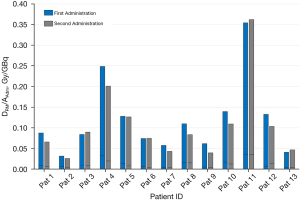
The absorbed doses delivered from single administration were between 6.3 and 300.9 Gy with a median 81.8 Gy while the total absorbed dose of the two administrations ranged from 44.2 and 585.8 Gy, with a median of 164.3 Gy. DLesion/AAdm was within the range of 0.9 to 26.1 Gy/GBq with a median of 8.6 Gy/GBq. The percentage difference in DLesion/AAdm between the second and the first administration was between −76.8% and 21.0% with a median of −44.7%. In Figure 5, DLesion/AAdm values of 13 lesions for each administration are reported. 10/13 (76.9%) lesions showed a reduction of the uptake at the second administration compared to the first (percentage differences ranged from −76.9% to −22.6%); 2/13 (15.4%) lesions had a similar values and only in one case there is an increase of the uptake equals to 21.0%.
We also assessed a possible therapeutic dose-response correlation showed in Figure 6.
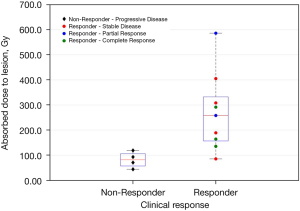
The median of the Non-Responder group was 82.0 Gy, while that one of the Responder group was 258.2 Gy. The minimum value present in the Responder group was 44.2 Gy. The Mann-Whitley U test showed that the median absorbed doses were significantly different in the two groups (P=0.0196).
Discussion
Our experience demonstrated feasibility of high activity therapy of 131I-mIBG in rrmHR-NBL children as two administration intensive strategy. As shown by Genolla et al. (23), the use of personalized dosimetry in combination with the clinical data confirms that protocol theorized by Gaze et al. (7) is a safe treatment strategy.
Dosimetric approaches allow a tailored high dose treatment maximizing the benefits of radionuclide therapy for pediatric patients.
The graph in Figure 1A shows that the summed dose value after the two administrations were close to the dose of 4 Gy in 8 patients (in the range of 3.5–4.5 Gy). Four patients (2,7,9,13) over 50 kg of weight reached an absorbed dose to WB within the range 1.5 to 3.0 Gy since it was not possible to administer a higher activity than 17 GBq due to legal limits. The remaining 2 patients (1 and 8 with a weight of 27 and 21 kg respectively) showed a variability of DWB/AAdm between the first and the second administration (around 25%) reaching an absorbed dose of 2.9 and 3.2 Gy respectively.
In 2015, Mínguez et al. (22) studied the correlation between DWB/AAdm and patient’s mass, using data of 3 different studies (22,24,25) and obtaining the following relationship:
From the comparison of the two results (Figure 4) it is possible to note that the two curves are very similar, with a percentage difference equal to 4.3%. The correlation is very strong as underlined by the R2 value of 0.9214. However, the maximum distance between data and fit is around 43%, consequently, equation number 9 can be used in a first approximation for the choice of the activity to be administered but patient’s dosimetry is still necessary in order to obtain a reliable dose value.
Generally the calculation of the absorbed dose to RM is not performed for patient affected by rrmHR-NBL treated with two administration of 131I-mIBG. Blood sample collection was not a painful procedure due to the presence of a CLC generally placed for clinical purpose in oncological patient submitted to heavy treatment. For this reason it has been possible to collect 6–7 blood samples from each patient. In the present protocol, the dose limit to WB of the entire treatment is set at 4 Gy without considering quantitative prescriptions on dosimetry to RM. Reviewing the literature about analogous dosimetric protocol, Giammarile et al. in EANM guideline procedure sets the dose limit to red marrow at 2 Gy (26) in order to avoid marrow toxicity. Analyzing the DRM, these values were higher than 2 Gy in 50% of patients (maximum value 2.6 Gy). This result is not in contradiction with the literature data because myeloablation is a deterministic effect required in the treatment protocol in preparation for bone marrow transplantation.
Considering the DWB/AAdm values (reported in Figure 1B and Figure 2 for WB and RM, respectively) and calculating the percentage difference between the first and the second administrations it is possible to assume that the first result is predictive of the second one; infact, in the majority of the cases (9/13), the differences is less than 10%.
Figure 3 shows the correlation between DWB and DRM confirming the linear correlation obtained by Mínguez et al. with a percentage differences between the angular coefficients equals to 6.25%. The small contribution of self blood to DRM (about 10%), showed in Figure 2 could explain the strong agreement between DWB and DRM reported in Figure 3.
It should be considered that dosimetric implementation in pediatric practice requires particular skills and a dedicated multidisciplinary team (pediatric nuclear medicine staff, oncologists, medical physicists) in order to obtain the best collaboration according to the patient’s age. Furthermore, in literature there is a limitated experience in dosimetric approach about radionuclide therapy in a pediatric setting; a dosimetric assessment including DRM and DWB could be helpful to have a complete data panel to define a reliable standardized procedure.
In order to have a deeper understanding regarding lesions uptake and relative metastatic response, we assessed absorbed dose to lesions by planar dosimetric approach, when feasible.
Concerning the lesions dosimetry, the doses distribution shows a wide range of calculated values on the first administration (33.9–284.8 Gy). This variability depends on multiple factors related to lesion volume, uptake and effective half-life. Furthermore, the obtained results are comparable with those reported in literature in similar studies (27).
Analysis of Figure 5 and Table 3, a high number of lesions appears to have an uptake reduction between the first and second administration, due to radiosensitivity phenomenon and to the reduction of the lesion size. Therefore it is possible to state that the first administration may not be predictive for the second which will potentially deliver a lower dose.
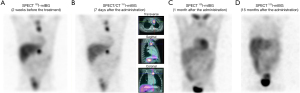
Figure 7 summarizes all clinical efforts to overcome the complex aspects linked to this challenge disease reporting a case of complete response. Otherwise, analysing “Non Responder” cases by dosimetric data allows to have a deeper understanding of limitative factors to obtain a therapeutic response.
In Figure 6, it is possible to note that there is a distinction between the two groups of response (Responder and Non-Responder) with a threshold value near to 120 Gy, confirming that the absorbed dose to lesions could be a good indicator for patients outcome as stated in (27). The value of 120 Gy is considerably higher than the 70 Gy administered in a single injection by Mhattay et al. Biological Effective Dose (BED) assessment could allow to overcome the limits in comparing studies, but in neuroblastoma disease a broad range of values have been reported in literature for radiobilogical factor (α/β: 1.85–17.59 Gy; µ: 0.46–1.28 h−1) (28) resulting in a high variability BED estimation.
Further investigations and a higher number of cases are needed to understand if the absorbed dose to lesions could give important prognostic information predicting patient’s treatment response. The small number of the analyzed sample and the numerical difference of the two groups lead to a large uncertainty in the estimation of the dose threshold.
The main drawbacks of the present study are due to the intrinsic limits of planar method which does not allow dosimetric assessment for overlapped uptake areas. In most of our cases, lesions were placed in retro-hepatic site and the physiological liver uptake limited the applicability of planar dosimetric approach. All this limits could justify the case of a low absorbed dose to lesions with a good clinical response (as reported in Figure 6). Despite the aforementioned limits, analizing each single case we observed a good agreement between the DLesion value (quantitatively calculated) and the clinical response status (assessed by locoregional and global qualitative comparison of pre/post treatment 123I-mIBG scan).
SPECT/CT imaging for tumor dosimetry (3D and voxel dosimetry) overcomes planar limits about critical site and lesion heterogeneity, especially in neuroblastoma disease.
A relatively limited number of whole-body measurements were performed compare to the gold standard protocol [as reported by Gear et al. (20)] due to the lack of a ceiling-mounted Geiger counter. However, 6–7 measurements performed by highly reproducible and accurate methods could still guarantee sufficiently reliable results.
Recent trials have focused on integrated treatment strategies in order to improve the poor prognosis in rrmHR-NBL patients. VERITAS trial (29) represents an encouraging therapeutic approach for very high-risk neuroblastoma clinical management applying intensive high-dose 131I-mIBG therapy in case of poor responder patients after induction chemotherapy. The efficacy of this international therapeutic program including the use of metabolic radiotherapy will be assessed compared with a classic treatment with myeloablative chemotherapy. This intensive treatment strategy combines the radiosensitiser effect of topotecan with the maximized therapeutic efficacy of radionuclide, followed by BuMel (busulfan and melphalan) as myeloablative regimen followed by peripheral blood stem cell rescue. The synergic treatments effect has the therapeutic goal to allow keeping a high anti-proliferative activity against disease. Furthermore, multicentric approach of VERITAS trial allows to obtain stronger results overcoming the limited number of patients with this rare disease submitted to high doses of 131I-mIBG therapy. Tailored treatment approaches based on dosimetry allows to include radionuclide therapy into systemic chemotherapy and myeloablative transplantation protocols maximizing the treatment benefits ensuring a high safety profile. Our experience enforces the confidence the personalized dosimetry could give a significant contribution in patient care affected by high-risk neuroblastoma. We applied high activity therapy 131I-mIBG protocol (as two administration intensive strategy) in heavily pre-treated rrmHR-NBL children; as soon as approved in Italy, we will take part to VERITAS protocol using radionuclide therapy to improve the understanding of its efficacy in poor responder children affected by very high-risk disease. The future implementation of 3D and voxel dosimetry on SPECT/CT imaging will overcome limits of planar dosimetric approach allowing a more accurate assessment of absorbed dose to lesions, regardless of tumor site.
Acknowledgments
We thank Dr. Maria Concetta Longo for taking part at the early phase of our experience presenting our preliminary data at EANM 18 congress; we thank Dr. Elisabetta Genovese for the support; we thank Dr Federica Martire and Davide Ciucci for helping us to calculate the absorbed dose.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/qims-21-548
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/qims-21-548). The authors have no conflicts of interest to declare
Ethics Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of IRCCS Bambino Gesù Children’s Hospital and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zage PE. Novel Therapies for Relapsed and Refractory Neuroblastoma. Children (Basel) 2018;5:148. [Crossref] [PubMed]
- London WB, Castel V, Monclair T, Ambros PF, Pearson AD, Cohn SL, Berthold F, Nakagawara A, Ladenstein RL, Iehara T, Matthay KK. Clinical and biologic features predictive of survival after relapse of neuroblastoma: a report from the International Neuroblastoma Risk Group project. J Clin Oncol 2011;29:3286-92. [Crossref] [PubMed]
- Matthay KK, Yanik G, Messina J, Quach A, Huberty J, Cheng SC, Veatch J, Goldsby R, Brophy P, Kersun LS, Hawkins RA, Maris JM. Phase II study on the effect of disease sites, age, and prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastoma. J Clin Oncol 2007;25:1054-60. [Crossref] [PubMed]
- Wilson JS, Gains JE, Moroz V, Wheatley K, Gaze MN. A systematic review of 131I-meta iodobenzylguanidine molecular radiotherapy for neuroblastoma. Eur J Cancer 2014;50:801-15. [Crossref] [PubMed]
- Matthay KK, Tan JC, Villablanca JG, Yanik GA, Veatch J, Franc B, Twomey E, Horn B, Reynolds CP, Groshen S, Seeger RC, Maris JM. Phase I dose escalation of iodine-131-metaiodobenzylguanidine with myeloablative chemotherapy and autologous stem-cell transplantation in refractory neuroblastoma: a new approaches to Neuroblastoma Therapy Consortium Study. J Clin Oncol 2006;24:500-6. [Crossref] [PubMed]
- French S, DuBois SG, Horn B, Granger M, Hawkins R, Pass A, Plummer E, Matthay K. 131I-MIBG followed by consolidation with busulfan, melphalan and autologous stem cell transplantation for refractory neuroblastoma. Pediatr Blood Cancer 2013;60:879-84. [Crossref] [PubMed]
- Gaze MN, Chang YC, Flux GD, Mairs RJ, Saran FH, Meller ST. Feasibility of dosimetry-based high-dose 131I-meta-iodobenzylguanidine with topotecan as a radiosensitizer in children with metastatic neuroblastoma. Cancer Biother Radiopharm 2005;20:195-9. [Crossref] [PubMed]
- Lewington V, Lambert B, Poetschger U, Sever ZB, Giammarile F, McEwan AJB, Castellani R, Lynch T, Shulkin B, Drobics M, Staudenherz A, Ladenstein R. 123I-mIBG scintigraphy in neuroblastoma: development of a SIOPEN semi-quantitative reporting, method by an international panel. Eur J Nucl Med Mol Imaging 2017;44:234-41. [Crossref] [PubMed]
- Park JR, Bagatell R, Cohn SL, Pearson AD, Villablanca JG, Berthold F, Burchill S, Boubaker A, McHugh K, Nuchtern JG, London WB, Seibel NL, Lindwasser OW, Maris JM, Brock P, Schleiermacher G, Ladenstein R, Matthay KK, Valteau-Couanet D. Revisions to the International Neuroblastoma Response Criteria: A Consensus Statement From the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol 2017;35:2580-7. [Crossref] [PubMed]
- Kletting P, Schimmel S, Kestler HA, Hänscheid H, Luster M, Fernández M, Bröer JH, Nosske D, Lassmann M, Glatting G. Molecular radiotherapy: the NUKFIT software for calculating the time-integrated activity coefficient. Med Phys 2013;40:102504. [Crossref] [PubMed]
- Glatting G, Kletting P, Reske SN, Hohl K, Ring C. Choosing the optimal fit function: comparison of the Akaike information criterion and the F-test. Med Phys 2007;34:4285-92. [Crossref] [PubMed]
- Hindorf C, Glatting G, Chiesa C, Lindén O, Flux GEANM Dosimetry Committee. EANM Dosimetry Committee guidelines for bone marrow and whole-body dosimetry. Eur J Nucl Med Mol Imaging 2010;37:1238-50. [Crossref] [PubMed]
- Buckley SE, Saran FH, Gaze MN, Chittenden S, Partridge M, Lancaster D, Pearson A, Flux GD. Dosimetry for fractionated (131)I-mIBG therapies in patients with primary resistant high-risk neuroblastoma: preliminary results. Cancer Biother Radiopharm 2007;22:105-12. [Crossref] [PubMed]
- Stabin MG, Siegel JA. Physical models and dose factors for use in internal dose assessment. Health Phys 2003;85:294-310. [Crossref] [PubMed]
- Giostra A, Richetta E, Pasquino M, Miranti A, Cutaia C, Brusasco G, Pellerito RE, Stasi M. Red marrow and blood dosimetry in (131)I treatment of metastatic thyroid carcinoma: pre-treatment versus in-therapy results. Phys Med Biol 2016;61:4316-26. [Crossref] [PubMed]
- Stabin MG, Siegel JA, Sparks RB. Sensitivity of model-based calculations of red marrow dosimetry to changes in patient-specific parameters. Cancer Biother Radiopharm 2002;17:535-43. [Crossref] [PubMed]
- Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 2005;46:1023-7. [PubMed]
- Siegel JA, Thomas SR, Stubbs JB, Stabin MG, Hays MT, Koral KF, Robertson JS, Howell RW, Wessels BW, Fisher DR, Weber DA, Brill AB. MIRD pamphlet no. 16: Techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates. J Nucl Med 1999;40:37S-61S. [PubMed]
- Ogawa K, Harata Y, Ichihara T, Kubo A, Hashimoto S. A practical method for position-dependent Compton-scatter correction in single photon emission CT. IEEE Trans Med Imaging 1991;10:408-12. [Crossref] [PubMed]
- Gear J, Chiesa C, Lassmann M, Gabiña PM, Tran-Gia J, Stokke C, Flux GEANM Dosimetry Committee. EANM Dosimetry Committee series on standard operational procedures for internal dosimetry for 131I mIBG treatment of neuroendocrine tumours. EJNMMI Phys 2020;7:15. [Crossref] [PubMed]
- Sgouros G. Blood and bone marrow dosimetry in radioiodine therapy of thyroid cancer. J Nucl Med 2005;46:899-900; author reply 901. [PubMed]
- Mínguez P, Flux G, Genollá J, Guayambuco S, Delgado A, Fombellida JC, Sjögreen Gleisner K. Dosimetric results in treatments of neuroblastoma and neuroendocrine tumors with (131)I-metaiodobenzylguanidine with implications for the activity to administer. Med Phys 2015;42:3969-78. [Crossref] [PubMed]
- Genolla J, Rodriguez T, Minguez P, Lopez-Almaraz R, Llorens V, Echebarria A. Dosimetry-based high-activity therapy with 131I-metaiodobenzylguanidine (131I-mIBG) and topotecan for the treatment of high-risk refractory neuroblastoma. Eur J Nucl Med Mol Imaging 2019;46:1567-75. [Crossref] [PubMed]
- Toporski J, Garkavij M, Tennvall J, Ora I, Gleisner KS, Dykes JH, Lenhoff S, Juliusson G, Scheding S, Turkiewicz D, Békássy AN. High-dose iodine-131-metaiodobenzylguanidine with haploidentical stem cell transplantation and posttransplant immunotherapy in children with relapsed/refractory neuroblastoma. Biol Blood Marrow Transplant 2009;15:1077-85. [Crossref] [PubMed]
- Buckley SE, Chittenden SJ, Saran FH, Meller ST, Flux GD. Whole-body dosimetry for individualized treatment planning of 131I-MIBG radionuclide therapy for neuroblastoma. J Nucl Med 2009;50:1518-24. [Crossref] [PubMed]
- Giammarile F, Chiti A, Lassmann M, Brans B, Flux G. EANM. EANM procedure guidelines for 131I-meta-iodobenzylguanidine (131I-mIBG) therapy. Eur J Nucl Med Mol Imaging 2008;35:1039-47. [Crossref] [PubMed]
- Matthay KK, Panina C, Huberty J, Price D, Glidden DV, Tang HR, Hawkins RA, Veatch J, Hasegawa B. Correlation of tumor and whole-body dosimetry with tumor response and toxicity in refractory neuroblastoma treated with (131)I-MIBG. J Nucl Med 2001;42:1713-21. [PubMed]
- Mínguez P, Gustafsson J, Flux G, Gleisner KS. Biologically effective dose in fractionated molecular radiotherapy--application to treatment of neuroblastoma with (131)I-mIBG. Phys Med Biol 2016;61:2532-51. [Crossref] [PubMed]
- Trial Evaluating and Comparing Two Intensification Treatment Strategies for Metastatic Neuroblastoma Patients With a Poor Response to Induction Chemotherapy (VERITAS). Available online: https://clinicaltrials.gov/ct2/show/NCT03165292


